Relationships of Pelvic Vein Diameter and Reflux with Clinical Manifestations of Pelvic Venous Disorder
Abstract
:1. Introduction
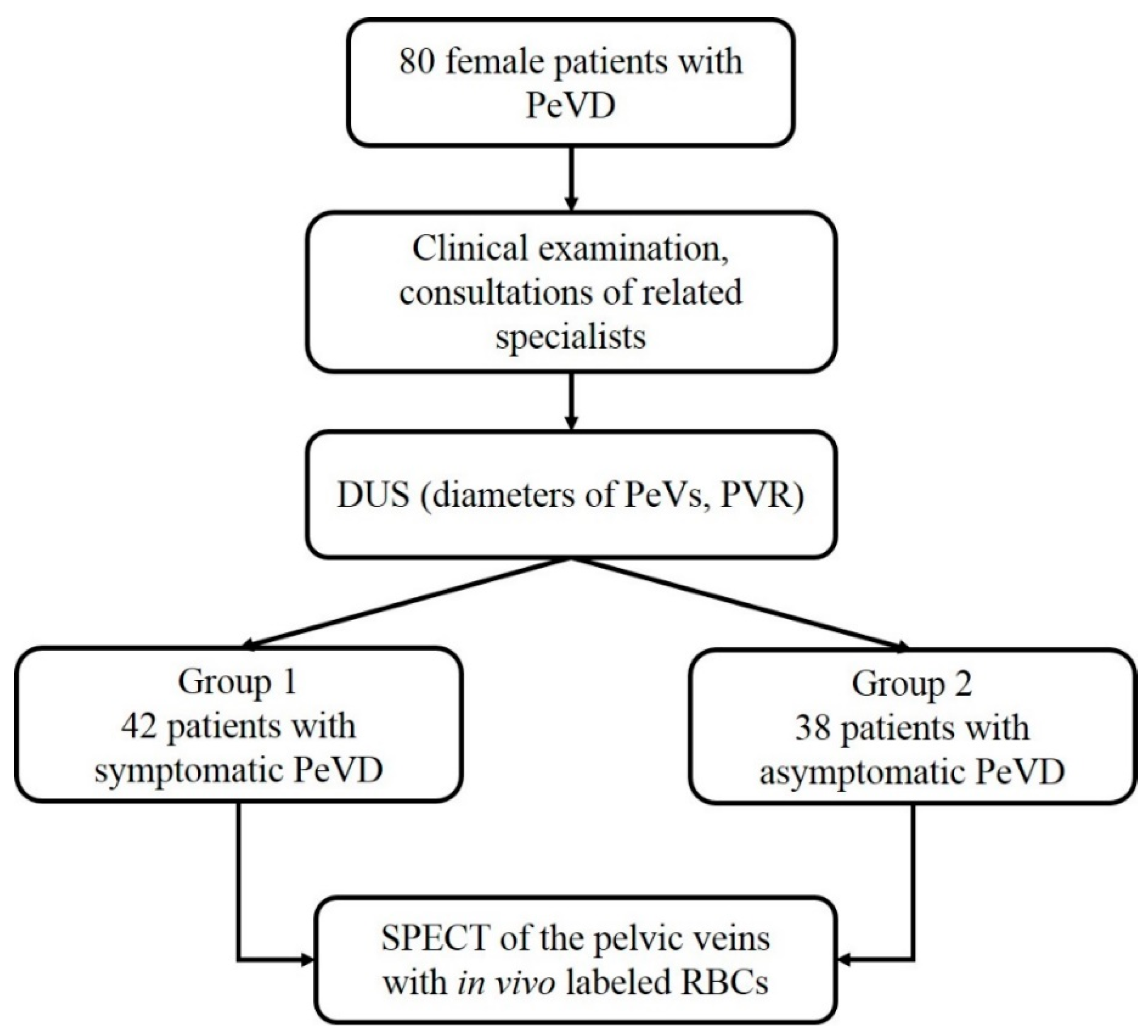
2. Materials and Methods
2.1. Assessment of the Pelvic Veins
2.2. Statistical Methods
3. Results
3.1. Symptomatic Patients with PeVD
3.2. Asymptomatic Patients with PeVD
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- El-Minawi, A.M. Pelvic varicosities and pelvic congestion syndrome. In Pelvic Pain: Diagnosis and Management; Howard, F.M., Perry, C.P., Carter, J.E., El-Minawi, A.M., Eds.; Lippincott, Williams & Wilkins: Philadelphia, PA, USA, 2000; pp. 171–183. [Google Scholar]
- Antignani, P.L.; Lazarashvili, Z.; Monedero, J.L.; Ezpeleta, S.Z.; Whiteley, M.S.; Khilnani, N.M.; Meissner, M.H.; Wittens, C.H.; Kurstjens, R.L.; Belova, L.; et al. Diagnosis and treatment of pelvic congestion syndrome: UIP consensus document. Int. Angiol. 2019, 38, 265–283. [Google Scholar] [CrossRef]
- Champaneria, R.; Shah, L.; Moss, J.; Gupta, J.K.; Birch, J.; Middleton, L.J.; Daniels, J.P. The relationship between pelvic vein incompetence and chronic pelvic pain in women: Systematic reviews of diagnosis and treatment effectiveness. Health Technol. Assess. 2016, 20, 1–108. [Google Scholar] [CrossRef]
- Gavrilov, S.; Moskalenko, Y.P.; Mishakina, N.Y.; Efremova, O.I.; Kulikov, V.M.; Grishenkova, A.S. Stratification of pelvic venous reflux in patients with pelvic varicose veins. J. Vasc. Surg. Venous Lymphat. Disord. 2021, 9, 1417–1424. [Google Scholar] [CrossRef]
