Characteristics of Hepatitis B Virus Genotype and Sub-Genotype in Hepatocellular Cancer Patients in Vietnam
Abstract
1. Introduction
2. Materials and Methods
2.1. DNA Extraction, Nested PCR, and Complete Genome Sequencing
2.2. HCV RNA Detection
2.3. Phylogenetic Analysis
2.4. Mutational Analysis
2.5. Statistical Analysis
3. Results
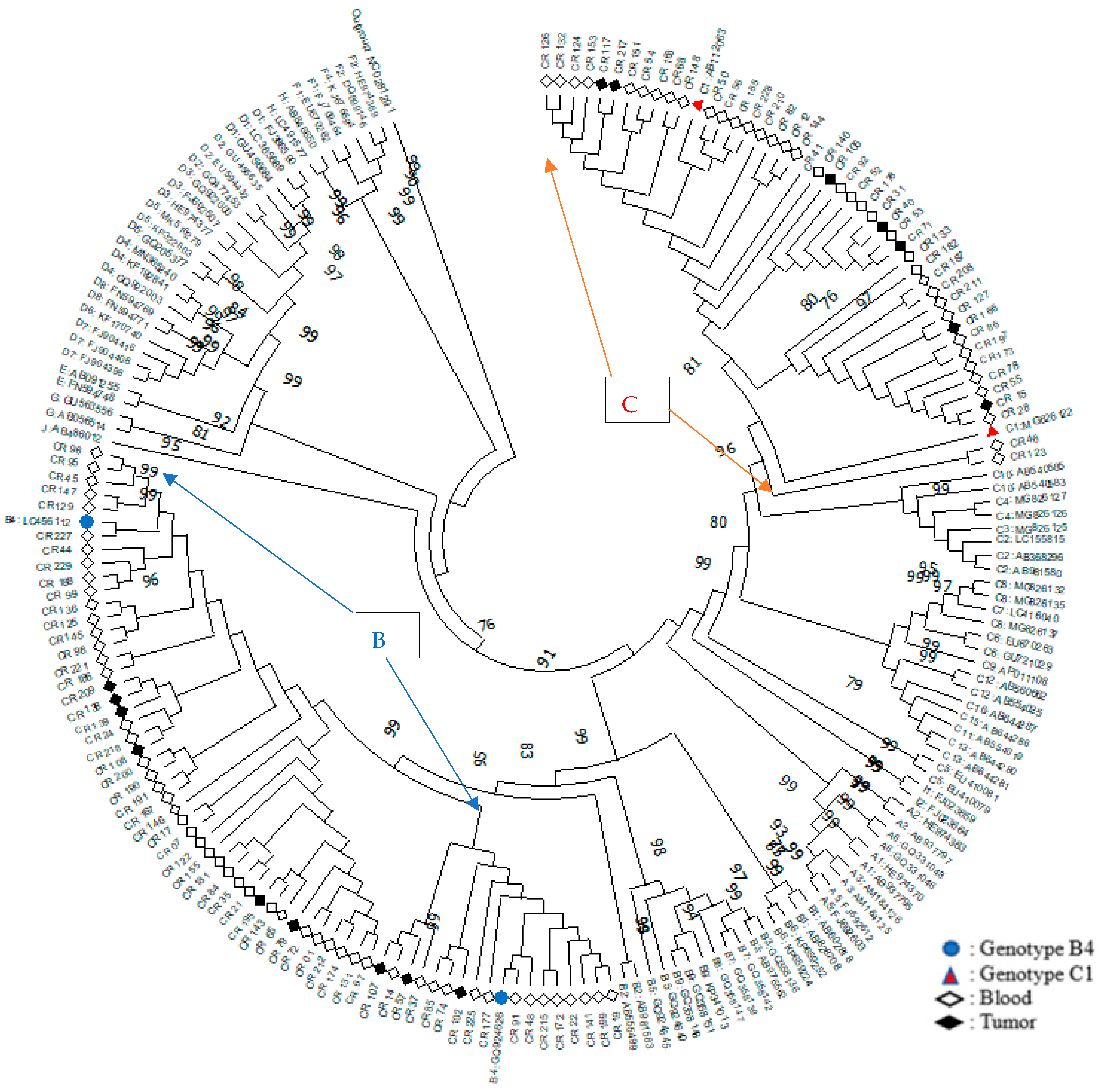
3.1. Phylogenetic Analysis of HBV Genotype
3.2. Distribution of Age and Gender Factors by Genotypes
3.3. Mutational Regions of HBV Genotypes B and C
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HBV | Hepatitis B Virus |
| WHO | World Health Organization |
| HCC | Hepatocellular Carcinoma |
| RFLP | Restriction Fragment Length Polymorphism reaction |
References
- Lavanchy, D.; Kane, M. Global Epidemiology of Hepatitis B Virus Infection. In Hepatitis B Virus in Human Diseases; Humana Press: Cham, Switzerland, 2016; pp. 187–203. [Google Scholar]
- World Health Organization. Global Hepatitis Report; World Health Organization: Geneva, Switzerland, 2017; p. 2017. [Google Scholar]
- Yuen, M.-F.; Chen, D.-S.; Dusheiko, G.M.; Janssen, H.L.; Lau, D.T.; Locarnini, S.A.; Peters, M.G.; Lai, C.L. Hepatitis B virus infection. Nat. Rev. Dis. Prim. 2018, 4, 18035. [Google Scholar] [CrossRef]
- Singal, A.G.; Lampertico, P.; Nahon, P. Epidemiology and surveillance for hepatocellular carcinoma: New trends. J. Hepatol. 2020, 72, 250–261. [Google Scholar] [CrossRef] [PubMed]
- London, W.T.; Petric, J.L.; McGlynn, K.A. Liver Cancer. In Cancer Epidemiology and Prevention, 4th ed.; Fraumeni, S., Ed.; Oxford University Press: Oxford, UK, 2018; pp. 635–659. [Google Scholar]
- Seeger, C.; Mason, W.S. Hepatitis B Virus Biology. Microbiol. Mol. Biol. Rev. 2000, 64, 51. [Google Scholar] [CrossRef]
- Yano, Y.; Azuma, T.; Hayashi, Y. Variations and mutations in the hepatitis B virus genome and their associations with clinical characteristics. World, J. Hepatol. 2015, 7, 583–592. [Google Scholar] [CrossRef]
- Locarnini, S. Hepatitis B viral resistance: Mechanisms and diagnosis. J. Hepatol. 2003, 39 (Suppl. 1), S124–S132. [Google Scholar] [CrossRef]
- Sunbul, M. Hepatitis B virus genotypes: Global distribution and clinical importance. World J. Gastroenterol. WJG 2014, 20, 5427–5434. [Google Scholar] [CrossRef] [PubMed]
