New Faecal Calprotectin Assay by IDS: Validation and Comparison to DiaSorin Method
Abstract
:1. Introduction
2. Materials and Methods
2.1. Comparison to DiaSorin Assay
2.2. IDS Method Validation
2.3. Statistical Analyses
3. Results
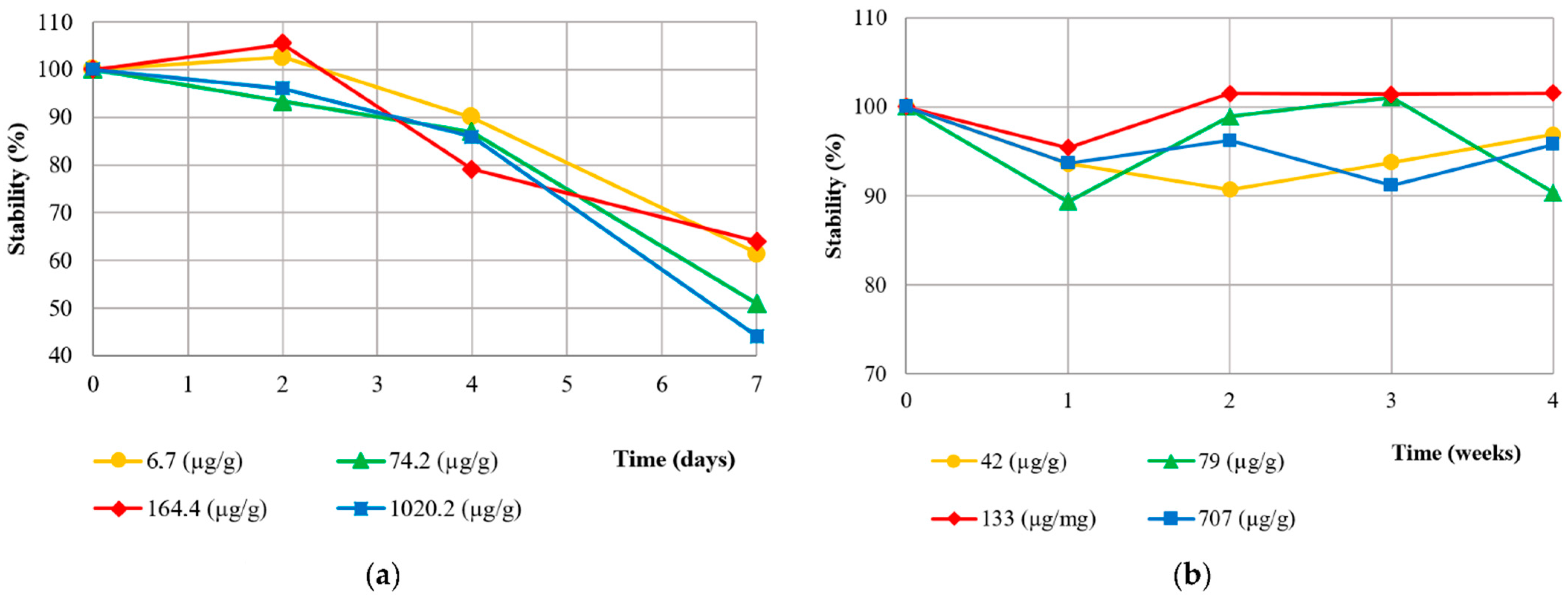
3.1. Method Validation
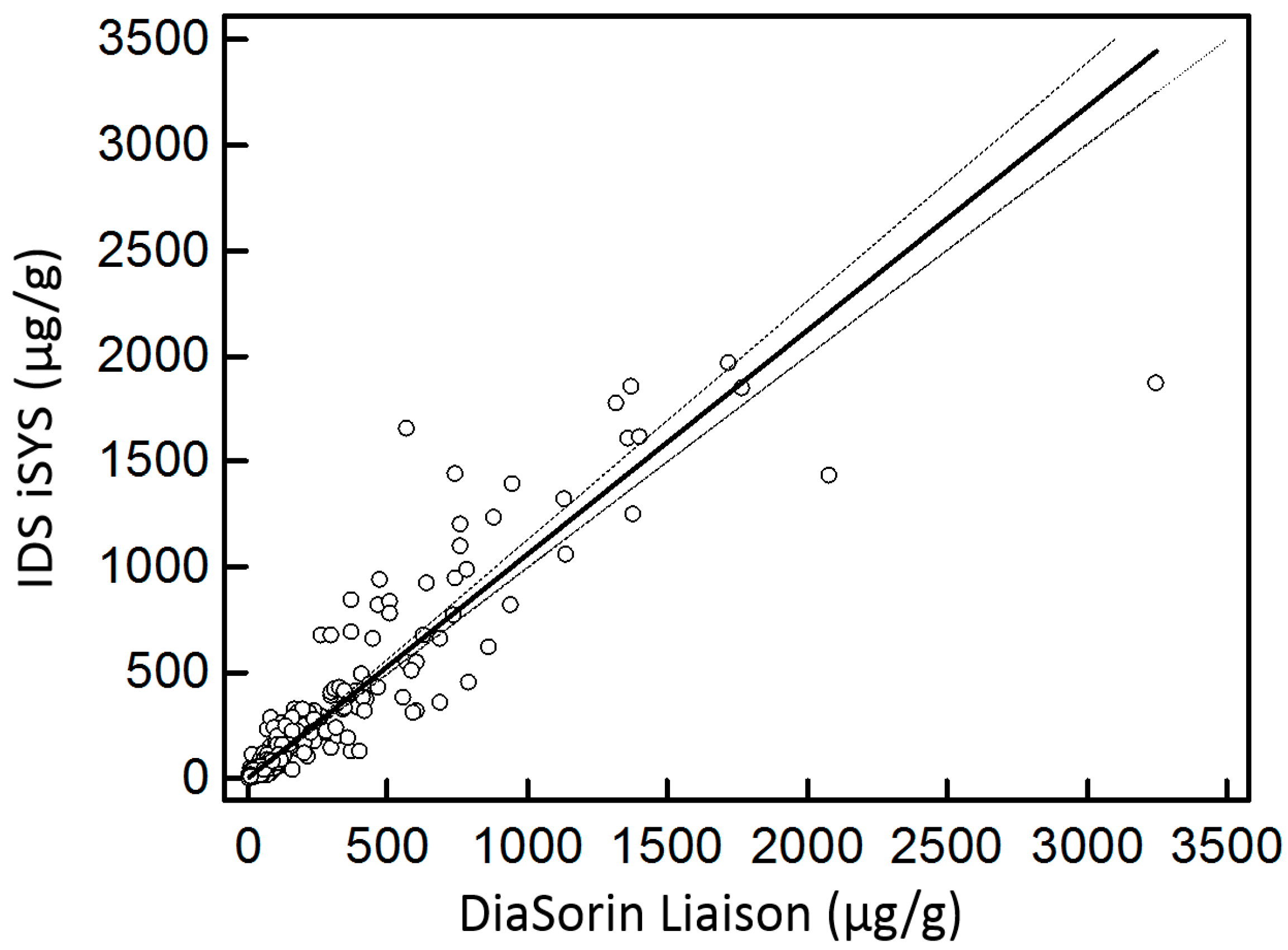
3.2. Diagnostic Performance
4. Discussion
4.1. Method Validation
4.2. Diagnostic Performance
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khaki-Khatibi, F.; Qujeq, D.; Kashifard, M.; Moein, S.; Maniati, M.; Vaghari-Tabari, M. Calprotectin in inflammatory bowel disease. Clin. Chim. Acta 2020, 510, 556–565. [Google Scholar] [CrossRef] [PubMed]
- Ayling, R.M.; Kok, K. Fecal Calprotectin. Adv. Clin. Chem. 2018, 87, 161–190. [Google Scholar] [CrossRef] [PubMed]
- Konikoff, M.R.; Denson, L.A. Role of fecal calprotectin as a biomarker of intestinal inflammation in inflammatory bowel disease. Inflamm. Bowel Dis. 2006, 12, 524–534. [Google Scholar] [CrossRef] [PubMed]
- Røseth, A.G.; Schmidt, P.N.; Fagerhol, M.K. Correlation between Faecal Excretion of Indium-111-Labelled Granulocytes and Calprotectin, a Granulocyte Marker Protein, in Patients with Inflammatory Bowel Disease. Scand. J. Gastroenterol. 1999, 34, 50–54. [Google Scholar] [CrossRef]
- van Rheenen, P.F.; Van de Vijver, E.; Fidler, V. Faecal calprotectin for screening of patients with suspected inflammatory bowel disease: Diagnostic meta-analysis. BMJ 2010, 341, c3369. [Google Scholar] [CrossRef]
- Limburg, P.J.; Ahlquist, D.A.; Sandborn, W.J.; Mahoney, D.W.; Devens, M.E.; Harrington, J.J.; Zinsmeister, A.R. Fecal Calprotectin Levels Predict Colorectal Inflammation among Patients with Chronic Diarrhea Referred for Colonoscopy. Am. J. Gastroenterol. 2000, 95, 2831–2837. [Google Scholar] [CrossRef]
