Diagnosis and Management of Pelvic Venous Disorders in Females
Abstract
1. Introduction
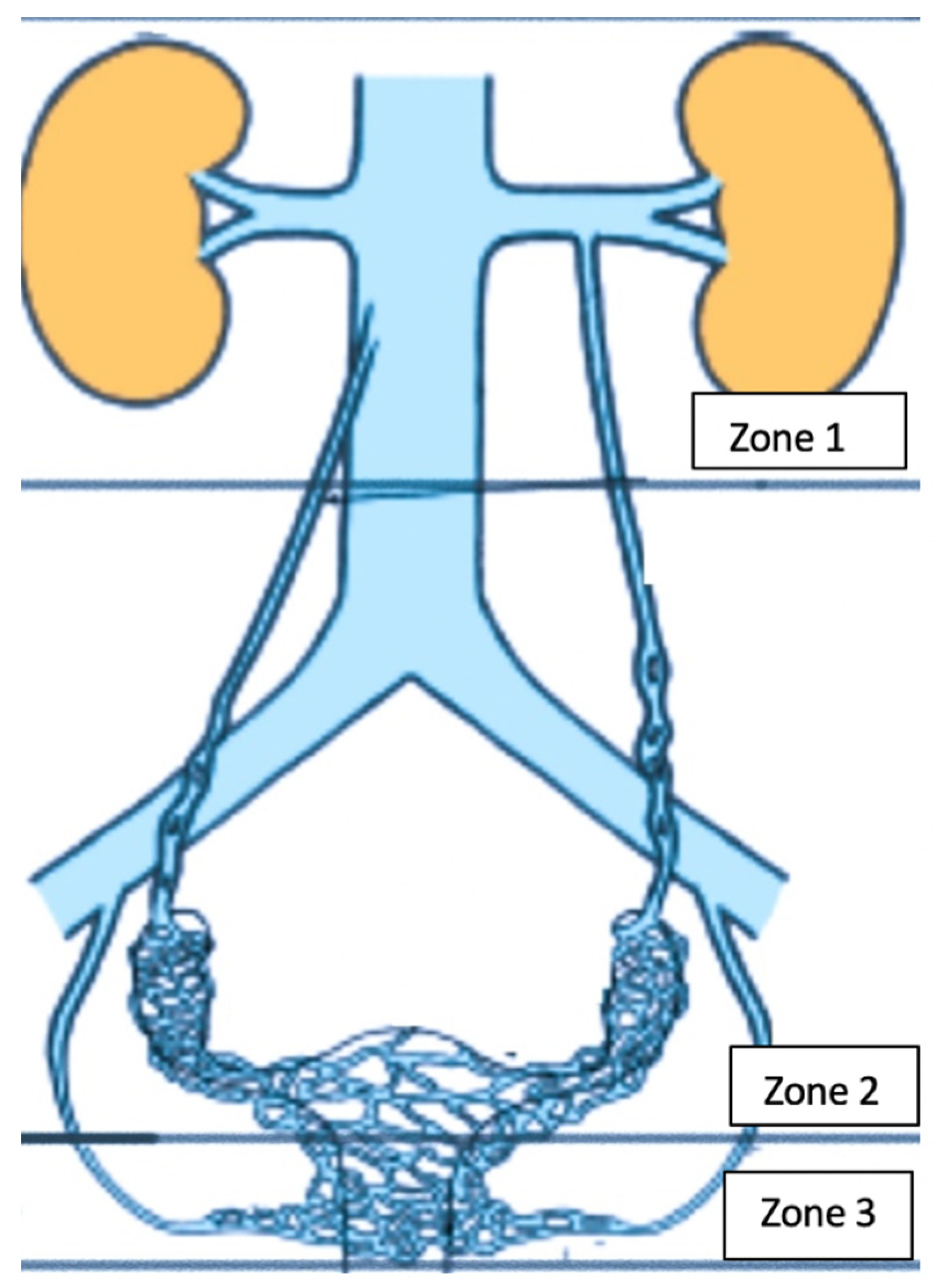
Physiology of PVD
2. Clinical Diagnosis
2.1. Left Renal Vein Symptoms of Venous Origin
2.2. Chronic Pelvic Pain of Venous Origin
2.3. Perineal and Lower Limb Venous Disease of Pelvic Origin
2.4. Proximal Iliac Vein Obstruction (May–Thurner Syndrome)
3. Imaging for PVDs
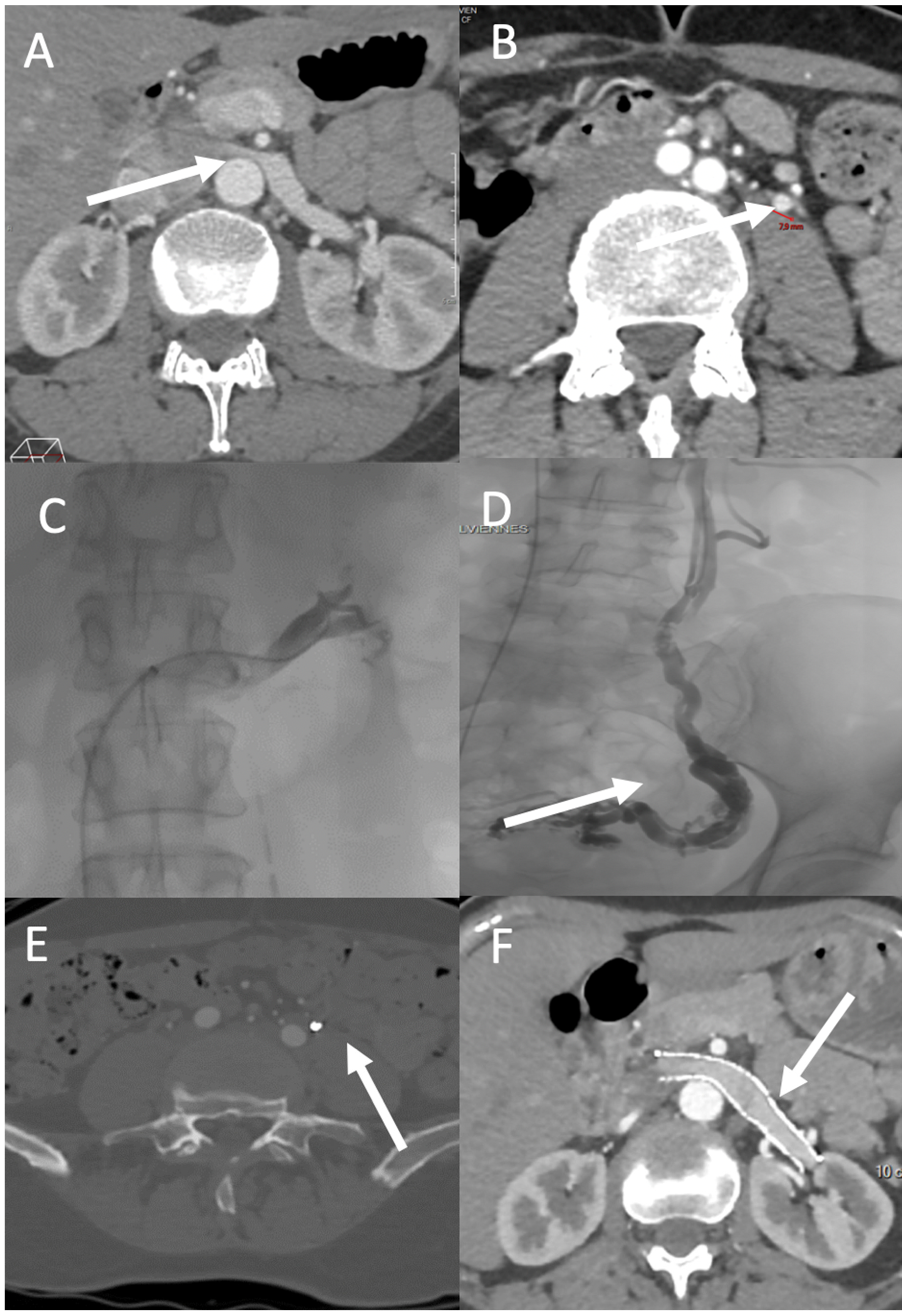
3.1. Renal Region
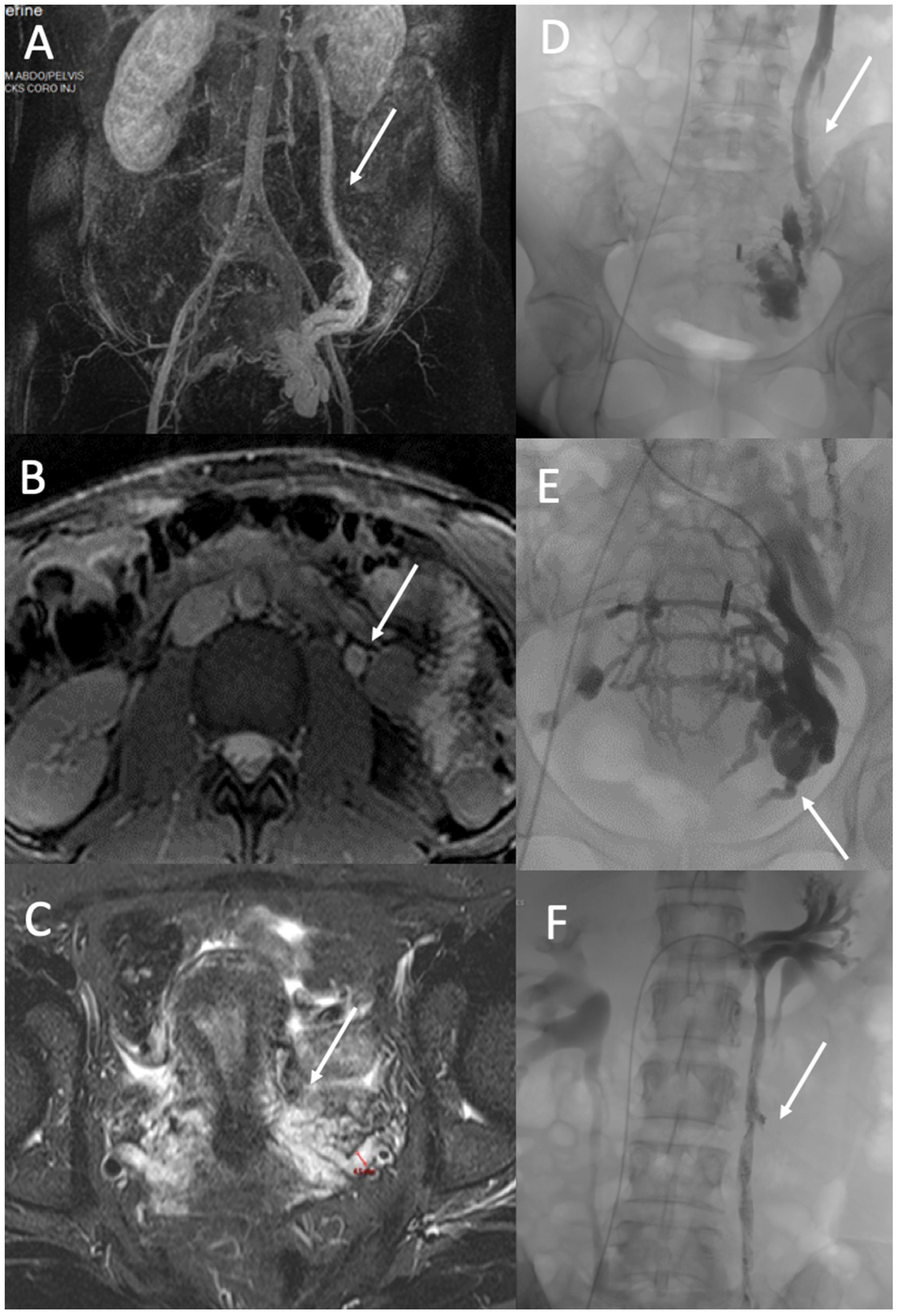
3.2. Gonadic Veins, Pelvic Venous Plexus, and Iliac Intern Veins
3.3. Perineal Varices of Pelvic Origin
3.4. Lower Limb Varices
4. PVD Treatment
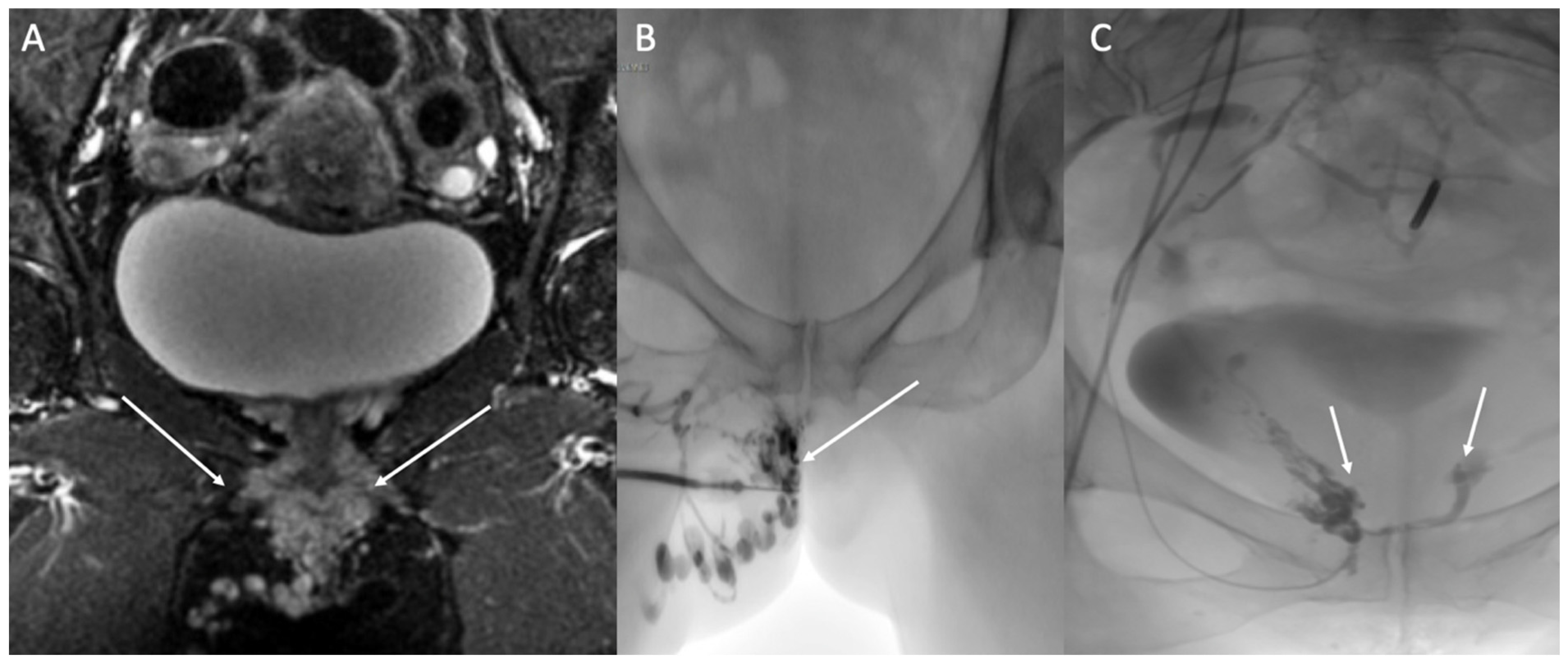
4.1. Nutcracker Syndrome and May–Thurner Syndrome
4.2. Ovarian and Iliac Venous Insufficiency
4.3. Vulvar and Lower Limb Varices of Pelvic Origin
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Gültaşli, N.Z.; Kurt, A.; Ipek, A.; Gümüş, M.; Yazicioğlu, K.R.; Dilmen, G.; Tas, I. The relation between pelvic varicose veins, chronic pelvic pain and lower extremity venous insufficiency in women. Diagn. Interv. Radiol. 2006, 12, 34–38. [Google Scholar] [PubMed]
- Bora, A.; Avcu, S.; Arslan, H.; Adali, E.; Bulut, M.D. The relation between pelvic varicose veins and lower extremity venous insufficiency in women with chronic pelvic pain. JBR-BTR 2012, 95, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Phillips, D.; Deipolyi, A.R.; Hesketh, R.L.; Midia, M.; Oklu, R. Pelvic congestion syndrome: Etiology of pain, diagnosis, and clinical management. J. Vasc. Interv. Radiol. 2014, 25, 725–733. [Google Scholar] [CrossRef] [PubMed]