- Pourkarim, M.R.; Amini-Bavil-Olyaee, S.; Kurbanov, F.; Van Ranst, M.; Tacke, F. Molecular identification of hepatitis B virus genotypes/subgenotypes: Revised classification hurdles and updated resolutions. World J. Gastroenterol. WJG 2014, 20, 7152–7168. [Google Scholar] [CrossRef] [PubMed]
- Do, S.H.; Yamada, H.; Fujimoto, M.; Ohisa, M.; Matsuo, J.; Akita, T.; Katayama, K.; Van Nguyen, N.; Miyakawa, Y.; Tanaka, J. High prevalences of hepatitis B and C virus infections among adults living in Binh Thuan province, Vietnam. Hepatol. Res. Off. J. Jpn. Soc. Hepatol. 2015, 45, 259–268. [Google Scholar] [CrossRef]
- Bui, T.T.T.; Tran, T.T.; Nghiem, M.N.; Rahman, P.; Tran, T.T.T.; Dinh, M.N.H.; Le, M.H.; Nguyen, V.V.C.; Thwaites, G.; Rahman, M. Molecular characterization of hepatitis B virus in Vietnam. BMC Infect. Dis. 2017, 17, 601. [Google Scholar] [CrossRef]
- Toan, N.L.; Song, L.H.; Kremsner, P.G.; Duy, D.N.; Binh, V.Q.; Koeberlein, B.; Kaiser, S.; Kandolf, R.; Torresi, J.; Bock, C.T. Impact of the hepatitis B virus genotype and genotype mixtures on the course of liver disease in Vietnam. Hepatology 2006, 43, 1375–1384. [Google Scholar] [CrossRef]
- Tatsukawa, M.; Takaki, A.; Shiraha, H.; Koike, K.; Iwasaki, Y.; Kobashi, H.; Fujioka, S.I.; Sakaguchi, K.; Yamamoto, K. Hepatitis B virus core promoter mutations G1613A and C1653T are significantly associated with hepatocellular carcinoma in genotype C HBV-infected patients. BMC Cancer 2011, 11, 458. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Deng, H.; Peng, Z.; Huang, Y.; Long, Q.; Huang, A. Mutations of Basal core promoter and precore regions in hepatitis B virus genotypes B and C. Hepat. Mon. 2015, 15, e23034. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Lee, S.A.; Do, S.Y.; Kim, B.J. Precore/core region mutations of hepatitis B virus related to clinical severity. World J. Gastroenterol. WJG 2016, 22, 4287–4296. [Google Scholar] [CrossRef]
- Liu, B.M.; Li, T.; Xu, J.; Li, X.G.; Dong, J.P.; Yan, P.; Yang, J.X.; Yan, L.; Gao, Z.Y.; Li, W.P.; et al. Characterization of potential antiviral resistance mutations in hepatitis B virus reverse transcriptase sequences in treatment-naïve Chinese patients. Antivir. Res. 2010, 85, 512–519. [Google Scholar] [CrossRef]
