HPV Tests Comparison in the Detection and Follow-Up after Surgical Treatment of CIN2+ Lesions
Abstract
1. Introduction
2. Patients and Methods
2.1. Population
2.2. Hybrid Capture 2
2.3. Linear Array
2.4. Cobas 4800 HPV Test
2.5. Real Time HR HPV
2.6. Onclarity
2.7. Anyplex II
2.8. Aptima
2.9. Cytology
2.10. Histology
2.11. Statistical Methods
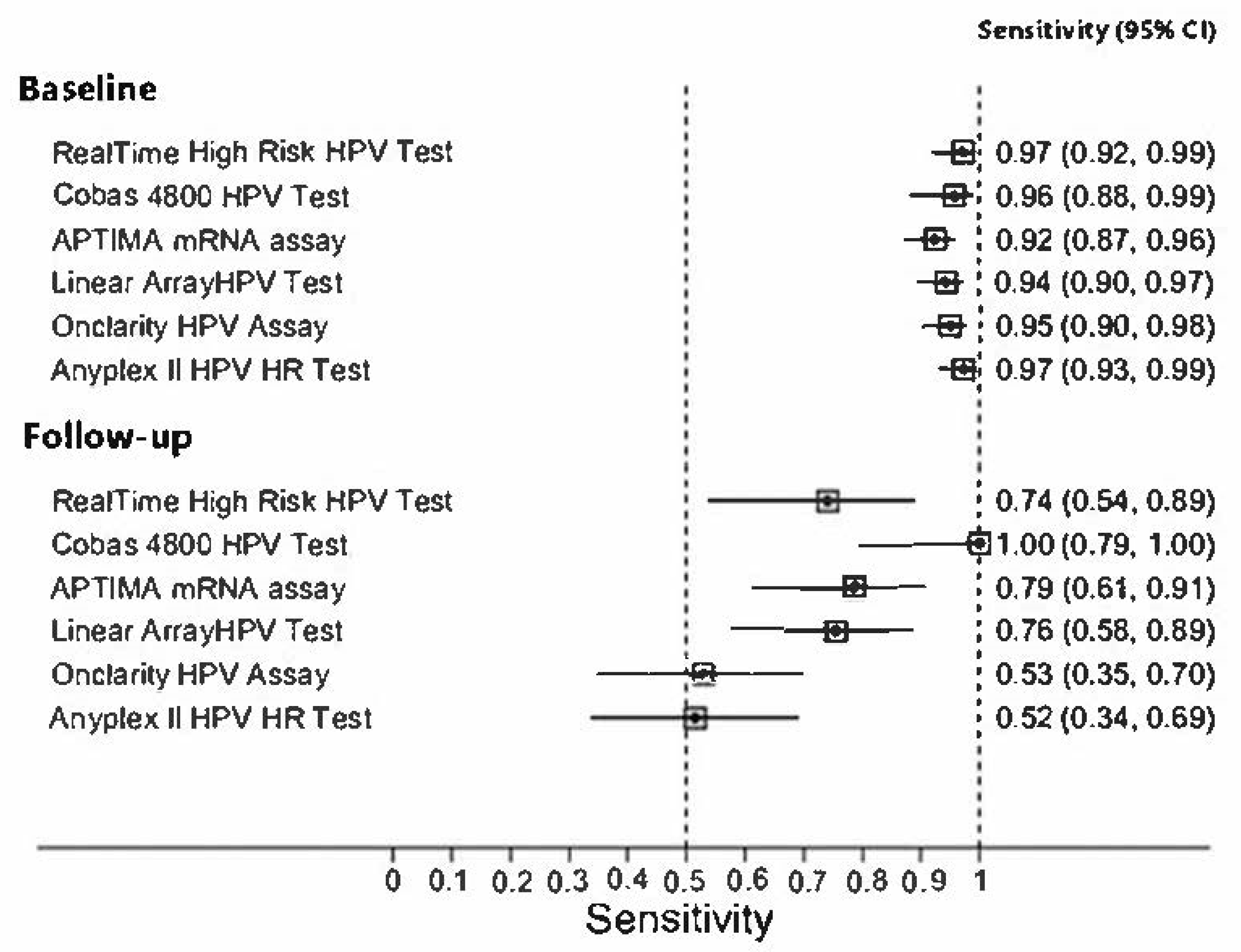
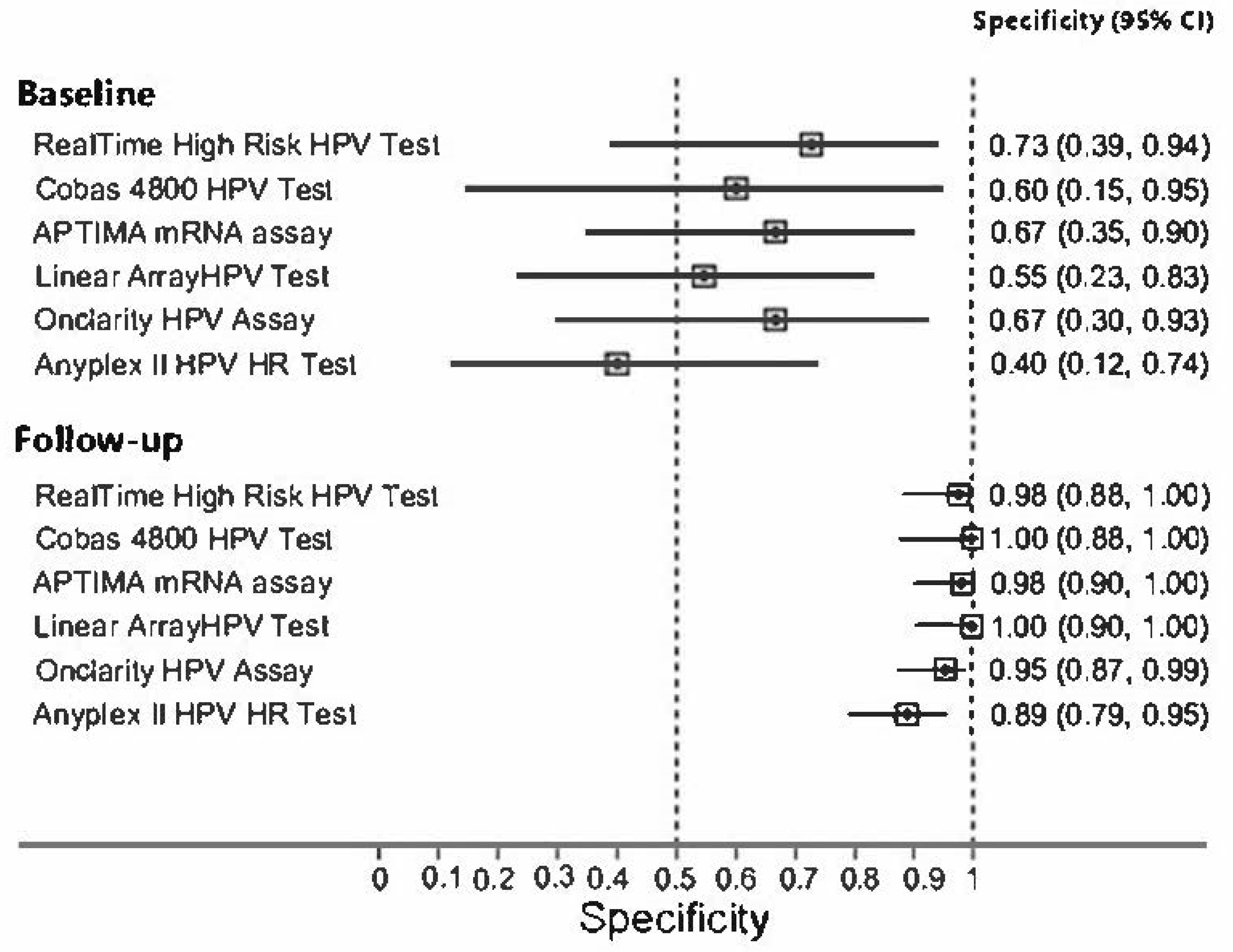
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations and Acronyms
| HPV = | Human Papillomavirus |
| CIN = | Cervical intraepithelial neoplasia |
| HR = | High risk |
| LR= | Low risk |
| IEO = | European Institute of Oncology |
| LBC = | Liquid based cytology |
| LSIL = | Low grade squamous intraepithelial lesion |
| HSIL= | High grade squamous intraepithelial lesion |
| PCR = | Polymerase chain reaction |
| HC2= | Qiagen Hybrid Capture 2 |
| PABAK = | Prevalence-Adjusted and Bias-Adjusted Kappa |
References
- Zur Hausen, H. Papillomaviruses in the causation of human cancers—A brief historical account. Virology 2009, 384, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Kjær, S.K.; Frederiksen, K.; Munk, C.; Iftner, T. Long-term absolute risk of cervical intraepithelial neoplasia grade 3 or worse following human papillomavirus infection: Role of persistence. J. Natl. Cancer Inst. 2010, 102, 1478–1488. [Google Scholar] [CrossRef] [PubMed]
- Poljak, M.; Oštrbenk Valenčak, A.; Gimpelj Domjanič, G.; Xu, L.; Arbyn, M. Commercially available molecular tests for human papillomaviruses: A global overview. Clin. Microbiol. Infect. 2020, 26, 1144–1150. [Google Scholar] [CrossRef] [PubMed]
- Arbyn, M.; Simon, M.; Peeters, E.; Xu, L.; Meijer, C.J.L.M.; Berkhof, J.; Cuschieri, K.; Bonde, J.; Ostrbenk Vanlencak, A.; Zhao, F.H.; et al. 2020 list of human papillomavirus assays suitable for primary cervical cancer screening. Clin. Microbiol. Infect. 2021, 27, 1083–1095. [Google Scholar] [CrossRef] [PubMed]