- Greiner, M.; Gilling-Smith, G.L. Leg varices originating from the pelvis: Diagnosis and treatment. Vascular 2007, 15, 70–78. [Google Scholar] [CrossRef]
- Meissner, M.H.; Khilnani, N.M.; Labropoulos, N.; Gasparis, A.P.; Gibson, K.; Greiner, M.; Learman, L.A.; Atashroo, D.; Lurie, F.; Passman, M.A.; et al. The Symptoms-Varices-Pathophysiology classification of pelvic venous disorders: A report of the American Vein & Lymphatic Society International Working Group on Pelvic Venous Disorders. J. Vasc. Surg. Venous Lymphat. Disord. 2021, 9, 568–584. [Google Scholar]
- Kim, H.S.; Malhotra, A.D.; Rowe, P.C.; Lee, J.M.; Venbrux, A.C. Embolotherapy for pelvic congestion syndrome: Long-term results. J. Vasc. Interv. Radiol. 2006, 17 Pt 1, 289–297. [Google Scholar] [CrossRef]
- Meissner, M.H.; Gibson, K. Clinical outcome after treatment of pelvic congestion syndrome: Sense and nonsense. Phlebology 2015, 30 (Suppl. 1), 73–80. [Google Scholar] [CrossRef]
- Asciutto, G.; Asciutto, K.C.; Mumme, A.; Geier, B. Pelvic venous incompetence: Reflux patterns and treatment results. Eur. J. Vasc. Endovasc. Surg. 2009, 38, 381–386. [Google Scholar] [CrossRef]
- Hansrani, V.; Dhorat, Z.; McCollum, C.N. Diagnosing of pelvic vein incompetence using minimally invasive ultrasound techniques. Vascular 2017, 25, 253–259. [Google Scholar] [CrossRef]
- Mahmoud, O.; Vikatmaa, P.; Aho, P.; Halmesmäki, K.; Albäck, A.; Rahkola-Soisalo, P.; Lappalainen, K.; Venermo, M. Efficacy of endovascular treatment for pelvic congestion syndrome. J. Vasc. Surg. Venous Lymphat. Disord. 2016, 4, 355–370. [Google Scholar] [CrossRef]