- Te, H.S.; Jensen, D.M. Epidemiology of hepatitis B and C viruses: A global overview. Clin. Liver Dis. 2010, 14, 1–21. [Google Scholar] [CrossRef]
- Huy, T.T.; Ushijima, H.; Quang, V.X.; Win, K.M.; Luengrojanakul, P.; Kikuchi, K.; Sata, T.; Abe, K. Genotype C of hepatitis B virus can be classified into at least two subgroups. J. Gen. Virol. 2004, 85, 283–292. [Google Scholar] [CrossRef]
- Kao, J.H.; Chen, P.J.; Lai, M.Y.; Chen, D.S. Hepatitis B genotypes correlate with clinical outcomes in patients with chronic hepatitis B. Gastroenterology 2000, 118, 554–559. [Google Scholar] [CrossRef]
- Orito, E.; Sugauchi, F.; Tanaka, Y.; Ichida, T.; Sata, M.; Tanaka, E.; Okanoue, T.; Sakugawa, H.; Watanabe, H.; Miyakawa, H.; et al. Differences of hepatocellular carcinoma patients with hepatitis B virus genotypes of Ba, Bj or C in Japan. Intervirology 2005, 48, 239–245. [Google Scholar] [CrossRef]
- Tangkijvanich, P.; Mahachai, V.; Komolmit, P.; Fongsarun, J.; Theamboonlers, A.; Poovorawan, Y. Hepatitis B virus genotypes and hepatocellular carcinoma in Thailand. World J. Gastroenterol. WJG 2005, 11, 2238–2243. [Google Scholar] [CrossRef]
- Li, Y.; Li, H.; Spitsbergen, J.M.; Gong, Z. Males develop faster and more severe hepatocellular carcinoma than females in kras(V12) transgenic zebrafish. Sci. Rep. 2017, 7, 41280. [Google Scholar] [CrossRef]
- Qu, L.S.; Liu, J.X.; Liu, T.T.; Shen, X.Z.; Chen, T.Y.; Ni, Z.P.; Lu, C.H. Association of hepatitis B virus pre-S deletions with the development of hepatocellular carcinoma in Qidong, China. PloS ONE 2014, 9, e98257. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.G.; Mahmud, M.M.; Nazir, K.; Ueda, K. PreS1 Mutations Alter the Large HBsAg Antigenicity of a Hepatitis B Virus Strain Isolated in Bangladesh. Int. J. Mol. Sci. 2020, 21, 546. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Qian, Y.-Y.; Fan, H. Pre-S2 and HBV associated hepatocellular carcinoma. Hepatoma Res. 2018, 4, 17. [Google Scholar] [CrossRef]
- Zhand, S.; Karami, C.; Hosseinzadeh Adli, A.; Tabarraei, A.; Khodabakhshi, B.; Moradi, A. Correlation Between Hepatitis B G1896A Precore Mutations and HBeAg in Chronic HBV Patients. Jundishapur. J. Microbiol. 2015, 8, e17126. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, S.; Che, Y.; Chen, D.; Liu, Y.; Shi, Y. Exploring new targets for the treatment of hepatitis-B virus and hepatitis-B virus-associated hepatocellular carcinoma: A new perspective in bioinformatics. Medicine 2021, 100, e26917. