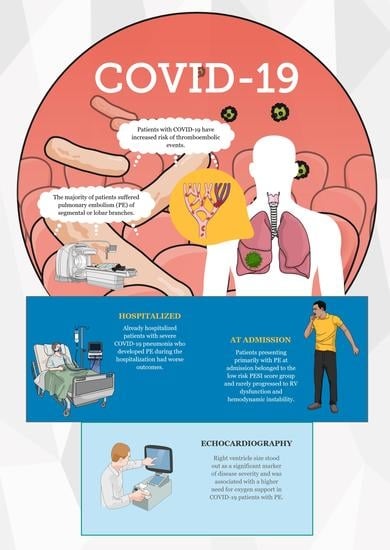
Point-of-Care Echocardiographic Characteristics of COVID-19 Patients with Pulmonary Embolism
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Demographic Data, Past Medical History, and Laboratory Parameters
2.3. Clinically Significant Parameters and Outcomes
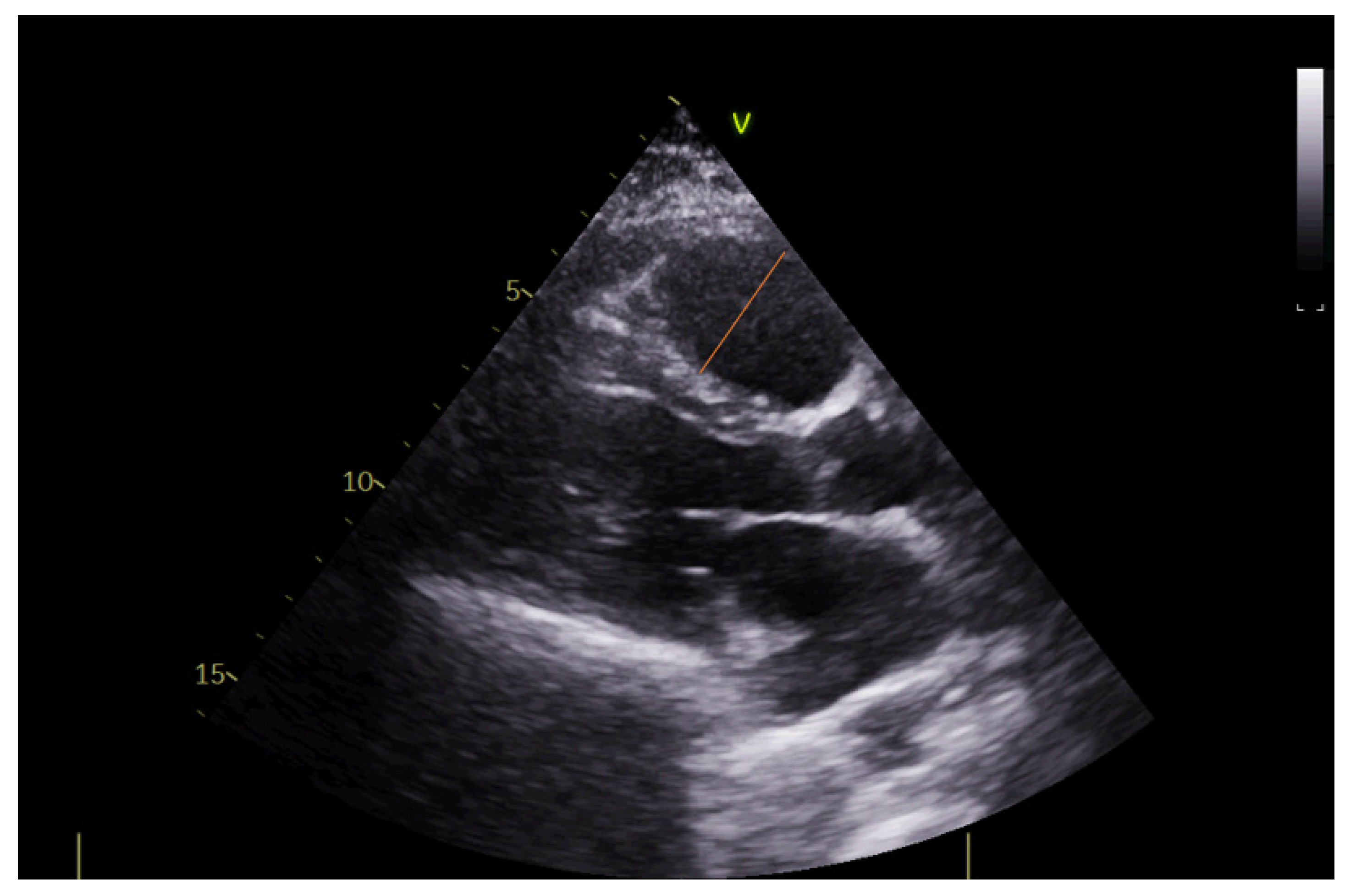
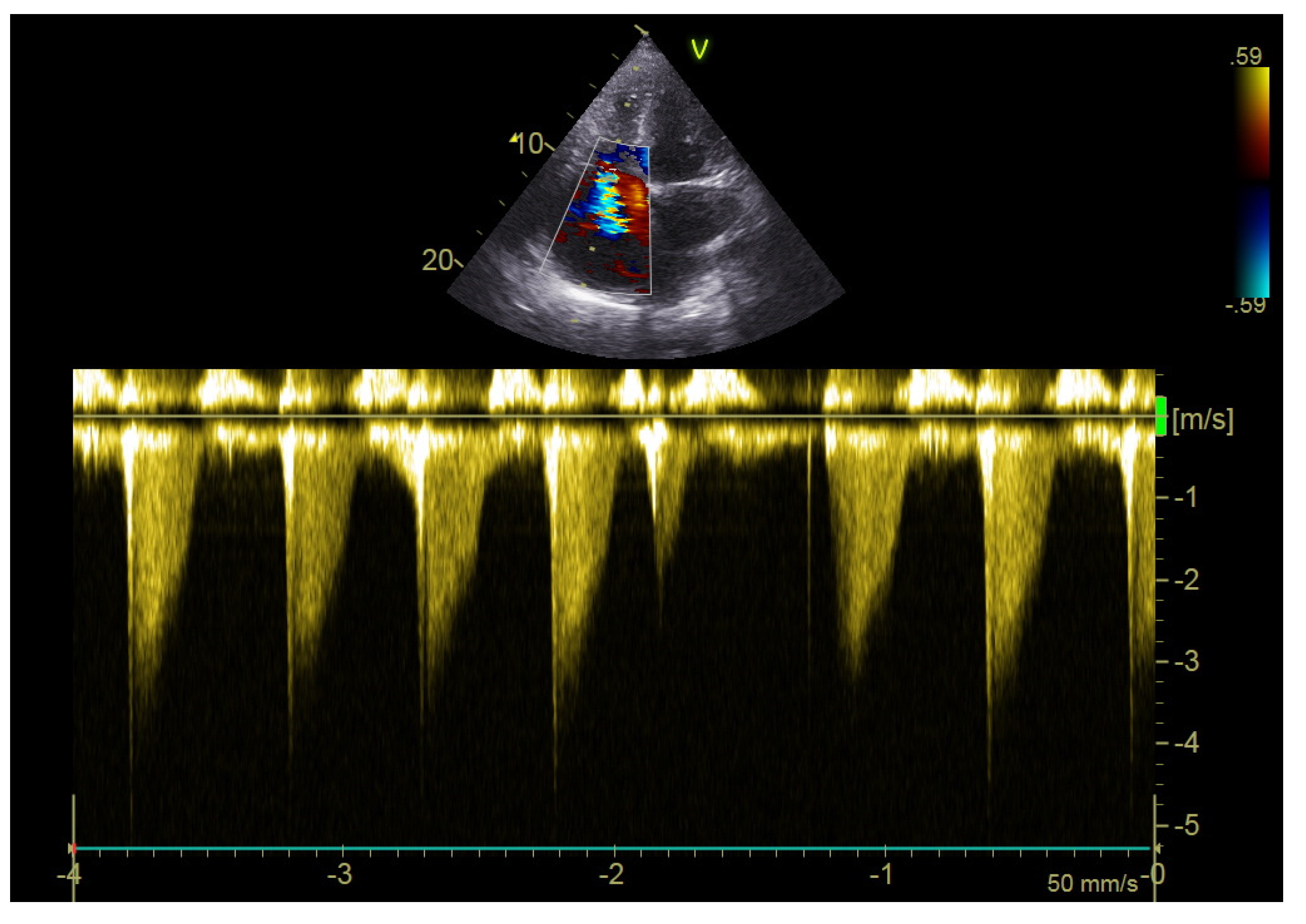
2.4. Echocardiography
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Klok, F.A.; Kruip, M.J.; Van der Meer, N.J.; Arbous, M.S.; Gommers, D.A.; Kant, K.M.; Kaptein, F.H.; van Paassen, J.; Stals, M.A.; Huisman, M.V.; et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Throm. Res. 2020, 191, 145–147. [Google Scholar] [CrossRef] [PubMed]
- Popadic, V.; Klasnja, S.; Milic, N.; Rajovic, N.; Aleksic, A.; Milenkovic, M.; Crnokrak, B.; Balint, B.; Todorovic-Balint, M.; Mrda, D.; et al. Predictors of mortality in critically ill COVID-19 patients demanding high oxygen flow: A thin line between inflammation, cytokine storm, and coagulopathy. Oxid. Med. Cell. Longev. 2021, 2021, 6648199. [Google Scholar] [CrossRef]
- Tang, N.; Li, D.; Wang, X.; Sun, Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J. Thromb. Haemost. 2020, 18, 844–847. [Google Scholar] [CrossRef]
- Cummings, M.J.; Baldwin, M.R.; Abrams, D.; Jacobson, S.D.; Meyer, B.J.; Balough, E.M.; Aaron, J.G.; Claassen, J.; Rabbani, L.E.; Hastie, J.; et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: A prospective cohort study. Lancet 2020, 395, 1763–1770. [Google Scholar] [CrossRef]
- Halaby, R.; Popma, C.J.; Cohen, A.; Chi, G.; Zacarkim, M.R.; Romero, G.; Goldhaber, S.Z.; Hull, R.; Hernandez, A.; Mentz, R.; et al. D-Dimer elevation and adverse outcomes. J. Thromb. Thrombolysis 2015, 39, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Poor, H.D. Pulmonary thrombosis and thromboembolism in COVID-19. Chest 2021, 160, 1471–1480. [Google Scholar] [CrossRef] [PubMed]
- Mirsadraee, S.; Gorog, D.A.; Mahon, C.F.; Rawal, B.; Semple, T.R.; Nicol, E.D.; Arachchillage, D.R.; Devaraj, A.; Price, S.; Desai, S.R.; et al. Prevalence of thrombotic complications in ICU-treated patients with coronavirus disease 2019 detected with systematic CT scanning. Crit. Care Med. 2021, 49, 804–815. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Yuan, B.; Yuan, Y. Incidence and prognostic value of pulmonary embolism in COVID-19: A systematic review and meta-analysis. PLoS ONE 2022, 17, e0263580. [Google Scholar] [CrossRef] [PubMed]