- Carroccio, A.; Iacono, G.; Cottone, M.; Di Prima, L.; Cartabellotta, F.; Cavataio, F.; Scalici, C.; Montalto, G.; Di Fede, G.; Rini, G.; et al. Diagnostic Accuracy of Fecal Calprotectin Assay in Distinguishing Organic Causes of Chronic Diarrhea from Irritable Bowel Syndrome: A Prospective Study in Adults and Children. Clin. Chem. 2003, 49, 861–867. [Google Scholar] [CrossRef]
- Waugh, N.; Cummins, E.; Royle, P.; Kandala, N.-B.; Shyangdan, D.; Arasaradnam, R.; Clar, C.; Johnston, R. Faecal calprotectin testing for differentiating amongst inflammatory and non-inflammatory bowel diseases: Systematic review and economic evaluation. Health Technol. Assess. 2013, 17, 1–131. [Google Scholar] [CrossRef]
- Røseth, A.G.; Aadland, E.; Jahnsen, J.; Raknerud, N. Assessment of Disease Activity in Ulcerative Colitis by Faecal Calprotectin, a Novel Granulocyte Marker Protein. Digestion 1997, 58, 176–180. [Google Scholar] [CrossRef]
- Burri, E.; Beglinger, C. Faecal calprotectin in the diagnosis of inflammatory bowel disease. Biochem. Medica 2011, 21, 245–253. [Google Scholar] [CrossRef]
- D’Haens, G.; Ferrante, M.; Vermeire, S.; Baert, F.; Noman, M.; Moortgat, L.; Geens, P.; Iwens, D.; Aerden, I.; Van Assche, G.; et al. Fecal calprotectin is a surrogate marker for endoscopic lesions in inflammatory bowel disease. Inflamm. Bowel Dis. 2012, 18, 2218–2224. [Google Scholar] [CrossRef] [PubMed]
- D’Amico, F.; Rubin, D.T.; Kotze, P.G.; Magro, F.; Siegmund, B.; Kobayashi, T.; Olivera, P.A.; Bossuyt, P.; Pouillon, L.; Louis, E.; et al. International consensus on methodological issues in standardization of fecal calprotectin measurement in inflammatory bowel diseases. United Eur. Gastroenterol. J. 2021, 9, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Pelkmans, L.P.J.; de Groot, M.J.M.; Curvers, J. Analytical Performance and Clinicopathologic Correlation of Four Fecal Calprotectin Methods. Am. J. Clin. Pathol. 