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Feng, J.; Wu, X.; Chu, W.; Zhang, Y.; Li, P. Bioinformatics Analysis of Candidate Genes and Pathways Related to Hepatocellular Carcinoma in China: A Study Based on Public Databases. Pathol. Oncol. Res. 2021, 27, 588532. [Google Scholar] [CrossRef] [PubMed]
| Region | Nucleotide | Amino Acid | Genotype B n= 62 | Genotype C n = 45 | p Value | |
|---|---|---|---|---|---|---|
| PreS1 (2848–3204) | A2962G | N38E | 60 (96.8) | 0 | <0.001 * | |
| C2964A | N38E | 60 (96.8) | 0 | <0.001 * | ||
| C3026A | A60V | 61 (98.4) | 44 (97.8) | 0.818 * | ||
| C3116T | A90V | 2 (3.2) | 1 (2.2) | 0.756 * | ||
| PreS2 (3205–155) | A3205G | M1V (Stop) | 5 (8.1) | 2 (4.4) | 0.696 * | |
| A3205C | M1L (Stop) | 1 (1.6) | 0 | 1.000 * | ||
| T3206C | M1T | 0 | 3 (6.7) | 0.071 * | ||
| T31C | No change | 1 (1.6) | 0 | 1.000 * | ||
| T53C | F22I | 9 (14.5) | 11 (24.4) | 0.193 ** | ||
| S (155–835) | A162G | N3S | 1 (1.6) | 45 (100) | <0.001 * | |
| C512T | P120S | 2 (3.2) | 0 | 0.508 * | ||
| C512A | P120T | 5 (8.1) | 0 | 0.072 * | ||
| C531T | T126I | 0 | 25 (55.6) | <0.001 * | ||
| C531A | T126N | 0 | 2 (4.4) | 0.175 * | ||
| A551T | M133L | 5 (8.1) | 0 | 0.072 * | ||
| T552C | M133T | 0 | 1 (2.2) | 0.421 * | ||
| G587A | G145R | 0 | 1 (2.2) | 0.421 * | ||
| G588C | G145A | 0 | 2 (4.4) | 0.175 * | ||
| T705C | V184A | 3 (4.8) | 1 (2.2) | 0.637 * | ||
| T766A | S204R | 11 (17.7) | 0 | 0.002 * | ||
| X (1374–1838) Enhancer II (1685–1773) | G1613A | NC | 23 (37.1) | 16 (35.6) | 0.870 ** | |
| C1653T | H94Y | 8 (12.9) | 6 (13.3) | 0.948 ** | ||
| G1721A | No change | 3 (4.8) | 39 (86.7) | <0.001 ** | ||
| X (1374–1838) BCP (1751–1769) | A1762T | K130M | 32 (51.6) | 43 (95.6) | 0.000 * | |
| G1764A | V131I | 31 (50) | 43 (95.6) | 0.000 * | ||
| G1764C | 0 | 1 (2.2) | 0.421 * | |||
| A1762T + G1764A | 31 (50) | 42 (93.3) | <0.001 * | |||
| C1766T + (G1764A) | V131I | 6 (9.9) | 3 (6.7) | 0.731 * | ||
| C1766T | No chance | 1 (1.6) | 0 | 1.000 * | ||
| T1768A | F132Y | 8 (12.9) | 4 (8.9) | 0.758 * | ||
| A1775G | No change | 1 (1.6) | 24 (53.3) | 0 * | ||
| Precore (1814–1900) | T1858C | No change | 1 (1.6) | 41 (91.1) | <0.001 * | |