- Ng, J.J.; Liang, Z.C.; Choong, A.M.T.L. The incidence of pulmonary thromboembolism in COVID-19 patients admitted to the intensive care unit: A meta-analysis and meta-regression of observational studies. J. Intensive Care 2021, 9, 20. [Google Scholar] [CrossRef] [PubMed]
- Moore, C.L.; Copel, J.A. Point-of-care ultrasonography. N. Engl. J. Med. 2011, 364, 749–757. [Google Scholar] [CrossRef] [PubMed]
- García-Cruz, E.; Manzur-Sandoval, D.; Rascón-Sabido, R.; Gopar-Nieto, R.; Barajas-Campos, R.L.; Jordán-Ríos, A.; Sierra-Lara Martínez, D.; Jiménez-Rodríguez, G.M.; Murillo-Ochoa, A.L.; Díaz-Méndez, A.; et al. Critical care ultrasonography during COVID-19 pandemic: The ORACLE protocol. Echocardiography 2020, 37, 1353–1361. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Zou, X.; Li, R.; Shu, H.; Yu, Y.; Yang, X.; Shang, Y. Application of POCUS in patients with COVID-19 for acute respiratory distress syndrome management: A narrative review. BMC Pulm. Med. 2022, 22, 52. [Google Scholar] [CrossRef] [PubMed]
- Konstantinides, S.V.; Meyer, G.; Becattini, C.; Bueno, H.; Geersing, G.J.; Harjola, V.P.; Huisman, M.V.; Humbert, M.; Jennings, C.S.; Jiménez, D.; et al. ESC Scientific Document Group. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur. Heart J. 2020, 41, 543–603. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, S.; Down, B.; Jha, S. Point-of-care lung ultrasound in intensive care during the COVID-19 pandemic. Clin. Radiol. 2020, 75, 710.e1–710.e4. [Google Scholar] [CrossRef] [PubMed]
- Shokoohi, H.; Boniface, K.S.; Pourmand, A.; Liu, Y.T.; Davison, D.L.; Hawkins, K.D.; Buhumaid, R.E.; Salimian, M.; Yadav, K. Bedside ultrasound reduces diagnostic uncertainty and guides resuscitation in patients with undifferentiated hypotension. Crit. Care Med. 2015, 43, 2562–2569. [Google Scholar] [CrossRef] [PubMed]