- Lopez, A.J. Female Pelvic Vein Embolization: Indications, Techniques, and Outcomes. Cardiovasc. Interv. Radiol. 2015, 38, 806–820. [Google Scholar] [CrossRef] [PubMed]
- Constans, J. Les Explorations Vasculaires (SFMV) Société Française de Médecine Vasculaire, (CEMV) Collège des Enseignants de Médecine Vasc, (CFPV) Collège Français de Pathologie Vasculaire; Elsevier Masson: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Williams, R.E.; Hartmann, K.E.; Steege, J.F. Documenting the current definitions of chronic pelvic pain: Implications for research. Obstet. Gynecol. 2004, 103, 686–691. [Google Scholar] [CrossRef] [PubMed]
- As-Sanie, S. A pathway to personalised pain care? BJOG 2018, 125, 1319. [Google Scholar] [CrossRef] [PubMed]
- Juhan, V. Chronic pelvic pain: An imaging approach. Diagn. Interv. Imaging 2015, 96, 997–1007. [Google Scholar] [CrossRef]
- Jambon, E.; Le Bras, Y.; Coussy, A.; Petitpierre, F.; Hans, H.; Lasserre, A.; Cazalas, G.; Grenier, N.; Marcelin, C. Embolization in pelvic venous disorders using ethylene vinyl alcohol copolymer (Onyx®) and Aetoxysclerol: A prospective evaluation of safety and long-term efficacy. Eur. Radiol. 2022, 32, 4679–4686. [Google Scholar] [CrossRef]
- Beard, R.W.; Reginald, P.W.; Wadsworth, J. Clinical features of women with chronic lower abdominal pain and pelvic congestion. Br. J. Obstet. Gynaecol. 1988, 95, 153–161. [Google Scholar] [CrossRef]
- Takebayashi, S.; Ueki, T.; Ikeda, N.; Fujikawa, A. Diagnosis of the nutcracker syndrome with color Doppler sonography: Correlation with flow patterns on retrograde left renal venography. AJR Am. J. Roentgenol. 1999, 172, 39–43. [Google Scholar] [CrossRef]
