Single CT Appointment for Double Lung and Colorectal Cancer Screening: Is the Time Ripe?
Abstract
1. Introduction
2. Epidemiology
3. Pathology
4. Screening Tests for LC and CRC, Their Organization and Adhesion
5. Screening Chest Low-Dose CT and CT Colonography
5.1. Operational Aspects
5.1.1. Technical Features
5.1.2. Reading the Screening CT Examinations
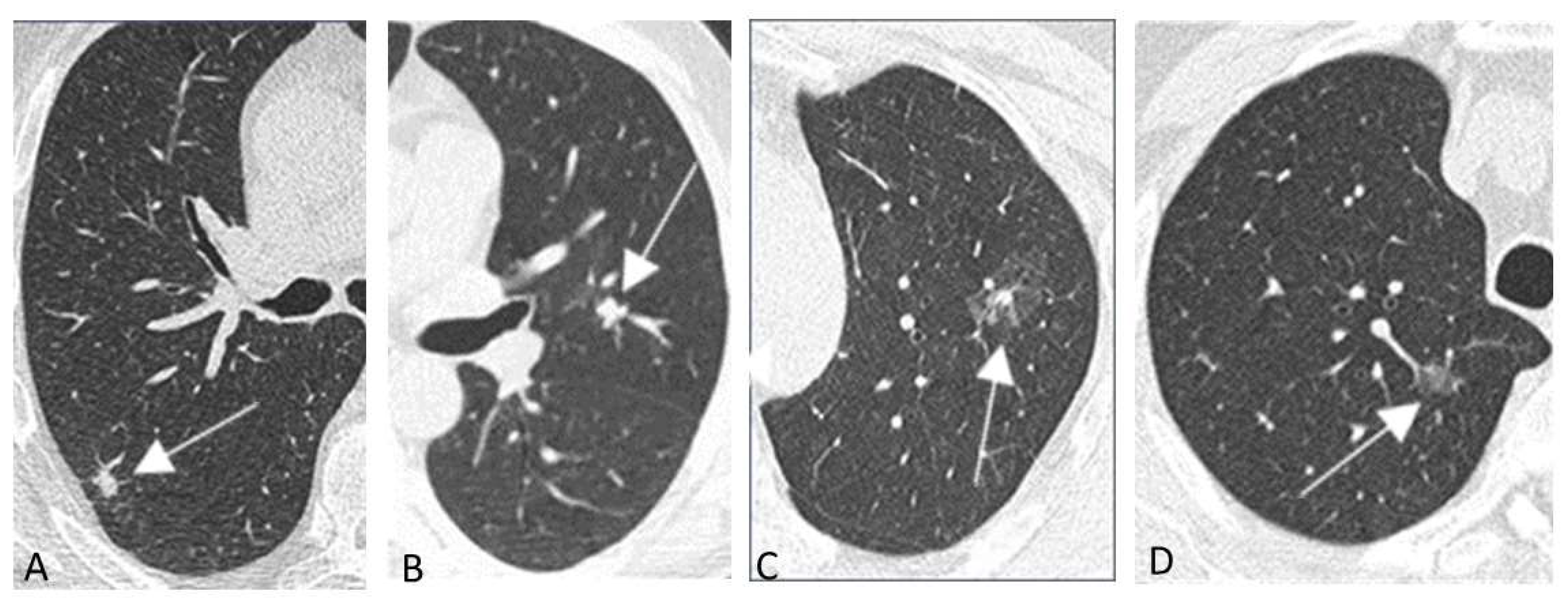
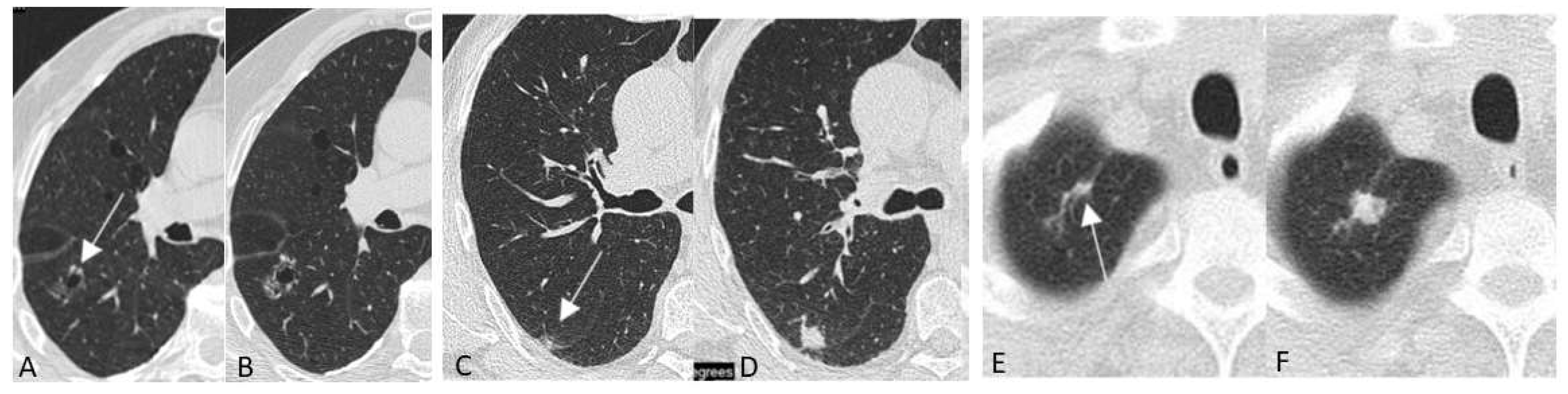
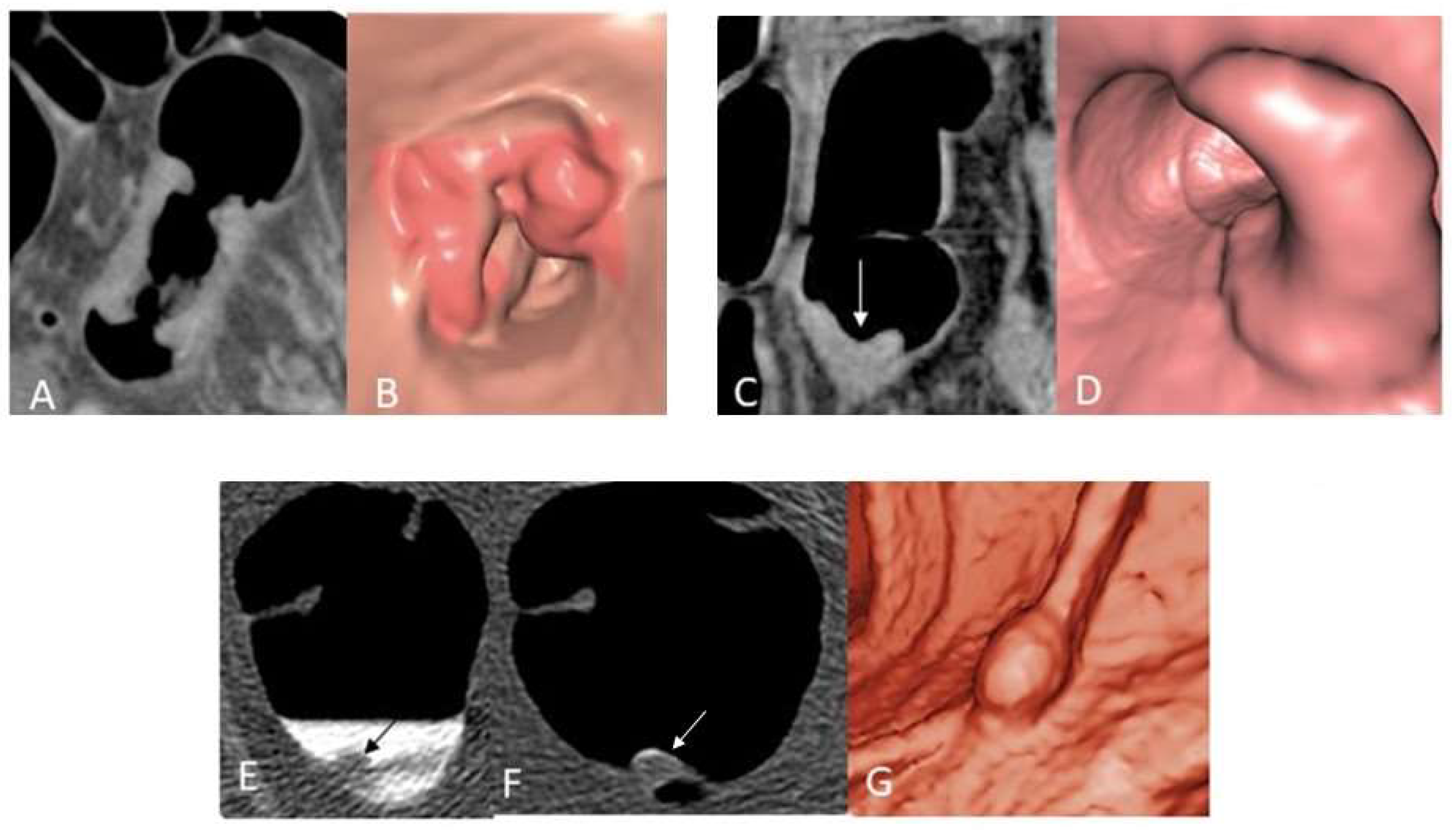
5.1.3. Typical Screening Findings
Chest LDCT
CT Colonography
5.1.4. Collateral and Incidental Findings
Chest LDCT
CT Colonography
5.2. Radiation Exposures
5.3. Costs
5.4. Harms
5.5. Cost Effectiveness
6. Single Appointment CT for Double LC and CRC Screening
6.1. Operational Aspects
6.1.1. Technical Features
6.1.2. Reading the Screening CT Examinations
6.1.3. Typical Screening Findings
6.1.4. Collateral and Incidental Findings
6.2. Radiation Exposure
6.3. Costs
6.4. Harms
6.5. Cost-Effectiveness
7. Open Issues in CT Screening of LC and CRC
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- US Preventive Services Task Force; Krist, A.H.; Davidson, K.W.; Mangione, M.; Barry, M.J.; Cabana, M.; Caughey, A.B.; Davis, E.M.; Donahue, K.E.; Doubeni, C.A.; et al. Screening for Lung Cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2021, 325, 962–970. [Google Scholar] [CrossRef] [PubMed]
- US Preventive Services Task Force; Davidson, K.W.; Barry, M.J.; Mangione, C.M.; Cabana, M.; Caughey, A.B.; Davis, E.M.; Donahue, K.E.; Doubeni, C.A.; Krist, A.H.; et al. Screening for Colorectal Cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2021, 325, 1965–1977, Erratum in: JAMA 2021, 326, 773. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- National Cancer Institute. Cancer Stat Facts: Lung and Bronchus Cancer. 2021. Available online: https://seer.cancer.gov/statfacts/html/lungb.html (accessed on 30 June 2022).
- Snyder, R.A.; Hu, C.Y.; Cuddy, A.; Francescatti, A.B.; Schumacher, J.R.; Van Loon, K.; You, Y.N.; Kozower, B.D.; Greenberg, C.C.; Schrag, D.; et al. Association Between Intensity of Posttreatment Surveillance Testing and Detection of Recurrence in Patients With Colorectal Cancer. JAMA 2018, 319, 2104–2115. [Google Scholar] [CrossRef] [PubMed]