- Karp, J.; Burke, K.; Daubaras, S.M.; McDermott, C. The role of PoCUS in the assessment of COVID-19 patients. J. Ultrasound 2022, 25, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Schrift, D.; Barron, K.; Arya, R.; Choe, C. The use of POCUS to manage ICU patients with COVID-19. J. Ultrasound Med. 2021, 40, 1749–1761. [Google Scholar] [CrossRef] [PubMed]
- Polito, M.V.; Silverio, A.; Di Maio, M.; Bellino, M.; Scudiero, F.; Russo, V.; Rasile, B.; Alfano, C.; Citro, R.; Parodi, G.; et al. Prognostic Implications of Right Ventricular Function and Pulmonary Pressures Assessed by Echocardiography in Hospitalized Patients with COVID-19. J. Pers. Med. 2021, 24, 1245. [Google Scholar] [CrossRef] [PubMed]
- Scudiero, F.; Silverio, A.; Di Maio, M.; Russo, V.; Citro, R.; Personeni, D.; Cafro, A.; D’Andrea, A.; Attena, E.; Pezzullo, S.; et al. Pulmonary embolism in COVID-19 patients: Prevalence, predictors and clinical outcome. Thromb. Res. 2021, 198, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Bikdeli, B.; Madhavan, M.V.; Jimenez, D.; Chuich, T.; Dreyfus, I.; Driggin, E.; Nigoghossian, C.D.; Ageno, W.; Madjid, M.; Guo, Y.; et al. COVID-19 and thrombotic or thromboembolic disease: Implications for prevention, antithrombotic therapy, and follow-up: JACC state-of-the-art review. J. Am. Coll. Cardiol. 2020, 75, 2950–2973. [Google Scholar] [CrossRef] [PubMed]


| Variable | n (%) |
|---|---|
| Gender | |
| Male | 17 (53.1) |
| Female | 15 (46.9) |
| Age, mean ± sd | 64.13 ± 17.3 |
| Smoking, yes | 4 (12.5) |
| Comorbidity | |
| Cardiovascular diseases | 3 (9.4) |
| Hypertension | 21 (65.6) |
| Heart failure | 3 (9.4) |
| Diabetes mellitus type 2 | 4 (12.5) |
| Previous deep vein thrombosis | 4 (12.5) |
| Previous pulmonary embolism | 0 (0.0) |
| Previous immobilization longer than 3 days, yes | 3 (9.4) |
| Current symptoms of deep vein thrombosis, yes | 3 (9.4) |
| Malignancy, yes | 5 (15.6) |
| Vaccination status | |
| Vaccinated | 12 (37.5) |
| Non-vaccinated | 20 (62.5) |
| Variable | n (%) |
|---|---|
| From beginning of symptoms to hospital admission (days), median (25th–75th percentile) | 8 (4.5–11.0) |
| Pulmonary embolism symptoms present at hospital admission, yes | 22 (68.8) |
| Time from hospital admission to pulmonary embolism symptoms (days), median (25th–75th percentile) | 8 (1.0–14.2) |
| Classification based on CT pulmonary angiography findings | |