- Kim, K.W.; Cho, J.Y.; Kim, S.H.; Yoon, J.H.; Kim, D.S.; Chung, J.W.; Park, J.H. Diagnostic value of computed tomographic findings of nutcracker syndrome: Correlation with renal venography and renocaval pressure gradients. Eur. J. Radiol. 2011, 80, 648–654. [Google Scholar] [CrossRef]
- Malgor, R.D.; Adrahtas, D.; Spentzouris, G.; Gasparis, A.P.; Tassiopoulos, A.K.; Labropoulos, N. The role of duplex ultrasound in the workup of pelvic congestion syndrome. J. Vasc. Surg. Venous Lymphat. Disord. 2014, 2, 34–38. [Google Scholar] [CrossRef]
- Yang, D.M.; Kim, H.C.; Nam, D.H.; Jahng, G.H.; Huh, C.Y.; Lim, J.W. Time-resolved MR angiography for detecting and grading ovarian venous reflux: Comparison with conventional venography. Br. J. Radiol. 2012, 85, e117–e122. [Google Scholar] [CrossRef]
- Kim, C.Y.; Miller, M.J.; Merkle, E.M. Time-resolved MR angiography as a useful sequence for assessment of ovarian vein reflux. AJR Am. J. Roentgenol. 2009, 193, W458–W463. [Google Scholar] [CrossRef] [PubMed]
- Jambon, E.; Le Bras, Y.; Petitpierre, F.; Balian, E.; Midy, D.; Grenier, N.; Marcelin, C. MRI associated factors of clinical efficacy of embolization in patients with pelvic venous insufficiency. Diagn. Interv. Imaging 2020, 101, 667–676. [Google Scholar] [CrossRef] [PubMed]
- Asciutto, G.; Mumme, A.; Marpe, B.; Köster, O.; Asciutto, K.C.; Geier, B. MR venography in the detection of pelvic venous congestion. Eur. J. Vasc. Endovasc. Surg. 2008, 36, 491–496. [Google Scholar] [CrossRef]