- Gavrilov, S.G.; Krasavin, G.V.; Mishakina, N.Y.; Efremova, O.I.; Zolotukhin, I.A. The effect of venoactive drug therapy on the development and severity of post-embolization syndrome in endovascular Interventions on the gonadal veins. J. Pers. Med. 2021, 11, 521. [Google Scholar] [CrossRef] [PubMed]
- Ganeshan, A.; Upponi, S.; Hon, L.-Q.; Uthappa, M.C.; Warakaulle, D.R.; Uberoi, R. Chronic pelvic pain due to pelvic congestion syndrome: The role of diagnostic and interventional radiology. Cardiovasc. Interv. Radiol. 2007, 30, 1105–1111. [Google Scholar] [CrossRef]
- Dos Santos, S.J.; Holdstock, J.M.; Harrison, C.C.; Lopez, A.J.; Whiteley, M.S. Ovarian Vein Diameter Cannot Be Used as an Indicator of Ovarian Venous Reflux. Eur. J. Vasc. Endovasc. Surg. 2015, 49, 90–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whiteley, A.M.; Taylor, D.C.; Dos Santos, S.J.; Whiteley, M.S. Pelvic venous reflux is a major contributory cause of recurrent varicose veins in more than a quarter of women. J. Vasc. Surg. Venous Lymphat. Disord. 2014, 2, 411–415. [Google Scholar] [CrossRef]
- Whiteley, M.S.; Dos Santos, S.J.; Harrison, C.C.; Holdstock, J.M.; Lopez, A.J. Transvaginal duplex ultrasonography appears to be the gold standard investigation for the haemodynamic evaluation of pelvic venous reflux in the ovarian and internal iliac veins in women. Phlebology 2015, 30, 706–713. [Google Scholar] [CrossRef]
- Dos Santos, S.J.; Holdstock, J.M.; Harrison, C.C.; Whiteley, M.S. Long-term results of transjugular coil embolisation for pelvic vein reflux—Results of the abolition of venous reflux at 6–8 years. Phlebology 2016, 31, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Whiteley, M.S. Objective measurements of pelvic venous reflux and stratification of severity of venous reflux in pelvic congestion syndrome due to pelvic venous reflux. Curr. Med. Res. Opin. 2019, 33, 2089–2095. [Google Scholar] [CrossRef] [Green Version]