- Mariani, L.; Sandri, M.T.; Preti, M.; Origoni, M.; Costa, S.; Cristoforoni, P.; Bottari, F.; Sideri, M. HPV-Testing in Follow-up of Patients Treated for CIN2+ Lesions. J. Cancer 2016, 7, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Bottari, F.; Iacobone, A.D.; Passerini, R.; Preti, E.P.; Sandri, M.T.; Cocuzza, C.E.; Gary, D.S.; Andrews, J.C. Human Papillomavirus Genotyping Compared with a Qualitative High-Risk Human Papillomavirus Test After Treatment of High-Grade Cervical Intraepithelial Neoplasia: A Systematic Review. Obstet. Gynecol. 2019, 134, 452–462. [Google Scholar] [CrossRef] [PubMed]
- Iacobone, A.D.; Radice, D.; Sandri, M.T.; Preti, E.P.; Guerrieri, M.E.; Vidal Urbinati, A.M.; Pino, I.; Franchi, D.; Passerini, R.; Bottari, F. Human Papillomavirus Same Genotype Persistence and Risk of Cervical Intraepithelial Neoplasia2+ Recurrence. Cancers 2021, 13, 3664. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Ostrbenk, A.; Poljak, M.; Arbyn, M. Assessment of the Roche linear array HPV genotyping test within the VALGENT framework. J. Clin. Virol. 2018, 98, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Heideman, D.A.M.; Hesselink, A.T.; Berkhof, J.; Van Kemenade, F.; Melchers, W.J.G.; Daalmeijer, N.F.; Verkuijten, M.; Meijer, C.J.L.M.; Snijders, P.J.F. Clinical validation of the cobas(R)4800 HPV test for cervical screening purposes. J. Clin. Microbiol. 2011, 49, 3983–3985. [Google Scholar] [CrossRef] [PubMed]
- Carozzi, F.M.; Burroni, E.; Bisanzi, S.; Puliti, D.; Confortini, M.; Giorgi Rossi, P.; Sani, C.; Scalisi, A.; Chini, F. Comparison of clinical performance of Abbott RealTime high risk HPV test with that of Hybrid Capture 2 assay in a screening setting. J. Clin. Microbiol. 2011, 49, 1446e51. [Google Scholar] [CrossRef] [PubMed]
- Ejegod, D.; Bottari, F.; Pedersen, H.; Sandri, M.T.; Bonde, J. The BD onclarity HPV assay on SurePath collected samples meets the international guidelines for human papillomavirus test requirements for cervical screening. J. Clin. Microbiol. 2016, 54, 2267–2272. [Google Scholar] [CrossRef] [PubMed]