- Meneses, L.Q.; Uribe, S.; Tejos, C.; Andía, M.E.; Fava, M.; Irarrazaval, P. Using magnetic resonance phase-contrast velocity mapping for diagnosing pelvic congestion syndrome. Phlebology 2011, 26, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Arnoldussen, C.W.K.P.; de Wolf, M.a.F.; Wittens, C.H.A. Diagnostic imaging of pelvic congestive syndrome. Phlebology 2015, 30 (Suppl. 1), 67–72. [Google Scholar] [CrossRef] [PubMed]
- Lasry, J.L.; Coppé, G.; Balian, E.; Borie, H. Pelvi-perineal venous insufficiency and varicose veins of the lower limbs: Duplex Doppler diagnosis and endoluminal treatment in thirty females. J. Mal. Vasc. 2007, 32, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Arnaoutoglou, C.; Variawa, R.S.; Zarogoulidis, P.; Ioannidis, A.; Machairiotis, N. Advances of Laparoscopy for the Diagnosis of Pelvic Congestion Syndrome. Medicina 2021, 57, 1041. [Google Scholar] [CrossRef]
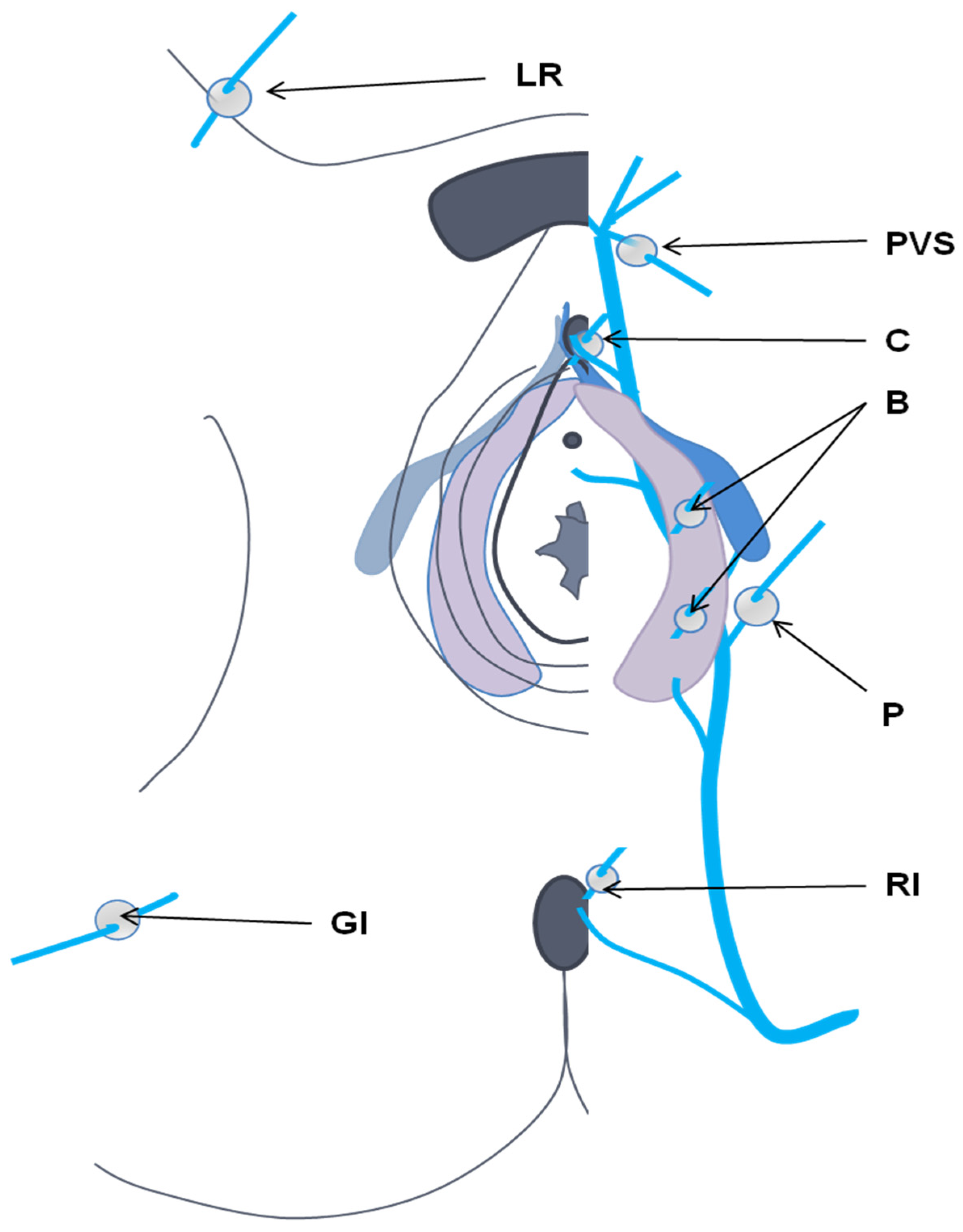
- Delfrate, R. Anatomy of Pelvic leak points in the context of varicose veins. Phlebologie 2021, 50, 42–50. [Google Scholar] [CrossRef]
- Franceschi, C.; Bahnini, A. Treatment of Lower Extremity Venous Insufficiency Due to Pelvic Leak Points in Women. Ann. Vasc. Surg. 2005, 19, 284–288. [Google Scholar] [CrossRef]
- Antignani, P.L.; Lazarashvili, Z.; Monedero, J.L.; Ezpeleta, S.Z.; Whiteley, M.S.; Khilnani, N.M.; Meissner, M.H.; Wittens, C.H.; Kurstjens, R.L.; Belova, L.; et al. Diagnosis and treatment of pelvic congestion syndrome: UIP consensus document. Int. Angiol. 2019, 38, 265–283. [Google Scholar] [CrossRef]
- Georgiev, M.; Myers, K.A.; Belcaro, G. The thigh extension of the lesser saphenous vein: From Giacomini’s observations to ultrasound scan imaging. J. Vasc. Surg. 2003, 37, 558–563. [Google Scholar] [CrossRef] [PubMed]