- GBD 2019 Respiratory Tract Cancers Collaborators. Global, regional, and national burden of respiratory tract cancers and associated risk factors from 1990 to 2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Respir. Med. 2021, 9, 1030–1049. [Google Scholar] [CrossRef]
- Su, S.Y.; Liaw, Y.P.; Jhuang, J.R.; Hsu, S.Y.; Chiang, C.J.; Yang, Y.W.; Lee, W.C. Associations between ambient air pollution and cancer incidence in Taiwan: An ecological study of geographical variations. BMC Public Health 2019, 19, 1496. [Google Scholar] [CrossRef]
- Lipfert, F.W.; Wyzga, R.E. Longitudinal relationships between lung cancer mortality rates, smoking, and ambient air quality: A comprehensive review and analysis. Crit. Rev. Toxicol. 2019, 49, 790–818. [Google Scholar] [CrossRef]
- Corrales, L.; Rosell, R.; Cardona, A.F.; Martín, C.; Zatarain-Barrón, Z.L.; Arrieta, O. Lung cancer in never smokers: The role of different risk factors other than tobacco smoking. Crit. Rev. Oncol. Hematol. 2020, 148, 102895. [Google Scholar] [CrossRef]
- Myers, R.; Brauer, M.; Dummer, T.; Atkar-Khattra, S.; Yee, J.; Melosky, B.; Ho, C.; McGuire, A.L.; Sun, S.; Grant, K.; et al. High-Ambient Air Pollution Exposure Among Never Smokers Versus Ever Smokers With Lung Cancer. J. Thorac. Oncol. 2021, 16, 1850–1858. [Google Scholar] [CrossRef]
- Dubin, S.; Griffin, D. Lung Cancer in Non-Smokers. Mo Med. 2020, 117, 375–379. [Google Scholar]
- Carreras, G.; Lugo, A.; Gallus, S.; Cortini, B.; Fernández, E.; López, M.J.; Soriano, J.B.; Nicolás, Á.L.; Semple, S.; Gorini, G.; et al. Burden of disease attributable to second-hand smoke exposure: A systematic review. Prev. Med. 2019, 129, 105833. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Hüsing, A.; Sookthai, D.; Bergmann, M.; Boeing, H.; Becker, N.; Kaaks, R. Selecting High-Risk Individuals for Lung Cancer Screening: A Prospective Evaluation of Existing Risk Models and Eligibility Criteria in the German EPIC Cohort. Cancer Prev. Res. 2015, 8, 777–785. [Google Scholar] [CrossRef] [PubMed]
- Tammemägi, M.C.; Ruparel, M.; Tremblay, A.; Myers, R.; Mayo, J.; Yee, J.; Atkar-Khattra, S.; Yuan, R.; Cressman, S.; English, J.; et al. USPSTF2013 versus PLCOm2012 lung cancer screening eligibility criteria (International Lung Screening Trial): Interim analysis of a prospective cohort study. Lancet Oncol. 2022, 23, 138–148. [Google Scholar] [CrossRef]