| G1896A | W28Stop | 46 (74.2) | 2 (4.4) | <0.001 * | ||
| G1899A | G29D | 6 (9.7) | 4 (8.9) | 1.000 * | ||
| Core region (1901–2452) | C2002T | No change | 1 (1.6) | 1 (2.2) | 1.000 * | |
| A2149T/C | E83D | 5 (8.1) | 3 (6.7) | 1.000 * | ||
| A2159G | 11 (17.7) | 6 (13.3) | 0.538 ** | |||
| A2189T | I97F | 2 (3.2) | 1 (2.2) | 1.000 * | ||
| A2189C–C2191T | ||||||
| A2189C | I97L | 19 (30.6) | 15 (33.3) | 0.768 ** | ||
| A2189T-C2191T | ||||||
| C2198A | L100I | 15 (24.2) | 10 (22.2) | 0.812 ** | ||
| G2203A | No change | 1 (1.6) | 0 | 1.000 * | ||
| Polymerase (2307–1623) | Putative Nar mutation | G287A | S53N | 47 (75.8) | 0 | <0.001 * |
| A286G-G287A | S53D | 7 (11.3) | 0 | 0.021 * | ||
| T382G | S85A | 0 | 1 (2.2) | 0.421 * | ||
| A400C | I91L | 56 (90.3) | 40 (88.9) | 0.810 ** | ||
| A400C-T402G | ||||||
| C505T | H126Y | 1 (1.6) | 0 | 1.000 * | ||
| C512A | T128N | 5 (8.1) | 0 | 0.072 * | ||
| C512T | T128I | 1 (1.6) | 0 | 1.000 * | ||
| A511G | T128A | 0 | 1 (2.2) | 0.421 * | ||
| A544C | N139H | 1 (1.6) | 0 | 1.000 * | ||
| A544C-C546A | N139Q | 1 (1.6) | 0 | 1.000 * | ||
| A544G-C546A | N139E | 0 | 1 (2.2) | 0.421 * | ||
| G587A | R153Q | 0 | 2 (4.4) | 0.175 * | ||
| G700A | V191I | 0 | 1 (2.2) | 0.421 * | ||
| G748A | V207M | 11 (17.7) | 0 | 0.002 * | ||
| G748C | V207L | 0 | 1 (2.2) | 0.421 * | ||
| T766A | S213T | 10 (16.1) | 0 | 0.005 * | ||
| A774C | Q215H | 2 (3.2) | 0 | 0.508 * | ||
| T779G | L217R | 1 (1.6) | 0 | 1.000 * | ||
| A/G783C | E218D | 2 (3.2) | 0 | 0.508 * | ||
| T791A | F221Y | 59 (95.2) | 5 (11.1) | <0.001 * | ||
| T790C-T791A | F221H | 1 (1.6) | 0 | 1.000 * | ||
| T814A | L229M | 2 (3.2) | 0 | 0.508 * | ||
| T814G | L229V | 1 (1.6) | 0 | 1.000 * | ||
| T815G | L229W | 0 | 1 (2.2) | 0.421 * | ||
| A826G | I233V | 2 (3.2) | 0 | 0.508 * | ||
| A841C-T843C | N238H | 62 (100) | 0 | <0.001 * | ||
| A841C-A842C | ||||||
| A842C | N238T | 0 | 11 (24.4) | <0.001 * | ||
| A841G-A842C | N238A | 0 | 3 (6.7) | 0.071 * | ||
| A895G | S256G | 12 (19.4) | 0 | 0.001 * | ||
| A895G-G896C | S256A | 4 (6.5) | 0 | 0.137 * | ||
| Pretreatment mutation | A241G | T38A | 0 | 1 (2.2) | 0.421 * | |
| C242A | T38K | 0 | 1 (2.2) | 0.421 * | ||
| T499C | Y124H | 61 (98.4) | 4 (8.9) | <0.001 * | ||
| T499A | Y124N | 1 (1.6) | 0 | 1.000 * | ||
| T499G | Y124D | 0 | 1 (2.2) | 0.421 * | ||