- Szary, C.; Wilczko, J.; Zawadzki, M.; Grzela, T. Hemodynamic and Radiological Classification of Ovarian Veins System Insufficiency. J. Clin. Med. 2021, 10, 646. [Google Scholar] [CrossRef]
- Ananthan, K.; Onida, S.; Davies, A.H. Nutcracker syndrome: An update on current diagnostic criteria and management guidelines. Eur. J. Vasc. Endovasc. Surg. 2017, 53, 886–894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Velasquez, C.A.; Saeyeldin, A.; Zafar, M.A.; Brownstein, A.J.; Erben, Y. A systematic review on management of nutcracker syndrome. J. Vasc. Surg. Venous Lymphat. Disord. 2018, 6, 271–278. [Google Scholar] [CrossRef]
- Gavrilov, S.G.; Karalkin, A.V.; Turischeva, O.O. Compression treatment of pelvic congestion syndrome. Phlebology 2018, 33, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Stones, R.W. Chronic pain in women: New perspectives on pathophysiology and management. Reprod. Med. Rev. 2000, 8, 229–240. [Google Scholar] [CrossRef]
- Daniels, J.P.; Khan, K.S. Chronic pelvic pain in women. BMJ 2010, 341, c4834. [Google Scholar] [CrossRef] [Green Version]
- Phillips, D.; Deipolyi, A.R.; Hesketh, R.L.; Midia, M.; Oklu, R. Pelvic congestion syndrome: Etiology of pain, diagnosis, and clinical management. J. Vasc. Interv. Radiol. 2014, 25, 725–733. [Google Scholar] [CrossRef]
- Gavrilov, S.G.; Vasiliev, A.; Moskalenko, Y.; Mishakina, N. Diagnostic value of pelvic venography in female patients with pelvic varicose veins and vulvar varicosities. Int. Angiol. 2020, 39, 452–460. [Google Scholar] [CrossRef]