- Cassidy, A.; Myles, J.P.; van Tongeren, M.; Page, R.D.; Liloglou, T.; Duffy, S.W.; Field, J.K. The LLP risk model: An individual risk prediction model for lung cancer. Br. J. Cancer 2008, 98, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Bach, P.B.; Kattan, M.W.; Thornquist, M.D.; Kris, M.G.; Tate, R.C.; Barnett, M.J.; Hsieh, L.J.; Begg, C.B. Variations in lung cancer risk among smokers. J. Natl. Cancer Inst. 2003, 95, 470–478. [Google Scholar] [CrossRef]
- Kanth, P.; Inadomi, J.M. Screening and prevention of colorectal cancer. BMJ 2021, 374, n1855. [Google Scholar] [CrossRef]
- Bouvard, V.; Loomis, D.; Guyton, K.Z.; Grosse, Y.; Ghissassi, F.E.; Benbrahim-Tallaa, L.; Guha, N.; Mattock, H.; Straif, K.; International Agency for Research on Cancer Monograph Working Group. Carcinogenicity of consumption of red and processed meat. Lancet Oncol. 2015, 16, 1599–1600. [Google Scholar] [CrossRef]
- Peto, R.; Lopez, A.D.; Boreham, J.; Thun, M. Mortality from Smoking in Developed Countries, 1950–2000, 2nd ed.; 2006; Available online: www.deathsfromsmoking.net (accessed on 30 June 2022).
- Silva, M.; Galeone, C.; Sverzellati, N.; Marchianò, A.; Calareso, G.; Sestini, S.; La Vecchia, C.; Sozzi, G.; Pelosi, G.; Pastorino, U. Screening with Low-Dose Computed Tomography Does Not Improve Survival of Small Cell Lung Cancer. J. Thorac. Oncol. 2016, 11, 187–193. [Google Scholar] [CrossRef]
- National Lung Screening Trial Research Team; Aberle, D.R.; Adams, A.M.; Berg, C.D.; Black, W.C.; Clapp, J.D.; Fagerstrom, R.M.; Gareen, I.F.; Gatsonis, C.; Marcus, P.M.; et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N. Engl. J. Med. 2011, 365, 395–409. [Google Scholar] [CrossRef]
- Paci, E.; Puliti, D.; Lopes Pegna, A.; Carrozzi, L.; Picozzi, G.; Falaschi, F.; Pistelli, F.; Aquilini, F.; Ocello, C.; Zappa, M.; et al. Mortality, survival and incidence rates in the ITALUNG randomised lung cancer screening trial. Thorax 2017, 72, 825–831. [Google Scholar] [CrossRef]
- de Koning, H.J.; van der Aalst, C.M.; de Jong, P.A.; Scholten, E.T.; Nackaerts, K.; Heuvelmans, M.A.; Lammers, J.J.; Weenink, C.; Yousaf-Khan, U.; Horeweg, N.; et al. Reduced Lung-Cancer Mortality with Volume CT Screening in a Randomized Trial. N. Engl. J. Med. 2020, 382, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Becker, N.; Motsch, E.; Trotter, A.; Heussel, C.P.; Dienemann, H.; Schnabel, P.A.; Kauczor, H.U.; Maldonado, S.G.; Miller, A.B.; Kaaks, R.; et al. Lung cancer mortality reduction by LDCT screening—Results from the randomized German LUSI trial. Int. J. Cancer 2020, 146, 1503–1513. [Google Scholar] [CrossRef] [PubMed]