- Velasquez, C.A.; Saeyeldin, A.; Zafar, M.A.; Brownstein, A.J.; Erben, Y. A systematic review on management of nutcracker syndrome. J. Vasc. Surg. Venous Lymphat. Disord. 2018, 6, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Kolber, M.K.; Cui, Z.; Chen, C.K.; Habibollahi, P.; Kalva, S.P. Nutcracker syndrome: Diagnosis and therapy. Cardiovasc. Diagn. Ther. 2021, 11, 1140–1149. [Google Scholar] [CrossRef] [PubMed]
- Avgerinos, E.D.; Saadeddin, Z.; Humar, R.; Salem, K.; Singh, M.; Hager, E.; Makaroun, M.; Chaer, R.A. Outcomes of left renal vein stenting in patients with nutcracker syndrome. J. Vasc. Surg. Venous Lymphat. Disord. 2019, 7, 853–859. [Google Scholar] [CrossRef] [PubMed]
- Daugherty, S.F.; Gillespie, D.L. Venous angioplasty and stenting improve pelvic congestion syndrome caused by venous outflow obstruction. J. Vasc. Surg. Venous Lymphat. Disord. 2015, 3, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Gloviczki, P.; Comerota, A.J.; Dalsing, M.C.; Eklof, B.G.; Gillespie, D.L.; Gloviczki, M.L.; Lohr, J.M.; McLafferty, R.B.; Meissner, M.H.; Murad, M.H.; et al. The care of patients with varicose veins and associated chronic venous diseases: Clinical practice guidelines of the Society for Vascular Surgery and the American Venous Forum. J. Vasc. Surg. 2011, 53 (Suppl. 5), 2S–48S. [Google Scholar] [CrossRef]
- Marcelin, C.; Izaaryene, J.; Castelli, M.; Barral, P.A.; Jacquier, A.; Vidal, V.; Bartoli, J.M. Embolization of ovarian vein for pelvic congestion syndrome with ethylene vinyl alcohol copolymer (Onyx®). Diagn. Interv. Imaging 2017, 98, 843–848. [Google Scholar] [CrossRef]
