Technical Outcome, Clinical Success, and Complications of Low-Milliampere Computed Tomography Fluoroscopy-Guided Drainage of Lymphoceles Following Radical Prostatectomy with Pelvic Lymph Node Dissection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Subjects
2.2. Peri-Interventional Imaging and Image Guidance
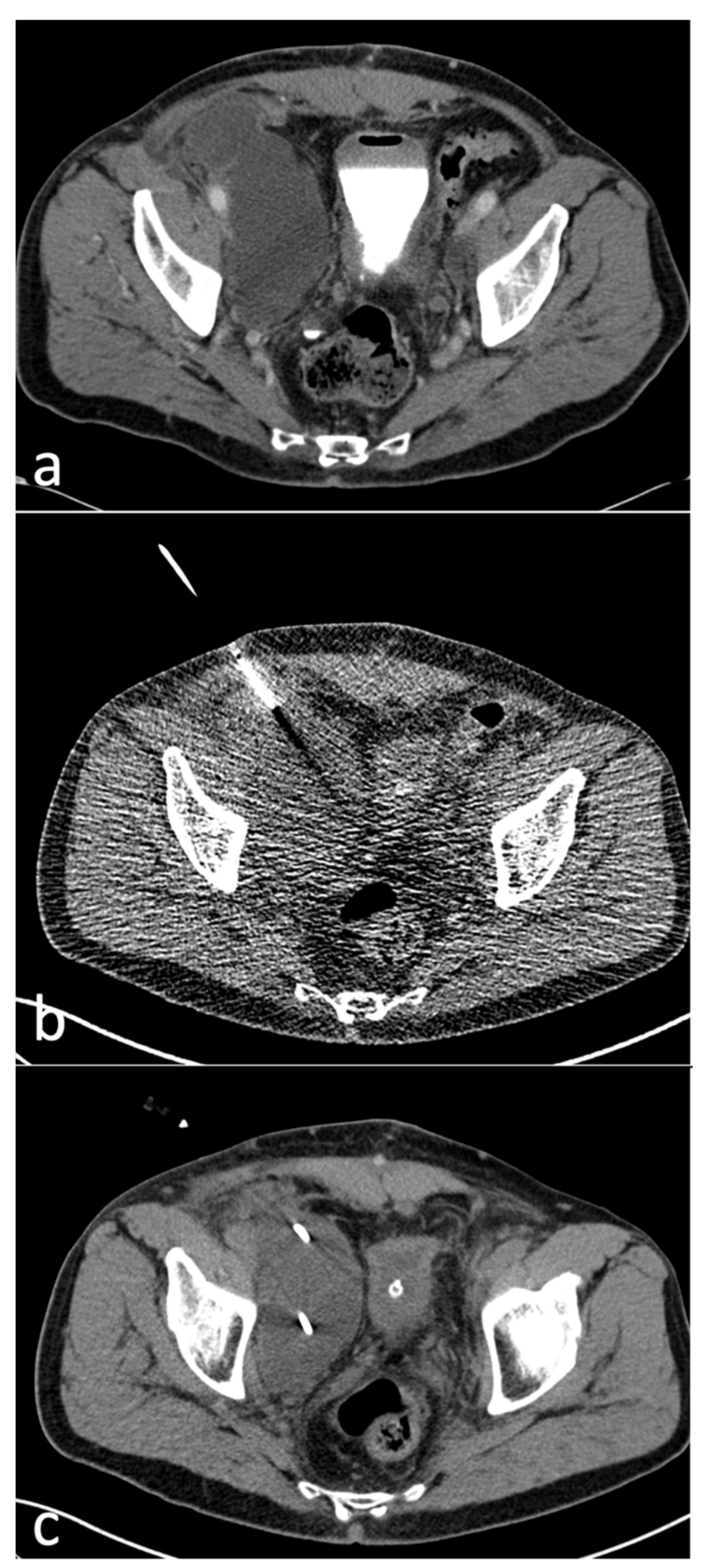
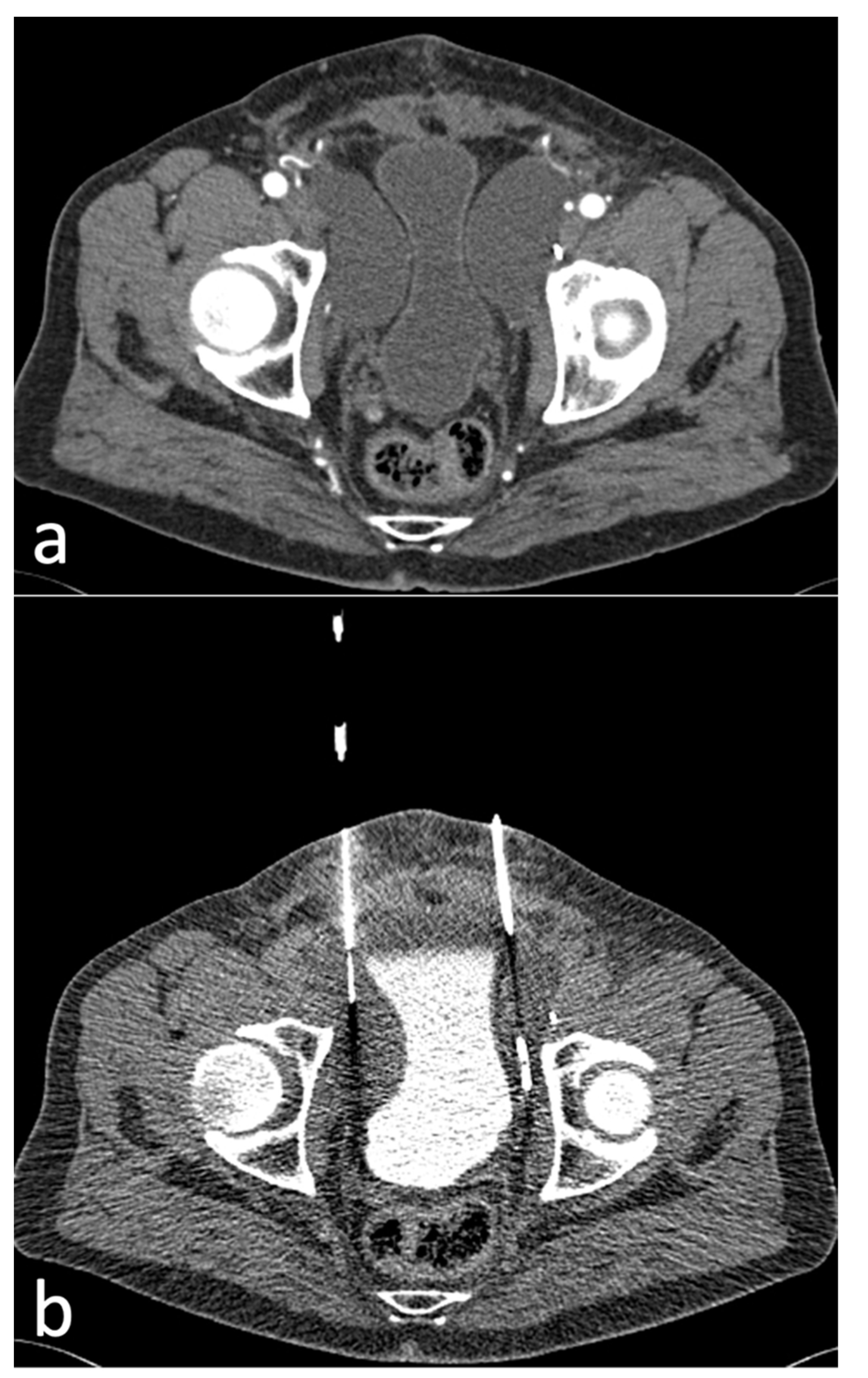
2.3. Procedure
2.4. Assessment of Technical and Clinical Outcome and Complications
2.5. Assessment of Patient Radiation Dose
2.6. Statistical Analysis
3. Results
3.1. Patient Characteristics
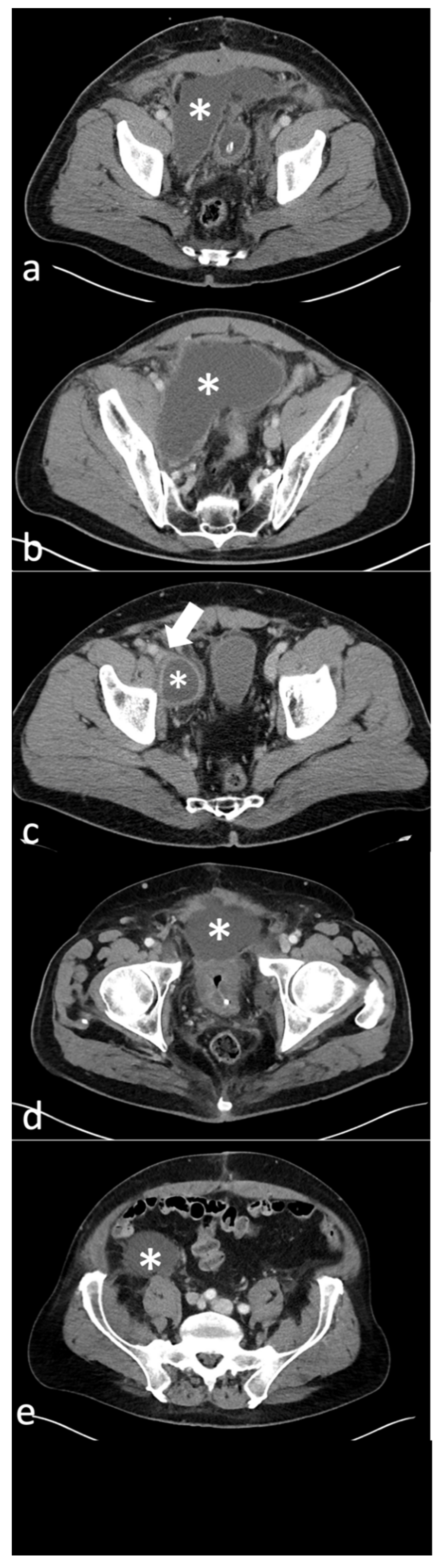
3.2. Classification of Lymphoceles and Characteristics
3.3. Interventions
3.4. Radiation Dose
3.5. Technical Outcome
3.6. Clinical Outcome
3.7. Complications
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Karcaaltincaba, M.; Akhan, O. Radiologic imaging and percutaneous treatment of pelvic lymphocele. Eur. J. Radiol. 2005, 55, 340–354. [Google Scholar] [CrossRef] [PubMed]
- Khoder, W.Y.; Trottmann, M.; Seitz, M.; Buchner, A.; Stuber, A.; Hoffmann, S.; Stief, C.G.; Becker, A.J. Management of pelvic lymphoceles after radical prostatectomy: A multicentre community based study. Eur. J. Med. Res. 2011, 16, 280–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lv, S.; Wang, Q.; Zhao, W.; Han, L.; Wang, Q.; Batchu, N.; Ulain, Q.; Zou, J.; Sun, C.; Du, J.; et al. A review of the postoperative lymphatic leakage. Oncotarget 2017, 8, 69062–69075. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahrer, A.; Ramchandani, P.; Trerotola, S.O.; Shlansky-Goldberg, R.D.; Itkin, M. Sclerotherapy in the management of postoperative lymphocele. J. Vasc. Interv. Radiol. 2010, 21, 1050–1053. [Google Scholar] [CrossRef]