- Ten Haaf, K.; van Rosmalen, J.; de Koning, H.J. Lung cancer detectability by test, histology, stage, and gender: Estimates from the NLST and the PLCO trials. Cancer Epidemiol. Biomark. Prev. 2015, 24, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Puliti, D.; Picozzi, G.; Gorini, G.; Carrozzi, L.; Mascalchi, M. Gender effect in the ITALUNG screening trial. A comparison with UKLS and other trials. Lancet Reg Health Eur. 2022, 13, 100300. [Google Scholar] [CrossRef]
- Lambe, G.; Durand, M.; Buckley, A.; Nicholson, S.; McDermott, R. Adenocarcinoma of the lung: From BAC to the future. Insights Imaging 2020, 11, 69. [Google Scholar] [CrossRef]
- Thakrar, R.M.; Pennycuick, A.; Borg, E.; Janes, S.M. Preinvasive disease of the airway. Cancer Treat. Rev. 2017, 58, 77–90. [Google Scholar] [CrossRef]
- Field, J.K.; Vulkan, D.; Davies, M.P.A.; Baldwin, D.R.; Brain, K.E.; Devaraj, A.; Eisen, T.; Gosney, J.; Green, B.A.; Holemans, J.A.; et al. Lung cancer mortality reduction by LDCT screening: UKLS randomised trial results and international meta-analysis. Lancet Reg. Health Eur. 2021, 10, 100179. [Google Scholar] [CrossRef]
- Kang, H.R.; Cho, J.Y.; Lee, S.H.; Lee, Y.J.; Park, J.S.; Cho, Y.J.; Yoon, H.I.; Lee, K.W.; Lee, J.H.; Lee, C.T. Role of Low-Dose Computerized Tomography in Lung Cancer Screening among Never-Smokers. J. Thorac. Oncol. 2019, 14, 436–444. [Google Scholar] [CrossRef]
- Kerpel-Fronius, A.; Tammemägi, M.; Cavic, M.; Henschke, C.; Jiang, L.; Kazerooni, E.; Lee, C.T.; Ventura, L.; Yang, D.; Lam, S.; et al. Screening for Lung Cancer in Individuals Who Never Smoked: An International Association for the Study of Lung Cancer Early Detection and Screening Committee Report. J. Thorac. Oncol. 2022, 17, 56–66. [Google Scholar] [CrossRef]
- Ollier, M.; Chamoux, A.; Naughton, G.; Pereira, B.; Dutheil, F. Chest CT scan screening for lung cancer in asbestos occupational exposure: A systematic review and meta-analysis. Chest 2014, 145, 1339–1346. [Google Scholar] [CrossRef]