| Subsegmental PE | 3 (9.7) |
| Segmental PE | 15 (48.4) |
| Lobar PE | 10 (32.3) |
| Massive PE | 3 (9.7) |
| Pulmonary Embolism Severity Index (PESI) score | |
| Very low (≤65) | 5 (15.6) |
| Low (66–85) | 11 (34.4) |
| Intermediate (86–105) | 6 (18.8) |
| High (106–125) | 6 (18.8) |
| Very high (≥125) | 4 (12.5) |
| Pneumonia, yes | 22 (68.8) |
| Pneumonia stages verified by chest CT | |
| Initial stage | 5 (19.2) |
| Progressive stage | 7 (26.9) |
| Peak stage | 9 (34.6) |
| Resolution | 5 (19.2) |
| CT severity score, median (25th–75th percentile) | 8 (0.0–16.75) |
| ICU stay (total days), median (25th–75th percentile) | 10 (7.25–14.75) |
| Anticoagulant therapy, yes | 11 (34.4) |
| Need for oxygen support, yes | 20 (62.5) |
| Non-invasive | 16 (50.0) |
| Mechanical ventilation | 4 (12.5) |
| Fatal outcome, yes | 4 (12.5) |
| Variable | |
|---|---|
| Leukocytes, median (25th–75th percentile) | 6.02 (4.81–9.20) |
| Neutrophils, median (25th–75th percentile) | 4.76 (2.60–6.32) |
| Lymphocytes, median (25th–75th percentile) | 1.03 (0.87–1.48) |
| Hemoglobin, mean ± sd | 129 ± 21 |
| Thrombocytes, median (25th–75th percentile) | 196 (146–284) |
| Urea, median (25th–75th percentile) | 5.9 (5.1–8.4) |
| Creatinine, median (25th–75th percentile) | 88 (75–102) |
| AST, median (25th–75th percentile) | 26 (19–38) |
| ALT, median (25th–75th percentile) | 30 (20–46) |
| D-dimer, median (25th–75th percentile) | 5072 (2709–11,527) |
| D-dimer max, median (25th–75th percentile) | 8110 (4241–18,355) |
| INR, median (25th–75th percentile) | 1.05 (1.0–1.10) |
| aPTT, mean ± sd | 23.7± 4.1 |
| NT-ProBNP, median (25th–75th percentile) | 1807 (278.4–5549.0) |
| CRP, median (25th–75th percentile) | 26.85 (6.4–42.7) |
| CRP max, median (25th–75th percentile) | 35.85 (21.5–125.8) |
| IL-6 max, median (25th–75th percentile) | 28.9 (9.11–49.6) |
| Variable | |
|---|---|
| Heart rate (/min), mean ± sd | 88 ± 23 |
| Systolic blood pressure (mm/Hg), mean ± sd | 125 ± 22 |
| Diastolic blood pressure (mm/Hg), mean ± sd | 78 ± 13 |
| D-dimer, median (25th–75th percentile) | 6811 (3428–14,128) |
| SaO2 (%), mean ± sd | 93 ± 7 |
| Variable | |
|---|---|
| LV ejection fraction (%), mean ± sd | 57 ± 10 |