| C/T531G | D134E | 0 | 3 (6.7) | 0.071 * | ||
| G529A-A530G | D134S | 0 | 1 (2.2) | 0.421 * | ||
| G529A | D134N | 1 (1.6) | 1 (2.2) | 0.818 * | ||
| A544C-C546A | N139Q | 1 (1.6) | 0 | 1.000 * | ||
| A544G-C546A | N139E | 0 | 1 (2.2) | 0.421 * | ||
| A544C | N139H | 2 (3.2) | 0 | 0.508 * | ||
| A799G | I224V | 61 (98.4) | 28 (62.2) | <0.001 * | ||
| Total n (%) | Genotype B n (%) | Genotype C n (%) | |
|---|---|---|---|
| 107 (100%) | 62 (57.9%) | 45 (42.1%) | |
| Subgenotype | |||
| B4 | 62 (57.9%) | ||
| C1 | 45 (42.1%) | ||
| Age (Mean ± SD) | 51.4 ± 11.4 | 52.9 ± 11.9 | 49.4 ± 10.4 |
| 30 | 1 (0.9%) | 1 (1.6%) | 0 |
| 31–40 | 20 (18.7%) | 9 (14.5%) | 11 (24.4%) |
| 41–50 | 35 (32.7%) | 20 (32.3%) | 15 (33.3%) |
| 51–60 | 27 (25.2%) | 15 (24.2%) | 12 (26.7%) |
| 61–70 | 17 (15.9%) | 12 (19.4%) | 5 (11.1%) |
| 71–80 | 7 (6.5%) | 5 (8.1%) | 2 (4.4%) |
| Gender | |||
| Male | 94 (87.9%) | 55 (88.7%) | 39 (86.7%) |
| Female | 13 (12.2%) | 7 (11.3%) | 6 (13.3%) |
| Biochemistry (Mean ± SD) | |||
| ALT (U/L) | 56.8 ± 47.6 | 45.2 ± 39.3 | 72.9 ± 53.4 |
| AST (U/L) | 61.9 ± 40.4 | 61.1 ± 46.1 | 62.9 ± 31.3 |
| Bilirubin (mg/dL) | 0.8 ± 0.6 | 0.8 ± 0.3 | 0.8 ± 0.9 |
| GGT (U/L) | 151.4 ± 122.6 | 143.6 ± 114.8 | 162.1 ± 113.2 |
| Albumin (g/dL) | 4.0 ± 0.4 | 4.0 ± 0.3 | 4.0 ± 0.4 |
| PT (s) | 13.5 ± 0.7 | 13.5 ± 0.7 | 13.5 ± 0.7 |
| Hb (g/L) | 139.3 ± 27.3 | 138.8 ± 24.0 | 140.0 ± 31.6 |
| WBC (G/L) | 10.0 ± 15.9 | 10.0 ± 16.2 | 10.0 ± 15.7 |
| Platelet (G/L) | 230.5 ± 74.4 | 243.2 ± 77.9 | 213.0 ± 66.2 |
| Region | Nucleotide | Amino Acid | Genotype B n= 62 | Genotype C n = 45 | p Value |
|---|---|---|---|---|---|
| PreS1 (2848–3204) | A2962G | N38E | 60 (96.8) | 0 | <0.001 * |
| C2964A | N38E | 60 (96.8) | 0 | <0.001 * | |
| C3026A | A60V | 61 (98.4) | 44 (97.8) | 0.818 * | |
| S (155–835) | A162G | N3S | 1 (1.6) | 45 (100) | <0.001 * |
| C531T | T126I | 0 | 25 (55.6) | <0.001 * | |
| T766A | S204R | 11 (17.7) | 0 | 0.002 * | |
| X (1374–1838) Enhancer II (1685–773) | G1721A | No change | 3 (4.8) | 39 (86.7) | <0.001 ** |
| X (1374–1838) BCP (1751–1769) | A1762T | K130M | 32 (51.6) | 43 (95.6) | <0.001 * |
| G1764A | V131I | 31 (50) | 43 (95.6) | <0.001 * | |
| A1762T + G1764A | 31 (50) | 42 (93.3) | <0.001 * | ||
| A1775G | No change | 1 (1.6) | 24 (53.3) | <0.001 * | |