2019, 152, 392–398. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, V.; Klepp, P.; Cvancarova, M.; Roseth, A.; Skar, V.; Moum, B. Prediction of Endoscopic Disease Activity in Ulcerative Colitis by Two Different Assays for Fecal Calprotectin. J. Crohn’s Colitis 2015, 9, 164–169. [Google Scholar] [CrossRef]
- Dhaliwal, A.; Zeino, Z.; Tomkins, C.; Cheung, M.; Nwokolo, C.; Smith, S.; Harmston, C.; Arasaradnam, R.P. Utility of faecal calprotectin in inflammatory bowel disease (IBD): What cut-offs should we apply? Frontline Gastroenterol. 2015, 6, 14–19. [Google Scholar] [CrossRef]
- Delefortrie, Q.; Schatt, P.; Grimmelprez, A.; Gohy, P.; Deltour, D.; Collard, G.; Vankerkhoven, P. Comparison of the Liaison® Calprotectin kit with a well established point of care test (Quantum Blue—Bühlmann-Alere®) in terms of analytical performances and ability to detect relapses amongst a Crohn population in follow-up. Clin. Biochem. 2016, 49, 268–273. [Google Scholar] [CrossRef]
- Miettinen, J.; Vuolteenaho, O.; Hedberg, P. Clinical Diagnostics of Fecal Calprotectin: A Comparative Study of Actim® Calprotectin and LIAISON® Calprotectin. Clin. Lab. 2022, 68. [Google Scholar] [CrossRef]
- Campbell, J.P.; Zierold, C.; Rode, A.M.; Blocki, F.A.; Vaughn, B.P. Clinical Performance of a Novel LIAISON Fecal Calprotectin Assay for Differentiation of Inflammatory Bowel Disease From Irritable Bowel Syndrome. J. Clin. Gastroenterol. 2021, 55, 239–243. [Google Scholar] [CrossRef]
- Oyaert, M.; Boel, A.; Jacobs, J.; Van den Bremt, S.; De Sloovere, M.; Vanpoucke, H.; Van Hoovels, L. Analytical performance and diagnostic accuracy of six different faecal calprotectin assays in inflammatory bowel disease. Clin. Chem. Lab. Med. 2017, 55, 1564–1573. [Google Scholar] [CrossRef]