- Oudkerk, M.; Devaraj, A.; Vliegenthart, R.; Henzler, T.; Prosch, H.; Heussel, C.P.; Bastarrika, G.; Sverzellati, N.; Mascalchi, M.; Delorme, S.; et al. European position statement on lung cancer screening. Lancet Oncol. 2017, 18, e754–e766. [Google Scholar] [CrossRef]
- Stoop, E.M.; de Haan, M.C.; de Wijkerslooth, T.R.; Bossuyt, P.M.; van Ballegooijen, M.; Nio, C.Y.; van de Vijver, M.J.; Biermann, K.; Thomeer, M.; van Leerdam, M.E.; et al. Participation and yield of colonoscopy versus non-cathartic CT colonography in population-based screening for colorectal cancer: A randomised controlled trial. Lancet Oncol. 2012, 13, 55–64. [Google Scholar] [CrossRef]
- Sali, L.; Mascalchi, M.; Falchini, M.; Ventura, L.; Carozzi, F.; Castiglione, G.; Delsanto, S.; Mallardi, B.; Mantellini, P.; Milani, S.; et al. Reduced and Full-Preparation CT Colonography, Fecal Immunochemical Test, and Colonoscopy for Population Screening of Colorectal Cancer: A Randomized Trial. J. Natl. Cancer Inst. 2015, 108, djv319. [Google Scholar] [CrossRef] [PubMed]
- Regge, D.; Iussich, G.; Segnan, N.; Correale, L.; Hassan, C.; Arrigoni, A.; Asnaghi, R.; Bestagini, P.; Bulighin, G.; Cassinis, M.C.; et al. Comparing CT colonography and flexible sigmoidoscopy: A randomised trial within a population-based screening programme. Gut 2017, 66, 1434–1440. [Google Scholar] [CrossRef]
- Randel, K.R.; Schult, A.L.; Botteri, E.; Hoff, G.; Bretthauer, M.; Ursin, G.; Natvig, E.; Berstad, P.; Jørgensen, A.; Sandvei, P.K.; et al. Colorectal Cancer Screening With Repeated Faecal Immunochemical Test Versus Sigmoidoscopy: Baseline Results From a Randomized Trial. Gastroenterology 2021, 160, 1085–1096.e5. [Google Scholar] [CrossRef]
- Forsberg, A.; Westerberg, M.; Metcalfe, C.; Steele, R.; Blom, J.; Engstrand, L.; Fritzell, K.; Hellström, M.; Levin, L.Å.; Löwbeer, C.; et al. Once-only colonoscopy or two rounds of faecal immunochemical testing 2 years apart for colorectal cancer screening (SCREESCO): Preliminary report of a randomised controlled trial. Lancet Gastroenterol. Hepatol. 2022, 7, 513–521. [Google Scholar] [CrossRef]
- European Union. Council Recommendation of 2 December 2003 on Cancer Screening. 2003. Available online: https://eurlex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2003:327:0034:0038:EN:PDF (accessed on 7 September 2022).