- Gloviczki, P.; Comerota, A.J.; Dalsing, M.C.; Eklof, B.G.; Gillespie, D.L.; Gloviczki, M.L.; Lohr, J.M.; McLafferty, R.B.; Meissner, M.H.; Murad, M.H.; et al. The care of patients with varicose veins and associated chronic venous diseases: Clinical practice guidelines of the Society for Vascular Surgery and the American Venous Forum. J. Vasc. Surg. 2011, 53, 2S–48S. [Google Scholar] [CrossRef] [Green Version]
- Herrera-Betancourt, A.L.; Villegas-Echeverri, J.D.; Lopez-Jaramillo, J.D.; Lopez-Isanoa, J.D.; Estrada-Alvarez, J.M. Sensitivity and specificity of clinical findings for the diagnosis of pelvic congestion syndrome in women with chronic pelvic pain. Phlebology 2018, 33, 303–308. [Google Scholar] [CrossRef]
- Asciutto, G.; Mumme, A.; Asciutto, K.C.; Geier, B. Pelvic vein incompetence influences pain levels in patients with lower limb varicosity. Phlebology 2010, 25, 179–183. [Google Scholar] [CrossRef]
- Khilnani, N.M.; Meissner, M.H.; Learman, L.A.; Gibson, K.D.; Daniels, J.P.; Winokur, R.S.; Marvel, R.P.; Machan, L.; Venbrux, A.C.; Tu, F.F.; et al. Research priorities in pelvic venous disorders in women: Recommendations from a multidisciplinary research consensus panel. J. Vasc. Interv. Radiol. 2019, 30, 781–789. [Google Scholar] [CrossRef]
- Ignacio, E.A.; Dua, R.; Sarin, S.; Amy Soltes Harper Yim, D.; Mathur, V.; Venbrux, A.C. Pelvic congestion syndrome: Diagnosis and treatment. Semin. Interv. Radiol. 2008, 25, 361–368. [Google Scholar] [CrossRef] [Green Version]
- Gavrilov, S.G.; Vasilieva, G.Y.; Vasiliev, I.M.; Efremova, O.I. Calcitonin gene-related peptide and substance P as predictors of venous pelvic pain. Acta Nat. 2019, 11, 88–92. [Google Scholar] [CrossRef]