- Cremer, A.; Ku, J.; Amininejad, L.; Bouvry, M.-R.; Brohet, F.; Liefferinckx, C.; Devière, J.; van Gossum, A.; Smet, J.; Stordeur, P.; et al. Variability of Faecal Calprotectin in Inflammatory Bowel Disease Patients: An Observational Case-control Study. J. Crohn’s Colitis 2019, 13, 1372–1379. [Google Scholar] [CrossRef]
- Westgard, J.O. WestgardQC. Available online: https://www.westgard.com (accessed on 13 June 2022).
- European Federation of Clinical Chemistry and Laboratory Medicine. EFLM European Federation of Clinical Chemistry and Laboratory Medicine. Biological Variation Database. Available online: https://biologicalvariation.eu/search?query=Calprotectinlevel (accessed on 22 September 2022).
- Parakh, R.; Greene, D.N.; Mathias, P.C.; Block, D.R.; Ranjitkar, P. Laboratory Utilization and Analytical Validation of Fecal Electrolyte Tests. J. Appl. Lab. Med. 2017, 1, 668–677. [Google Scholar] [CrossRef] [PubMed]
- Schött, H.-F.; Krautbauer, S.; Höring, M.; Liebisch, G.; Matysik, S. A Validated, Fast Method for Quantification of Sterols and Gut Microbiome Derived 5α/β-Stanols in Human Feces by Isotope Dilution LC–High-Resolution MS. Anal. Chem. 2018, 90, 8487–8494. [Google Scholar] [CrossRef]
- van Amsterdam, P.; Companjen, A.; Brudny-Kloeppel, M.; Golob, M.; Luedtke, S.; Timmerman, P. The European Bioanalysis Forum community’s evaluation, interpretation and implementation of the European Medicines Agency guideline on Bioanalytical Method Validation. Bioanalysis 2013, 5, 645–659. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration. FDA Guidance for Industry: Bioanalytical Method Validation; U.S. Department of Health and Human Services: Washington, DC, USA, 2001. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/bioanalytical-method-validation-guidance-industry (accessed on 22 September 2022).