- Wolf, A.M.D.; Fontham, E.T.H.; Church, T.R.; Flowers, C.R.; Guerra, C.E.; LaMonte, S.J.; Etzioni, R.; McKenna, M.T.; Oeffinger, K.C.; Shih, Y.T.; et al. Colorectal cancer screening for average-risk adults: 2018 guideline update from the American Cancer Society. CA Cancer J. Clin. 2018, 68, 250–281. [Google Scholar] [CrossRef]
- Ventura, L.; Mantellini, P.; Grazzini, G.; Castiglione, G.; Buzzoni, C.; Rubeca, T.; Sacchettini, C.; Paci, E.; Zappa, M. The impact of immunochemical faecal occult blood testing on colorectal cancer incidence. Dig. Liver Dis. 2014, 46, 82–86. [Google Scholar] [CrossRef]
- Elmunzer, B.J.; Hayward, R.A.; Schoenfeld, P.S.; Saini, S.D.; Deshpande, A.; Waljee, A.K. Effect of flexible sigmoidoscopy-based screening on incidence and mortality of colorectal cancer: A systematic review and meta-analysis of randomized controlled trials. PLoS Med. 2012, 9, e1001352. [Google Scholar] [CrossRef]
- Atkin, W.; Edwards, R.; Kralj-Hans, I.; Wooldrage, K.; Hart, A.R.; Northover, J.M.; Parkin, D.M.; Wardle, J.; Duffy, S.W.; Cuzick, J.; et al. Once-only flexible sigmoidoscopy screening in prevention of colorectal cancer: A multicentre randomised controlled trial. Lancet 2010, 375, 1624–1633. [Google Scholar] [CrossRef]
- Meester, R.G.; Doubeni, C.A.; Lansdorp-Vogelaar, I.; Goede, S.L.; Levin, T.R.; Quinn, V.P.; Ballegooijen, M.V.; Corley, D.A.; Zauber, A.G. Colorectal cancer deaths attributable to nonuse of screening in the United States. Ann. Epidemiol. 2015, 25, 208–213.e1. [Google Scholar] [CrossRef]
- Zauber, A.G. The impact of screening on colorectal cancer mortality and incidence: Has it really made a difference? Dig. Dis. Sci. 2015, 60, 681–691. [Google Scholar] [CrossRef] [PubMed]
- Pickhardt, P.J. CT Colonography: The Role of Radiologist Training. Radiology 2022, 303, 371–372. [Google Scholar] [CrossRef] [PubMed]
- Sali, L.; Ventura, L.; Mascalchi, M.; Falchini, M.; Mallardi, B.; Milani, M.; Grazzini, G.; Zappa, M.; Mantellini, P. Single CT colonography versus three rounds of faecal immunochemical test for population-based screening of colorectal cancer: The SAVE randomised clinical trial. Lancet Gastroenterol. Hepatol. 2022; Online ahead of print. [Google Scholar] [CrossRef]
- Rex, D.K.; Boland, C.R.; Dominitz, J.A.; Giardiello, F.M.; Johnson, D.A.; Kaltenbach, T.; Levin, T.R.; Lieberman, D.; Robertson, D.J. Colorectal Cancer Screening: Recommendations for Physicians and Patients from the U.S. Multi-Society Task Force on Colorectal Cancer. Am. J. Gastroenterol. 2017, 112, 1016–1030. [Google Scholar] [CrossRef] [PubMed]
- Winawer, S.J.; Fletcher, R.H.; Miller, L.; Godlee, F.; Stolar, M.H.; Mulrow, C.D.; Woolf, S.H.; Glick, S.N.; Ganiats, T.G.; Bond, J.H.; et al. Colorectal cancer screening: Clinical guidelines and rationale. Gastroenterology 1997, 112, 594–642, Erratum in: Gastroenterology 1997, 112, 1060. Erratum in: Gastroenterology 1998, 114, 625. [Google Scholar] [CrossRef] [PubMed]
- Ladabaum, U.; Ferrandez, A.; Lanas, A. Cost-effectiveness of colorectal cancer screening in high-risk Spanish patients: Use of a validated model to inform public policy. Cancer Epidemiol. Biomark. Prev. 2010, 19, 2765–2776. [Google Scholar] [CrossRef]