- Gavrilov, S.G.; Vassilieva, G.Y.; Vasilev, I.M.; Grishenkova, A.S. The role of vasoactive neuropeptides in the genesis of venous pelvic pain: A review. Phlebology 2020, 35, 4–9. [Google Scholar] [CrossRef] [PubMed]
- White, J.V. Moving toward more appropriate care for women with pelvic, labial, or perineal venous disease. J. Vasc. Surg. Venous Lymphat. Disord. 2017, 5, 475–476. [Google Scholar] [CrossRef]
- Gibson, K.; Minjarez, R.; Ferris, B.; Neradilek, M.; Wise, M.; Stoughton, J.; Meissner, M. Clinical presentation of women with pelvic source varicose veins in the perineum as a first step in the development of a disease-specific patient assessment tool. J. Vasc. Surg. Venous Lymphat. Disord. 2017, 5, 493–499. [Google Scholar] [CrossRef] [PubMed]
- White, A.M.; Holdstock, J.M. Ultrasound assessment of pelvic venous reflux. Indian J. Vasc. Endovasc. Surg. 2018, 5, 234–243. [Google Scholar] [CrossRef]
- Greiner, M.; Gilling-Smith, G.L. Leg varices originating from the pelvis: Diagnosis and treatment. Vascular 2007, 15, 70–78. [Google Scholar] [CrossRef]
- Malgor, R.D.; Adrahtas, D.; Spentzouris, G.; Gasparis, A.P.; Tassiopoulos, A.K.; Labropoulos, N. The role of duplex ultrasound in the workup of pelvic congestion syndrome. J. Vasc. Surg. Venous Lymphat. Disord. 2014, 2, 34–38. [Google Scholar] [CrossRef] [PubMed]
| Variable | Symptoms of PeVD (n = 42) | No Symptoms of PeVD (n = 38) | p Value |
|---|---|---|---|
| Age, mean ± SD, years | 32.5 ± 1.4 | 33.3 ± 1.2 | 0.6 |
| BMI, mean ± SD, kg/m2 | 21.1 ± 1.2 | 24.5 ± 0.8 | 0.02 |