- Senechal, Q.; Echegut, P.; Bravetti, M.; Florin, M.; Jarboui, L.; Bouaboua, M.; Teriitehau, C.; Feignoux, J.; Legou, F.; Pessis, E. Endovascular Treatment of Pelvic Congestion Syndrome: Visual Analog Scale Follow-Up. Front. Cardiovasc. Med. 2021, 8, 751178. [Google Scholar] [CrossRef]
- Laborda, A.; Medrano, J.; de Blas, I.; Urtiaga, I.; Carnevale, F.C.; de Gregorio, M.A. Endovascular treatment of pelvic congestion syndrome: Visual analog scale (VAS) long-term follow-up clinical evaluation in 202 patients. Cardiovasc. Interv. Radiol. 2013, 36, 1006–1014. [Google Scholar] [CrossRef]
- Nasser, F.; Cavalcante, R.N.; Affonso, B.B.; Messina, M.L.; Carnevale, F.C.; de Gregorio, M.A. Safety, efficacy, and prognostic factors in endovascular treatment of pelvic congestion syndrome. Int. J. Gynaecol. Obstet. 2014, 125, 65–68. [Google Scholar] [CrossRef]
- De Gregorio, M.A.; Guirola, J.A.; Alvarez-Arranz, E.; Sánchez-Ballestin, M.; Urbano, J.; Sierre, S. Pelvic Venous Disorders in Women due to Pelvic Varices: Treatment by Embolization: Experience in 520 Patients. J. Vasc. Interv. Radiol. 2020, 31, 1560–1569. [Google Scholar] [CrossRef] [PubMed]
- Gibson, K.; Minjarez, R.; Ferris, B.; Neradilek, M.; Wise, M.; Stoughton, J.; Meissner, M. Clinical presentation of women with pelvic source varicose veins in the perineum as a first step in the development of a disease-specific patient assessment tool. J. Vasc. Surg. Venous Lymphat. Disord. 