- Hesselink, A.T.; Sahli, R.; Berkhof, J.; Snijders, P.J.; van der Salm, M.L.; Agard, D.; Bleeker, M.C.; Heideman, D.A. Clinical validation of Anyplex II HPV HR detection according to the guidelines for HPV test requirements for cervical cancer screening. J. Clin. Virol. 2016, 76, 36–39. [Google Scholar] [CrossRef] [PubMed]
- Heideman, D.A.; Hesselink, A.T.; Van Kemenade, F.J.; Iftner, T.; Berkhof, J.; Topal, F.; Agard, D.; Meijer, C.J.; Snijders, P.J. The APTIMA HPV assay fulfills the cross-sectional clinical and reproducibility criteria of international guidelines for HPV test requirements for cervical screening. J. Clin. Microbiol. 2013, 51, 3653–3657. [Google Scholar] [CrossRef] [PubMed]
- Byrt, T.; Bishop, J.; Carlin, J.B. Bias, prevalence and kappa. J. Clin. Epidemiol. 1993, 46, 423–429. [Google Scholar] [CrossRef]
- Ruano, Y.; Torrents, M.; Ferrer, F.J. Human papillomavirus combined with cytology and margin status identifies patients at risk for recurrence after conization for high-grade cervical intraepithelial neoplasia. Eur. J. Gynaecol. Oncol. 2015, 36, 245–251. [Google Scholar] [PubMed]
| Test | Company | Method | HPV TARGET | TARGET Region | HR HPV Genotypes | Validation References | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 16 | 18 | 31 | 33 | 35 | 39 | 45 | 51 | 52 | 56 | 58 | 59 | 66 | 68 | ||||||
| Hybrid Capture II | Qiagen | Hybridization and signal amplification | DNA | Whole genome | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | Gold standard, NTCC study | |
| Linear Array HPV Test | Roche | PCR and oligonucleotide hybridization | DNA | L1 | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | [8] |
| Cobas 4800 HPV Test | Roche | Real Time PCR | DNA | L1 | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | [9], ATHENA study |
| RealTime High Risk HPV Test | Abbott | Real Time PCR | DNA | L1 | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | [10] |