- Khorshidi, F.; Majdalany, B.S.; Peters, G.; Tran, A.N.; Shaikh, J.; Liddell, R.P.; Perez Lozada, J.C.; Kokabi, N.; Nezami, N. Minimally invasive treatment of abdominal lymphocele: A review of contemporary options and how to approach them. Lymphology 2021, 54, 56–67. [Google Scholar] [CrossRef]
- Ten Hove, A.S.; Tjiong, M.Y.; Zijlstra, I.A.J. Treatment of symptomatic postoperative pelvic lymphoceles: A systematic review. Eur. J. Radiol. 2021, 134, 109459. [Google Scholar] [CrossRef]
- Khoder, W.Y.; Gratzke, C.; Haseke, N.; Herlemann, A.; Stief, C.G.; Becker, A.J. Laparoscopic marsupialisation of pelvic lymphoceles in different anatomic locations following radical prostatectomy. Eur. Urol. 2012, 62, 640–648. [Google Scholar] [CrossRef]
- Khoder, W.Y.; Trottmann, M.; Buchner, A.; Stuber, A.; Hoffmann, S.; Stief, C.G.; Becker, A.J. Risk factors for pelvic lymphoceles post-radical prostatectomy. Int. J. Urol. 2011, 18, 638–643. [Google Scholar] [CrossRef]
- Magistro, G.; Tuong-Linh Le, D.; Westhofen, T.; Buchner, A.; Schlenker, B.; Becker, A.; Stief, C.G. Occurrence of symptomatic lymphocele after open and robot-assisted radical prostatectomy. Cent. European J. Urol. 2021, 74, 341–347. [Google Scholar] [CrossRef]
- Tsaur, I.; Thomas, C. Risk factors, complications and management of lymphocele formation after radical prostatectomy: A mini-review. Int. J. Urol. 2019, 26, 711–716. [Google Scholar] [CrossRef]
- Gossler, C.; Hillinger, J.; Burger, M.; Brundl, J.; Denzinger, S.; Gierth, M.; Breyer, J. Epidemiology and therapy of symptomatic lymphoceles after robot-assisted radical prostatectomy (RARP). Transl. Androl. Urol. 2021, 10, 821–829. [Google Scholar] [CrossRef] [PubMed]