| PeVD duration, mean ± SD, years | 4.2 ± 2.3 | 3.3 ± 1.8 | 0.4 |
| CPP, mean ± SD, VAS scores | 7.4 ± 1.6 | 0 | n.a. |
| Dyspareunia, mean ± SD, VAS scores | 6.3 ± 0.9 | 0 | n.a. |
| Heaviness in hypogastrium, n (%) | 42 (100) | 0 | n.a. |
| Dysuria, n (%) | 15 (36) | 0 | n.a. |
| Vulvar varicosities, n (%) | 11 (26) | 0 | n.a. |
| VVLE, n (%) | 6 (14) | 17 (45) | <0.01 |
| CVD of class 1 CEAP, n (%) | 15 (36) | 11 (29) | >0.01 |
| Number of pregnancies, n | 2–4 | 2–4 | n.a. |
| Number of births, n | 1–3 | 1–3 | n.a. |
| Small uterine fibroids, n (%) | 3 (7) | 4 (11) | >0.01 |
| Polycystic ovaries, n (%) | 5 (12) | 3 (8) | >0.01 |
| Chronic colitis, n (%) | 2 (5) | 1 (3) | >0.01 |
| Cholelithiasis, n (%) | 4 (10) | 6 (16) | >0.01 |
| Variable | DUS | SPECT | ||||
|---|---|---|---|---|---|---|
| Without Symptoms, n = 38 | With Symptoms, n = 42 | p Value * | Without Symptoms, n = 38 | With Symptoms, n = 42 | p Value * | |
| No reflux in GVs, n (%) | 36 (95) | 31 (74) | 0.03 | - | - | - |
| Diameter of non-refluxing GVs, mean ± SD, mm | - | - | - | |||
| Left | 3.8 ± 0.3 | 4.2 ± 0.4 | 0.6 | |||
| Right | 3.2 ± 0.2 | 3.5 ± 0.3 | 0.43 | |||
| Reflux in GVs, n (%) | 2 (5) | 11 (26) | 0.0 | - | - | - |
| Diameter of refluxing GVs, mean ± SD, mm | - | - | - | |||
| Left | 8.5 ± 0.5 | 7.7 ± 1.3 | 0.5 | |||
| Right | no | 6.8 ± 0.5 | - | |||
| Duration of reflux in GVs, M [IQR], s | 1.0 [0; 2.0] | 4.0 [3.0; 5.0] | 0.008 | - | - | - |
| Diameter of PVs, mean ± SD, mm | 9.5 ± 0.9 | 9.8 ± 0.9 | 0.7 | - | - | - |
| Reflux in PVs, n (%) | 38 (100) | 42 (100) | n.a. | - | - | - |
| Duration of reflux in PVs, M [IQR], s | 1.5 [1.0; 2.0] | 4.0 [3.0; 5.0] | 0.007 | - | - | - |