| Precore (1814–1900) | T1858C | No change | 1 (1.6) | 41 (91.1) | <0.001 * |
| G1896A | W28Stop | 46 (74.2) | 2 (4.4) | 0.000 * | |
| Polymerase (2307–1623) Putative Nar mutation | G287A | S53N | 47 (75.8) | 0 | <0.001 * |
| A286G-G287A | S53D | 7 (11.3) | 0 | 0.021 * | |
| G748A | V207M | 11 (17.7) | 0 | 0.002 * | |
| T766A | S213T | 10 (16.1) | 0 | 0.005 * | |
| T791A | F221Y | 59 (95.2) | 5 (11.1) | <0.001 * | |
| A841C-T843C | N238H | 62 (100) | 0 | <0.001 * | |
| A841C-A842C | N238T | 0 | 11 (24.4) | <0.001 * | |
| A842C | |||||
| A895G | S256G | 12 (19.4) | 0 | 0.001 * | |
| Polymerase (2307–1623) Pretreatment mutation | T499C | Y124H | 61 (98.4) | 4 (8.9) | <0.001 * |
| A799G | I224V | 61 (98.4) | 28 (62.2) | <0.001 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ho, P.T.; Balzanelli, M.G.; Distratis, P.; Lazzaro, R.; Tran, D.K.; Nguyen, K.C.D.; Bui, T.M.; Nguyen, T.T.; Pham, S.T.; Nguyen, H.S.D.; et al. Characteristics of Hepatitis B Virus Genotype and Sub-Genotype in Hepatocellular Cancer Patients in Vietnam. Diagnostics 2022, 12, 2393. https://doi.org/10.3390/diagnostics12102393
Ho PT, Balzanelli MG, Distratis P, Lazzaro R, Tran DK, Nguyen KCD, Bui TM, Nguyen TT, Pham ST, Nguyen HSD, et al. Characteristics of Hepatitis B Virus Genotype and Sub-Genotype in Hepatocellular Cancer Patients in Vietnam. Diagnostics. 2022; 12(10):2393. https://doi.org/10.3390/diagnostics12102393
Chicago/Turabian StyleHo, Phat Tan, Mario Giosuè Balzanelli, Pietro Distratis, Rita Lazzaro, Duy Khanh Tran, Kieu C. D. Nguyen, Tri Minh Bui, Thinh Tien Nguyen, Son Truong Pham, Huy Song Dinh Nguyen, and et al. 2022. "Characteristics of Hepatitis B Virus Genotype and Sub-Genotype in Hepatocellular Cancer Patients in Vietnam" Diagnostics 12, no. 10: 2393. https://doi.org/10.3390/diagnostics12102393
APA StyleHo, P. T., Balzanelli, M. G., Distratis, P., Lazzaro, R., Tran, D. K., Nguyen, K. C. D., Bui, T. M., Nguyen, T. T., Pham, S. T., Nguyen, H. S. D., Tran, V. T., Ho, T. T., Dipalma, G., Inchingolo, F., Quek, C., Pham, H. T., Isacco, C. G., Santacroce, L., & Pham, V. H. (2022). Characteristics of Hepatitis B Virus Genotype and Sub-Genotype in Hepatocellular Cancer Patients in Vietnam. Diagnostics, 12(10), 2393. https://doi.org/10.3390/diagnostics12102393