- Chu, H.H.; Shin, J.H.; Kim, J.W.; Noh, S.Y.; Yang, W.J.; Park, S. Lymphangiography and Lymphatic Embolization for the Management of Pelvic Lymphocele After Radical Prostatectomy in Prostatic Cancer. Cardiovasc. Intervent. Radiol. 2019, 42, 873–879. [Google Scholar] [CrossRef] [PubMed]
- White, M.; Mueller, P.R.; Ferrucci, J.T., Jr.; Butch, R.J.; Simeone, J.F.; Neff, C.C.; Yoder, I.; Papanicolaou, N.; Pfister, R.C. Percutaneous drainage of postoperative abdominal and pelvic lymphoceles. AJR Am. J. Roentgenol. 1985, 145, 1065–1069. [Google Scholar] [CrossRef] [PubMed]
- Khoder, W.Y.; Becker, A.J.; Seitz, M.; Haseke, N.; Schlenker, B.; Stief, C.G. Modified laparoscopic lymphocele marsupialization for the treatment of lymphoceles after radical prostatectomy: First results. J. Laparoendosc. Adv. Surg. Tech. A 2011, 21, 145–148. [Google Scholar] [CrossRef] [PubMed]
- Carlson, S.K.; Bender, C.E.; Classic, K.L.; Zink, F.E.; Quam, J.P.; Ward, E.M.; Oberg, A.L. Benefits and safety of CT fluoroscopy in interventional radiologic procedures. Radiology 2001, 219, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Young, A.S.; Shyn, P.B.; Johnson, O.W.; Sainani, N.I.; Nawfel, R.D.; Silverman, S.G. Bending percutaneous drainage catheters to facilitate CT-guided insertion using curved trocar technique. Abdom. Radiol. 2017, 42, 2160–2167. [Google Scholar] [CrossRef]
- Filippiadis, D.K.; Binkert, C.; Pellerin, O.; Hoffmann, R.T.; Krajina, A.; Pereira, P.L. Cirse Quality Assurance Document and Standards for Classification of Complications: The Cirse Classification System. Cardiovasc. Intervent. Radiol. 2017, 40, 1141–1146. [Google Scholar] [CrossRef]