2017, 5, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Castenmiller, P.H.; de Leur, K.; de Jong, T.E.a.M.; van der Laan, L. Clinical results after coil embolization of the ovarian vein in patients with primary and recurrent lower-limb varices with respect to vulval varices. Phlebology 2013, 28, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Ninia, J.G. Treatment of vulvar varicosities by injection-compression sclerotherapy. Dermatol. Surg. 1997, 23, 573–574; discussion 574–575. [Google Scholar] [CrossRef] [PubMed]
- Rabe, E.; Pannier, F. Embolization is not essential in the treatment of leg varices due to pelvic venous insufficiency. Phlebology 2015, 30 (Suppl. 1), 86–88. [Google Scholar] [CrossRef] [PubMed]
- Hartung, O. Embolization is essential in the treatment of leg varicosities due to pelvic venous insufficiency. Phlebology 2015, 30 (Suppl. 1), 81–85. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marcelin, C.; Le Bras, Y.; Molina Andreo, I.; Jambon, E.; Grenier, N. Diagnosis and Management of Pelvic Venous Disorders in Females. Diagnostics 2022, 12, 2337. https://doi.org/10.3390/diagnostics12102337
Marcelin C, Le Bras Y, Molina Andreo I, Jambon E, Grenier N. Diagnosis and Management of Pelvic Venous Disorders in Females. Diagnostics. 2022; 12(10):2337. https://doi.org/10.3390/diagnostics12102337
Chicago/Turabian StyleMarcelin, Clément, Yann Le Bras, Isabelle Molina Andreo, Eva Jambon, and Nicolas Grenier. 2022. "Diagnosis and Management of Pelvic Venous Disorders in Females" Diagnostics 12, no. 10: 2337. https://doi.org/10.3390/diagnostics12102337
APA StyleMarcelin, C., Le Bras, Y., Molina Andreo, I., Jambon, E., & Grenier, N. (2022). Diagnosis and Management of Pelvic Venous Disorders in Females. Diagnostics, 12(10), 2337. https://doi.org/10.3390/diagnostics12102337