| Onclarity HPV Assay | BD | Real Time PCR | DNA | E6 E7 | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | [11] |
| Anyplex II HPV HR Test | Seegene | TOCE Real Time PCR | DNA | L1 | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | [12] |
| APTIMA mRNA assay | Hologic | Transcription-Mediated Amplification | mRNA | E6 E7 | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | [13] |
| in pool | |||||||||||||||||||
| in small pool | |||||||||||||||||||
| Characteristic | Level | Statistic a |
|---|---|---|
| Age, years | 39.0 (7.8) b | |
| Histology | CIN 1 | 14 (8.1) |
| CIN 2+ | 158 (91.9) | |
| HC2 | Negative | 12 (7.0) |
| Positive | 160 (93.0) | |
| Cytology | Negative | 6 (3.5) |
| ASCUS | 6 (3.5) | |
| LSIL | 18 (10.5) | |
| HSIL/ASC-H | 107 (62.2) | |
| AGC | 2 (1.2) | |
| SCC | 7 (4.1) | |
| missing | 26 (15.1) |
| HC2, N (col %) a | ||||||
|---|---|---|---|---|---|---|
| HPV Test | Negative | Positive | PABAK (95% CI) | p-Value b | Agreement % (95% CI) | |
| Abbott | Negative | 8 (72.7) | 3 (2.9) | 0.90 | 95% | |
| Positive | 3 (27.3) | 100 (97.1) | (0.81, 0.98) | 1.00 | (88.9, 98.0) | |
| Roche | Negative | 3 (60.0) | 3 (4.3) | 0.87 | 93% | |
| Positive | 2 (40.0) | 67 (95.7) | (0.75, 0.98) | 1.00 | (85.1, 97.8) | |
| Aptima | Negative | 8 (66.7) | 12 (7.6) | 0.81 | 91% | |
| Positive | 4 (33.3) | 147 (92.5) | (0.73, 0.90) | 0.08 | (85.3, 94.6) | |
| Linear Array | Negative | 6 (54.6) | 9 (5.7) | 0.84 | 92% | |
| Positive | 5 (45.4) | 150 (94.3) | (0.75, 0.91) | 0.42 | (86.6, 95.4) | |
| BD Onclarity | Negative | 6 (66.7) | 7 (4.9) | 0.87 | 93% | |
| Positive | 3 (33.3) | 136 (95.1) | (0.79, 0.95) | 0.34 | (88.2, 96.8) | |
| Seegene | Negative | 4 (40.0) | 4 (2.8) | 0.87 | 93% | |
| Positive | 6 (60.0) | 137 (97.2) | (0.79, 0.95) | 0.75 | (88.2, 96.8) | |
| HC2, N (col %) b | ||||||
|---|---|---|---|---|---|---|
| HPV Test | Negative | Positive | PABAK (95% CI) | p-Value c | Agreement % (95% CI) | |
| Abbott | Negative | 43 (97.7) | 7 (25.9) | 0.78 | 89% | |