| Diameter of UVs, mean ± SD, mm | 5.5 ± 0.6 | 5.6 ± 0.2 | 0.87 | - | - | - |
| Reflux in UVs, n (%) | 7 (18) | 33 (79) | 0.004 | - | - | - |
| Duration of reflux in UVs, M [IQR], s | 1.0 [1.0; 2.0] | 2.0 [2.0; 3.0] | 0.04 | - | - | - |
| Visible GVs | - | - | - | - | ||
| Left GV, n (%) | 0 | 8 | ||||
| Right GV, n (%) | 0 | 0 | ||||
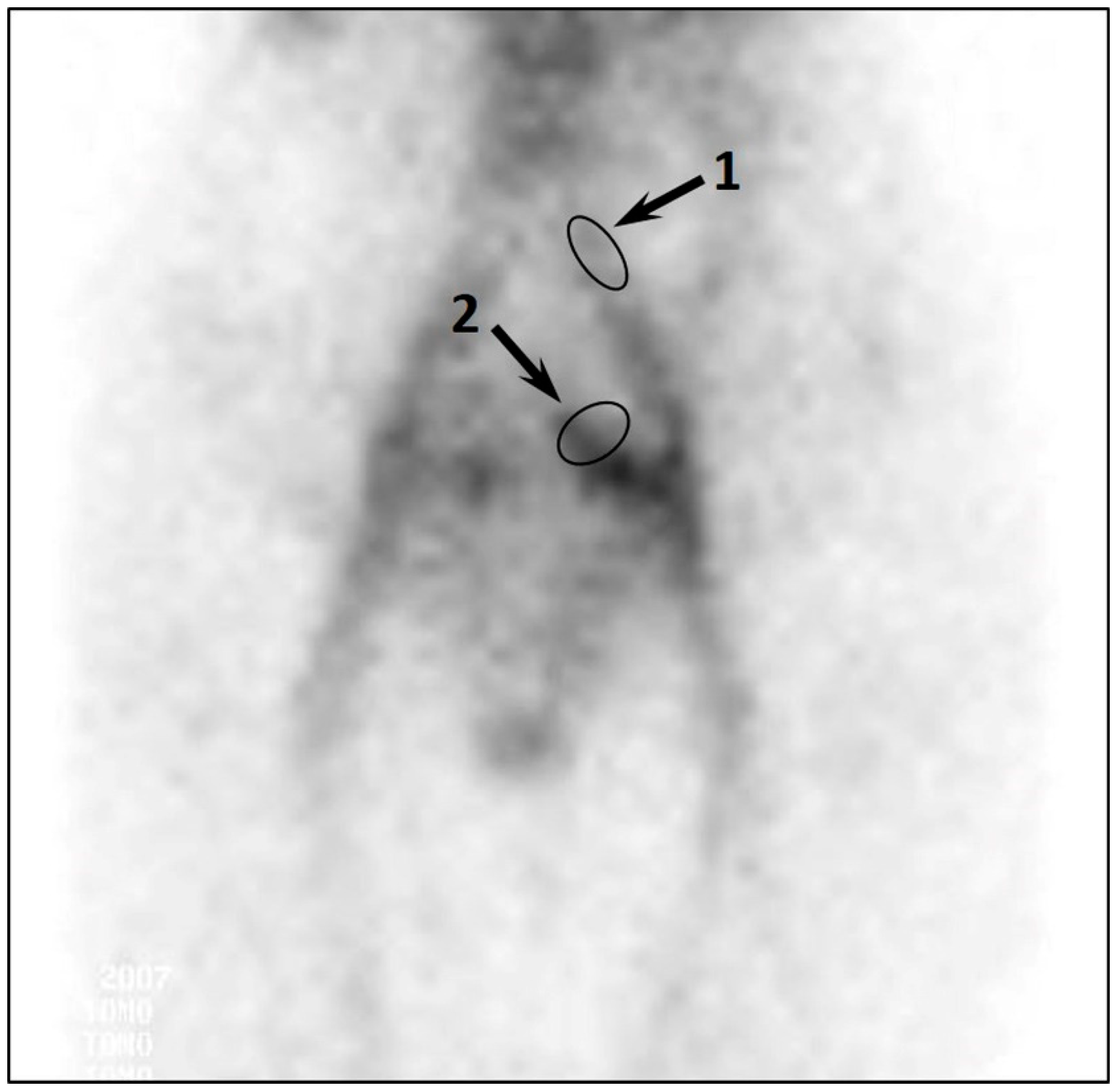
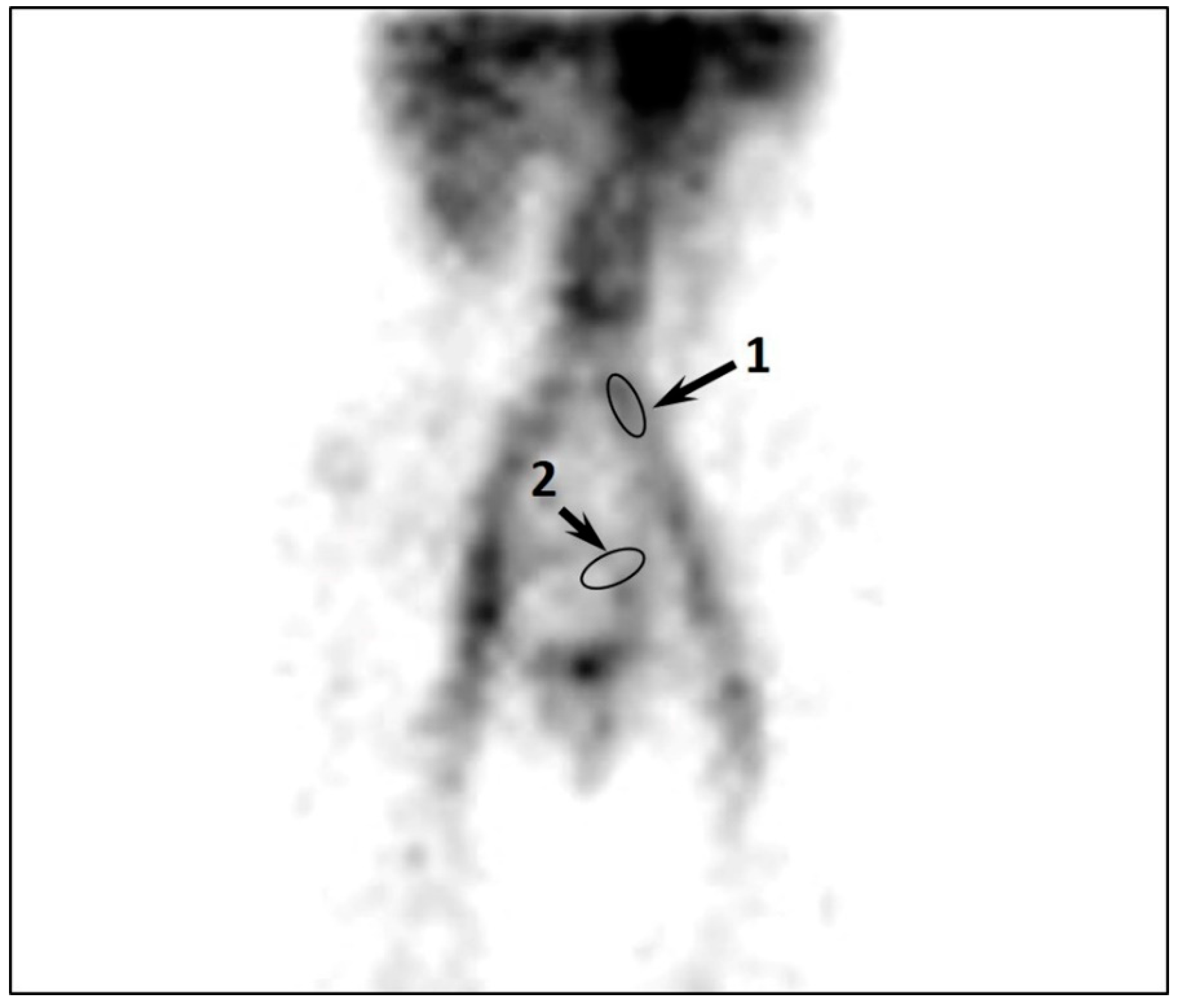
| Excessive accumulation of RPH in PVs and UVs | - | - | - | + | +++ | - |
| CPVC | - | - | - | 0.7 ± 0.2 | 1.9 ± 0.4 | 0.008 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gavrilov, S.; Karalkin, A.; Mishakina, N.; Efremova, O.; Grishenkova, A. Relationships of Pelvic Vein Diameter and Reflux with Clinical Manifestations of Pelvic Venous Disorder. Diagnostics 2022, 12, 145. https://doi.org/10.3390/diagnostics12010145
Gavrilov S, Karalkin A, Mishakina N, Efremova O, Grishenkova A. Relationships of Pelvic Vein Diameter and Reflux with Clinical Manifestations of Pelvic Venous Disorder. Diagnostics. 2022; 12(1):145. https://doi.org/10.3390/diagnostics12010145
Chicago/Turabian StyleGavrilov, Sergey, Anatoly Karalkin, Nadezhda Mishakina, Oksana Efremova, and Anastasia Grishenkova. 2022. "Relationships of Pelvic Vein Diameter and Reflux with Clinical Manifestations of Pelvic Venous Disorder" Diagnostics 12, no. 1: 145. https://doi.org/10.3390/diagnostics12010145
APA StyleGavrilov, S., Karalkin, A., Mishakina, N., Efremova, O., & Grishenkova, A. (2022). Relationships of Pelvic Vein Diameter and Reflux with Clinical Manifestations of Pelvic Venous Disorder. Diagnostics, 12(1), 145. https://doi.org/10.3390/diagnostics12010145