| Right ventricular diameter (mm), mean ± sd | 29 ± 6 |
| Right/left ventricular diameter ratio, mean ± sd | 0.64 ± 0.11 |
| Right atrial area (cm2), mean ± sd | 17.3 ± 5.3 |
| TAPSE (mm), mean ± sd | 23 ± 4 |
| S’RV (cm/s), mean ± sd | 16 ± 3 |
| PVAT (msec), mean ± sd | 120 ± 25 |
| RV ESP (mmHg), mean ± sd | 36 ± 11 |
| VCI (mm), mean ± sd | 15 ± 2 |
| Signs of deep vein thrombosis on color Doppler, yes, n (%) | 9 (28.1) |
| Variable | Need for Oxygen Support | p | |
|---|---|---|---|
| No (n = 12) | Yes (n = 20) | ||
| Age, mean ± sd | 53.1 ± 21.5 | 70.7 ± 9.8 | 0.003 |
| Pulmonary embolism symptoms present at hospital admission, yes | 12 (100.0) | 10 (50.0) | 0.003 |
| PESI score | |||
| Very low/low | 10 (83.3) | 6 (30.0) | 0.003 |
| Intermediate–high/very high | 2 (16.7) | 14 (70.0) | |
| CRP max, median (25th–75th percentile) | 26.9 (6.2–46.0) | 47.0 (28.4–174.7) | 0.045 |
| CT severity score, median (25th–75th percentile) | 0 (0–5) | 14 (8–18) | 0.001 |
| Heart rate (/min), mean ± sd | 82.4 ± 20.1 | 91.6 ± 24.0 | 0.280 |
| Systolic blood pressure (mm/Hg), mean ± sd | 127.1 ± 24.3 | 122.9 ± 20.6 | 0.610 |
| Diastolic blood pressure (mm/Hg), mean ± sd | 81.7 ± 9.6 | 76.3 ± 15.1 | 0.283 |
| D-dimer, median (25th–75th percentile) | 3766 (3105–6700) | 9590 (5610–20,750) | 0.023 |
| SaO2 (%), mean ± sd Point-of-care echocardiographic parameters | 97.3 ± 1.0 | 90.6 ± 7.6 | 0.005 |
| LV ejection fraction (%), mean ± sd | 55.8 ± 14.6 | 57.8 ± 5.4 | 0.579 |
| Right ventricular diameter (mm), mean ± sd | 26.2 ± 3.5 | 31.3 ± 5.8 | 0.012 |
| Right/left ventricular diameter ratio, mean ± sd | 0.60 ± 0.11 | 0.66 ± 0.11 | 0.461 |
| Right atrial area (cm2), mean ± sd | 17.5 ± 6.7 | 17.2 ± 4.2 | 0.922 |
| TAPSE (mm), mean ± sd | 21.3 ± 5.1 | 23.7 ± 2.2 | 0.112 |
| S’RV (cm/s), mean ± sd | 13.2 ± 1.7 | 17.4 ± 3.4 | 0.061 |
| PVAT (msec), mean ± sd | 131.5 ± 14.0 | 114.7 ± 27.7 | 0.278 |
| RV ESP (mmHg), mean ± sd | 35.5 ± 13.7 | 35.7 ± 10.7 | 0.964 |
| Variable | PESI Score | |
|---|---|---|
| Rho | p | |
| CRP max | 0.445 | 0.011 |
| CT severity score | 0.292 | 0.105 |
| Heart rate | 0.254 | 0.168 |
| Systolic blood pressure | −0.328 | 0.071 |
| Diastolic blood pressure | −0.357 | 0.049 |
| D-dimer at PE diagnosis | 0.388 | 0.031 |