- Ait Ouakrim, D.; Boussioutas, A.; Lockett, T.; Winship, I.; Giles, G.G.; Flander, L.B.; Keogh, L.; Hopper, J.L.; Jenkins, M.A. Screening practices of unaffected people at familial risk of colorectal cancer. Cancer Prev. Res. 2012, 5, 240–247. [Google Scholar] [CrossRef]
- Wilkins, T.; McMechan, D.; Talukder, A.; Herline, A. Colorectal Cancer Screening and Surveillance in Individuals at Increased Risk. Am. Fam. Physician 2018, 97, 111–116. [Google Scholar]
- Paszat, L.; Sutradhar, R.; Luo, J.; Tinmouth, J.; Rabeneck, L.; Baxter, N.N. Uptake and Short-term Outcomes of High-risk Screening Colonoscopy Billing Codes: A Population-based Study Among Young Adults. J. Can. Assoc. Gastroenterol. 2021, 5, 86–95. [Google Scholar] [CrossRef]
- U.S. Preventive Services Task Force. Lung Cancer: Screening. Recommendation Summary. Available online: www.uspreventiveservicestaskforce.org (accessed on 9 July 2018).
- Allison, J.E.; Tekawa, I.S.; Ransom, L.J.; Adrain, A.L. A comparison of fecal occult-blood tests for colorectal-cancer screening. N. Engl. J. Med. 1996, 334, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Castiglione, G.; Zappa, M.; Grazzini, G.; Mazzotta, A.; Biagini, M.; Salvadori, P.; Ciatto, S. Immunochemical vs guaiac faecal occult blood tests in a population-based screening programme for colorectal cancer. Br. J. Cancer 1996, 74, 141–144. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rustagi, A.S.; Byers, A.L.; Keyhani, S. Likelihood of Lung Cancer Screening by Poor Health Status and Race and Ethnicity in US Adults, 2017 to 2020. JAMA Netw. Open 2022, 5, e225318. [Google Scholar] [CrossRef] [PubMed]
- Barsouk, A.; Saginala, K.; Aluru, J.S.; Rawla, P.; Barsouk, A. US Cancer Screening Recommendations: Developments and the Impact of COVID-19. Med. Sci. 2022, 10, 16. [Google Scholar] [CrossRef] [PubMed]
- Battisti, F.; Falini, P.; Gorini, G.; Sassoli de Bianchi, P.; Armaroli, P.; Giubilato, P.; Giorgi Rossi, P.; Zorzi, M.; Battagello, J.; Senore, C.; et al. Cancer screening programmes in Italy during the COVID-19 pandemic: An update of a nationwide survey on activity volumes and delayed diagnoses. Ann. Ist. Super. Sanità 2022, 58, 16–24. [Google Scholar] [CrossRef]
- Silva, M.; Picozzi, G.; Sverzellati, N.; Anglesio, S.; Bartolucci, M.; Cavigli, E.; Deliperi, A.; Falchini, M.; Falaschi, F.; Ghio, D.; et al. Low-dose CT for lung cancer screening: Position paper from the Italian college of thoracic radiology. Radiol. Med. 2022, 127, 543–559. [Google Scholar] [CrossRef]
- Sali, L.; Ventura, L.; Grazzini, G.; Borgheresi, A.; Delsanto, S.; Falchini, M.; Mallardi, B.; Mantellini, P.; Milani, S.; Pallanti, S.; et al. Patients’ experience of screening CT colonography with reduced and full bowel preparation in a randomised trial. Eur. Radiol. 2019, 29, 2457–2464. [Google Scholar] [CrossRef]
- Sali, L.; Grazzini, G.; Carozzi, F.; Castiglione, G.; Falchini, M.; Mallardi, B.; Mantellini, P.; Ventura, L.; Regge, D.; Zappa, M.; et al. Screening for colorectal cancer with FOBT, virtual colonoscopy and optical colonoscopy: Study protocol for a randomized controlled trial in the Florence district (SAVE study). Trials 2013, 14, 74. [Google Scholar] [CrossRef]