- Whitehead, S.J.; French, J.; Brookes, M.J.; Ford, C.; Gama, R. Between-assay variability of faecal calprotectin enzyme-linked immunosorbent assay kits. Ann. Clin. Biochem. Int. J. Lab. Med. 2013, 50, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Morris, L.S.; Marchesi, J.R. Assessing the impact of long term frozen storage of faecal samples on protein concentration and protease activity. J. Microbiol. Methods 2016, 123, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Oyaert, M.; Van den Bremt, S.; Boel, A.; Bossuyt, X.; Van Hoovels, L. Do not forget about pre-analytics in faecal calprotectin measurement! Clin. Chim. Acta 2017, 473, 124–126. [Google Scholar] [CrossRef]
- Lasson, A.; Stotzer, P.-O.; Öhman, L.; Isaksson, S.; Sapnara, M.; Strid, H. The intra-individual variability of faecal calprotectin: A prospective study in patients with active ulcerative colitis. J. Crohn’s Colitis 2015, 9, 26–32. [Google Scholar] [CrossRef]
- Gihyeon, K.; Kyoung Wan, Y.; Changho, P.; Kyu Hyuck, K.; Sujeong, K.; Youngmin, Y.; Sang Eun, L.; Yeongmin, K.; Hansoo, P. Fecal storage condition induces variations of microbial composition and differential interpretation of metagenomic analysis. Ann. Biomed. Sci. Eng. 2021, 5, 6–12. [Google Scholar] [CrossRef]
- Maesa, J.M.; Martin, S.; Elena, C.; Gallego, A.M.; Perez-Perez, A. Comparison of fecal calprotectine determination with turbidimetry and chemiluminiscence. Clin. Chim. Acta 2019, 493, S30. [Google Scholar] [CrossRef]
- Oyaert, M.; Trouvé, C.; Baert, F.; De Smet, D.; Langlois, M.; Vanpoucke, H. Comparison of two immunoassays for measurement of faecal calprotectin in detection of inflammatory bowel disease: (Pre)-analytical and diagnostic performance characteristics. Clin. Chem. Lab. Med. 2014, 52, 391–397. [Google Scholar] [CrossRef]
- Sun, S.; Cavey, T.; Peltier, L.; Bendavid, C.; Bouguen, G. Letter: Wide variation in faecal calprotectin values according to the assay. Aliment. Pharmacol. Ther. 2016, 43, 177–178. [Google Scholar] [CrossRef] [PubMed]
- Fauny, M.; D’Amico, F.; Bonovas, S.; Netter, P.; Danese, S.; Loeuille, D.; Peyrin-Biroulet, L. Faecal Calprotectin for the Diagnosis of Bowel Inflammation in Patients With Rheumatological Diseases: A Systematic Review. J. Crohn’s Colitis 2020, 14, 688–693. [Google Scholar] [CrossRef] [PubMed]
- Cypers, H.; Varkas, G.; Beeckman, S.; Debusschere, K.; Vogl, T.; Roth, J.; Drennan, M.B.; Lavric, M.; Foell, D.; Cuvelier, C.A.; et al. Elevated calprotectin levels reveal bowel inflammation in spondyloarthritis. Ann. Rheum. Dis. 2016, 75, 1357–1362. [Google Scholar] [CrossRef] [PubMed]
- Gundling, F.; Schmidtler, F.; Hapfelmeier, A.; Schulte, B.; Schmidt, T.; Pehl, C.; Schepp, W.; Seidl, H. Fecal calprotectin is a useful screening parameter for hepatic encephalopathy and spontaneous bacterial peritonitis in cirrhosis. Liver Int. 2011, 31, 1406–1415. [Google Scholar] [CrossRef]
- Tibble, J.; Sigthorsson, G.; Foster, R.; Sherwood, R.; Fagerhol, M.; Bjarnason, I. Faecal calprotectin and faecal occult blood tests in the diagnosis of colorectal carcinoma and adenoma. Gut 2001, 49, 402–408. [Google Scholar] [CrossRef]
- Vavricka, S.R.; Heinrich, H.; Buetikofer, S.; Breitenmoser, F.; Burri, E.; Schneider-Yin, X.; Barman-Aksoezen, J.; Biedermann, L.; Scharl, M.; Zeitz, J.; et al. The Vampire Study: Significant elevation of faecal calprotectin in healthy volunteers after 300 ml blood ingestion mimicking upper gastrointestinal bleeding. United Eur. Gastroenterol. J. 2018, 6, 1007–1014. [Google Scholar] [CrossRef]