- Kloeckner, R.; dos Santos, D.P.; Schneider, J.; Kara, L.; Dueber, C.; Pitton, M.B. Radiation exposure in CT-guided interventions. Eur. J. Radiol. 2013, 82, 2253–2257. [Google Scholar] [CrossRef]
- Mottet, N.; van den Bergh, R.C.N.; Briers, E.; Van den Broeck, T.; Cumberbatch, M.G.; De Santis, M.; Fanti, S.; Fossati, N.; Gandaglia, G.; Gillessen, S.; et al. EAU-EANM-ESTRO-ESUR-ISUP-SIOG Guidelines on Prostate Cancer. In Proceedings of the EAU Annual Congress 2022, Amsterdam, The Netherlands, 1—4 July 2022. [Google Scholar]
- Heers, H.; Laumeier, T.; Olbert, P.J.; Hofmann, R.; Hegele, A. Lymphoceles post-radical retropubic prostatectomy: A retrospective evaluation of epidemiology, risk factors and outcome. Urol. Int. 2015, 95, 400–405. [Google Scholar] [CrossRef]
- Franke, M.; Saager, C.; Kroger, J.R.; Borggrefe, J.; Muckner, K. Vacuum-assisted suction drainage as a successful treatment option for postoperative symptomatic lymphoceles. Rofo 2022, 194, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Trumm, C.G.; Burgard, C.; Deger, C.; Stahl, R.; Forbrig, R.; D’Anastasi, M. Intermittent quick-check CT fluoroscopy-guided percutaneous drainage placement in patients with infected renal and perirenal fluid collections: 11-year experience. Diagn. Interv. Radiol. 2021, 27, 378–385. [Google Scholar] [CrossRef] [PubMed]



| Lymphocele Type | Description | Number | % |
|---|---|---|---|
| Type 1 | Paravesical | 2 | 2.2 |
| Type 2A | Lateral pelvic | 48 | 53.9 |
| Type 2B | Deep Pelvic | 17 | 19.1 |
| Type 3 | Prevesical | 5 | 5.6 |
| Type 4 | Pelvic with retroperitoneal extension | 15 | 16.9 |
| Mixed | Mixed | 2 | 2.2 |
| Total | 89 | 100 |
| mAs | No of Interventions | % |
|---|---|---|
| 10 | 52/77 | 67.5 |
| 15 | 2/77 | 2.5 |
| 20 | 11/77 | 14.2 |
| 25 | 10/77 | 12.9 |