| SaO2 at PE diagnosis | −0.366 | 0.043 |
| LV ejection fraction | −0.387 | 0.028 |
| Right ventricular diameter | 0.315 | 0.084 |
| Right/left ventricular diameter ratio | 0.014 | 0.960 |
| Right atrial area | 0.192 | 0.493 |
| TAPSE | −0.161 | 0.441 |
| S’RV | 0.429 | 0.249 |
| PVAT | −0.535 | 0.049 |
| RV ESP | 0.257 | 0.248 |
| VCI | 0.188 | 0.722 |
| Variable | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| p | OR | 95% CI | p | OR | 95% CI | |
| Age | 0.013 | 1.075 | 1.015–1.138 | |||
| PESI score | 0.014 | 4.452 | 1.361–14.560 | 0.042 | 4.614 | 1.057–20.132 |
| CT score | 0.005 | 1.275 | 1.075–1.513 | 0.035 | 1.319 | 1.020–1.706 |
| D-dimer | 0.041 | 1.000 | 1.000–1.000 | |||
| RV diameter | 0.030 | 1.272 | 1.023–1.582 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klasnja, S.; Manojlovic, A.; Popadic, V.; Ivankovic, T.; Ninkovic, N.; Rajovic, N.; Popovic, M.; Nikolic, N.; Brajkovic, M.; Radojevic, A.; et al. Point-of-Care Echocardiographic Characteristics of COVID-19 Patients with Pulmonary Embolism. Diagnostics 2022, 12, 2380. https://doi.org/10.3390/diagnostics12102380
Klasnja S, Manojlovic A, Popadic V, Ivankovic T, Ninkovic N, Rajovic N, Popovic M, Nikolic N, Brajkovic M, Radojevic A, et al. Point-of-Care Echocardiographic Characteristics of COVID-19 Patients with Pulmonary Embolism. Diagnostics. 2022; 12(10):2380. https://doi.org/10.3390/diagnostics12102380
Chicago/Turabian StyleKlasnja, Slobodan, Andrea Manojlovic, Viseslav Popadic, Tatjana Ivankovic, Nebojsa Ninkovic, Nina Rajovic, Maja Popovic, Novica Nikolic, Milica Brajkovic, Aleksandra Radojevic, and et al. 2022. "Point-of-Care Echocardiographic Characteristics of COVID-19 Patients with Pulmonary Embolism" Diagnostics 12, no. 10: 2380. https://doi.org/10.3390/diagnostics12102380
APA StyleKlasnja, S., Manojlovic, A., Popadic, V., Ivankovic, T., Ninkovic, N., Rajovic, N., Popovic, M., Nikolic, N., Brajkovic, M., Radojevic, A., Lasica, R., Rajsic, S., Todorovic, Z., Brankovic, M., Radonjic, T., Memon, L., Mrda, D., Milic, N., & Zdravkovic, M. (2022). Point-of-Care Echocardiographic Characteristics of COVID-19 Patients with Pulmonary Embolism. Diagnostics, 12(10), 2380. https://doi.org/10.3390/diagnostics12102380