| Positive | 1 (2.3) | 20 (74.1) | (0.63, 0.92) | 0.07 | (79.0, 95.0) | |
| Roche | Negative | 28 (100) | 0 | 1.00 | 100% | |
| Positive | 0 | 16 (100) | (1.00, 1.00) | 1.00 | (92.0, 100) | |
| Aptima | Negative | 52 (98.1) | 7 (21.2) | 0.81 | 88% | |
| Positive | 1 (1.9) | 26 (78.8) | (0.69, 0.94) | 0.07 | (80.1, 93.1) | |
| Linear Array | Negative | 36 (100) | 8 (24.2) | 0.77 | 88% | |
| Positive | 0 | 25 (75.8) | (0.62, 0.92) | 0.008 | (78.4, 94.9) | |
| BD Onclarity | Negative | 63 (95.5) | 16 (47.1) | 0.62 | 81% | |
| Positive | 3 (4.6) | 18 (52.9) | (0.47, 0.77) | 0.004 | (71.9, 88.2) | |
| Seegene | Negative | 57 (89.1) | 16 (48.5) | 0.53 | 76% | |
| Positive | 7 (10.9) | 17 (51.5) | (0.36, 0.70) | 0.09 | (66.6, 84.3) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bottari, F.; Iacobone, A.D.; Radice, D.; Preti, E.P.; Preti, M.; Franchi, D.; Boveri, S.; Sandri, M.T.; Passerini, R. HPV Tests Comparison in the Detection and Follow-Up after Surgical Treatment of CIN2+ Lesions. Diagnostics 2022, 12, 2359. https://doi.org/10.3390/diagnostics12102359
Bottari F, Iacobone AD, Radice D, Preti EP, Preti M, Franchi D, Boveri S, Sandri MT, Passerini R. HPV Tests Comparison in the Detection and Follow-Up after Surgical Treatment of CIN2+ Lesions. Diagnostics. 2022; 12(10):2359. https://doi.org/10.3390/diagnostics12102359
Chicago/Turabian StyleBottari, Fabio, Anna Daniela Iacobone, Davide Radice, Eleonora Petra Preti, Mario Preti, Dorella Franchi, Sara Boveri, Maria Teresa Sandri, and Rita Passerini. 2022. "HPV Tests Comparison in the Detection and Follow-Up after Surgical Treatment of CIN2+ Lesions" Diagnostics 12, no. 10: 2359. https://doi.org/10.3390/diagnostics12102359
APA StyleBottari, F., Iacobone, A. D., Radice, D., Preti, E. P., Preti, M., Franchi, D., Boveri, S., Sandri, M. T., & Passerini, R. (2022). HPV Tests Comparison in the Detection and Follow-Up after Surgical Treatment of CIN2+ Lesions. Diagnostics, 12(10), 2359. https://doi.org/10.3390/diagnostics12102359