- de Seny, D.; Fillet, M.; Ribbens, C.; Marée, R.; Meuwis, M.-A.; Lutteri, L.; Chapelle, J.-P.; Wehenkel, L.; Louis, E.; Merville, M.-P.; et al. Monomeric Calgranulins Measured by SELDI-TOF Mass Spectrometry and Calprotectin Measured by ELISA as Biomarkers in Arthritis. Clin. Chem. 2008, 54, 1066–1075. [Google Scholar] [CrossRef]
- Adhikari, J.; Stephan, J.R.; Rempel, D.L.; Nolan, E.M.; Gross, M.L. Calcium Binding to the Innate Immune Protein Human Calprotectin Revealed by Integrated Mass Spectrometry. J. Am. Chem. Soc. 2020, 142, 13372–13383. [Google Scholar] [CrossRef]
- Hoskin, T.S.; Crowther, J.M.; Cheung, J.; Epton, M.J.; Sly, P.D.; Elder, P.A.; Dobson, R.C.J.; Kettle, A.J.; Dickerhof, N. Oxidative cross-linking of calprotectin occurs in vivo, altering its structure and susceptibility to proteolysis. Redox Biol. 2019, 24, 101202. [Google Scholar] [CrossRef]
- Edwards, T.S.; Dickerhof, N.; Magon, N.J.; Paton, L.N.; Sly, P.D.; Kettle, A.J. Formation of Calprotectin-Derived Peptides in the Airways of Children with Cystic Fibrosis. J. Immunol. 2022, 208, 979–990. [Google Scholar] [CrossRef]
| Concentration (µg/g) | Nature | Intra-Assay CV (%) | Inter-Assay CV (%) |
|---|---|---|---|
| 39.6 | Sample | 1.9 | 2.6 |
| 69.1 | Sample | 3.0 | 3.5 |
| 72.8 | Quality control | 2.9 | 3.6 |
| 76.7 | Quality control | 3.0 | 3.8 |
| 154.1 | Sample | 1.8 | 2.5 |
| 692.7 | Sample | 2.0 | 3.4 |
| 776.1 | Quality control | 2.1 | 3.5 |
| 780.9 | Quality control | 2.9 | 4.1 |
| 1545.8 | Sample | 1.9 | 3.1 |
| IDS iSYS | ||||
|---|---|---|---|---|
| Negative (n = 203, 53.6%) | Borderline (n = 59, 15.6%) | Positive (n = 117, 30.9%) | ||
| DiaSorin Liaison XL | Negative (n = 192, 50.7%) | 183 | 9 | 0 |
| Borderline (n = 73, 19.3%) | 19 | 44 | 10 | |
| Positive (n = 114, 30.1%) | 1 | 6 | 107 |
| IDS iSYS | ||||
|---|---|---|---|---|
| Negative (n = 203, 53.6%) | Borderline (n = 98, 25.9%) | Positive (n = 78, 20.6%) | ||
| DiaSorin Liaison XL | Negative (n = 190, 50.4%) | 183 | 8 | 0 |
| Borderline (n = 112, 29.6%) | 20 | 82 | 10 | |
| Positive (n = 76, 20.1%) | 0 | 8 | 68 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castiglione, V.; Berodes, M.; Lukas, P.; Louis, E.; Cavalier, E.; Lutteri, L. New Faecal Calprotectin Assay by IDS: Validation and Comparison to DiaSorin Method. Diagnostics 2022, 12, 2338. https://doi.org/10.3390/diagnostics12102338
Castiglione V, Berodes M, Lukas P, Louis E, Cavalier E, Lutteri L. New Faecal Calprotectin Assay by IDS: Validation and Comparison to DiaSorin Method. Diagnostics. 2022; 12(10):2338. https://doi.org/10.3390/diagnostics12102338
Chicago/Turabian StyleCastiglione, Vincent, Maëlle Berodes, Pierre Lukas, Edouard Louis, Etienne Cavalier, and Laurence Lutteri. 2022. "New Faecal Calprotectin Assay by IDS: Validation and Comparison to DiaSorin Method" Diagnostics 12, no. 10: 2338. https://doi.org/10.3390/diagnostics12102338
APA StyleCastiglione, V., Berodes, M., Lukas, P., Louis, E., Cavalier, E., & Lutteri, L. (2022). New Faecal Calprotectin Assay by IDS: Validation and Comparison to DiaSorin Method. Diagnostics, 12(10), 2338. https://doi.org/10.3390/diagnostics12102338