| 30 | 2/77 | 2.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Anastasi, M.; Ebenberger, S.; Alghamdi, A.; Helck, A.; Herlemann, A.; Stief, C.; Khoder, W.; Trumm, C.G.; Stahl, R. Technical Outcome, Clinical Success, and Complications of Low-Milliampere Computed Tomography Fluoroscopy-Guided Drainage of Lymphoceles Following Radical Prostatectomy with Pelvic Lymph Node Dissection. Diagnostics 2022, 12, 2394. https://doi.org/10.3390/diagnostics12102394
D’Anastasi M, Ebenberger S, Alghamdi A, Helck A, Herlemann A, Stief C, Khoder W, Trumm CG, Stahl R. Technical Outcome, Clinical Success, and Complications of Low-Milliampere Computed Tomography Fluoroscopy-Guided Drainage of Lymphoceles Following Radical Prostatectomy with Pelvic Lymph Node Dissection. Diagnostics. 2022; 12(10):2394. https://doi.org/10.3390/diagnostics12102394
Chicago/Turabian StyleD’Anastasi, Melvin, Simone Ebenberger, Abdulmajeed Alghamdi, Andreas Helck, Annika Herlemann, Christian Stief, Wael Khoder, Christoph G. Trumm, and Robert Stahl. 2022. "Technical Outcome, Clinical Success, and Complications of Low-Milliampere Computed Tomography Fluoroscopy-Guided Drainage of Lymphoceles Following Radical Prostatectomy with Pelvic Lymph Node Dissection" Diagnostics 12, no. 10: 2394. https://doi.org/10.3390/diagnostics12102394
APA StyleD’Anastasi, M., Ebenberger, S., Alghamdi, A., Helck, A., Herlemann, A., Stief, C., Khoder, W., Trumm, C. G., & Stahl, R. (2022). Technical Outcome, Clinical Success, and Complications of Low-Milliampere Computed Tomography Fluoroscopy-Guided Drainage of Lymphoceles Following Radical Prostatectomy with Pelvic Lymph Node Dissection. Diagnostics, 12(10), 2394. https://doi.org/10.3390/diagnostics12102394