- Hwang, E.J.; Goo, J.M.; Kim, H.Y.; Yoon, S.H.; Jin, G.Y.; Yi, J.; Kim, Y. Variability in interpretation of low-dose chest CT using computerized assessment in a nationwide lung cancer screen ing program: Comparison of prospective reading at individual institutions and retrospective central reading. Eur. Radiol. 2021, 31, 2845–2855. [Google Scholar] [CrossRef]
- Huang, P.; Park, S.; Yan, R.; Lee, J.; Chu, L.C.; Lin, C.T.; Hussien, A.; Rathmell, J.; Thomas, B.; Chen, C.; et al. Added Value of Computer-aided CT Image Features for Early Lung Cancer Diagnosis with Small Pulmonary Nodules: A Matched Case-Control Study. Radiology 2018, 286, 286–295. [Google Scholar] [CrossRef]
- Cui, S.; Ming, S.; Lin, Y.; Chen, F.; Shen, Q.; Li, H.; Chen, G.; Gong, X.; Wang, H. Development and clinical application of deep learning model for lung nodules screening on CT images. Sci. Rep. 2020, 10, 13657. [Google Scholar] [CrossRef]
- Cui, X.; Zheng, S.; Heuvelmans, M.A.; Du, Y.; Sidorenkov, G.; Fan, S.; Li, Y.; Xie, Y.; Zhu, Z.; Dorrius, M.D.; et al. Performance of a deep learning-based lung nodule detection system as an alternative reader in a Chinese lung cancer screening program. Eur. J. Radiol. 2022, 146, 110068. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.; Wang, G.; Yi, L.; Wang, C.; Lan, T.; Xu, X.; Guo, J.; Deng, T.; Liu, D.; Chen, B.; et al. Deep Learning Empowers Lung Cancer Screening Based on Mobile Low-Dose Computed Tomography in Resource-Constrained Sites. Front. Biosci. 2022, 27, 212. [Google Scholar] [CrossRef] [PubMed]
- Obaro, A.; Plumb, A.A.; North, M.P.; Halligan, S.; Burling, D.N. Computed tomographic colonography: How many and how fast should radiologists report? Eur. Radiol. 2019, 29, 5784–5790. [Google Scholar] [CrossRef] [PubMed]
- Iussich, G.; Correale, L.; Senore, C.; Segnan, N.; Laghi, A.; Iafrate, F.; Campanella, D.; Neri, E.; Cerri, F.; Hassan, C.; et al. CT colonography: Preliminary assessment of a double-read paradigm that uses computer-aided detection as the first reader. Radiology 2013, 268, 743–751. [Google Scholar] [CrossRef] [PubMed]
- Neri, E.; Halligan, S.; Hellström, M.; Lefere, P.; Mang, T.; Regge, D.; Stoker, J.; Taylor, S.; Laghi, A.; ESGAR CT Colonography Working Group. The second ESGAR consensus statement on CT colonography. Eur. Radiol. 2013, 23, 720–729. [Google Scholar] [CrossRef]
- Plumb, A.A.; Halligan, S.; Nickerson, C.; Bassett, P.; Goddard, A.F.; Taylor, S.A.; Patnick, J.; Burling, D. Use of CT colonography in the English Bowel Cancer Screening Programme. Gut 2014, 63, 964–973. [Google Scholar] [CrossRef]
- Obaro, A.E.; Plumb, A.A.; Halligan, S.; Mallett, S.; Bassett, P.; McCoubrie, P.; Baldwin-Cleland, R.; Ugarte-Cano, C.; Lung, P.; Muckian, J.; et al. Colorectal Cancer: Performance and Evaluation for CT Colonography Screening- A Multicenter Cluster-randomized Controlled Trial. Radiology 2022, 303, 361–370. [Google Scholar] [CrossRef]
- Sali, L.; Delsanto, S.; Sacchetto, D.; Correale, L.; Falchini, M.; Ferraris, A.; Gandini, G.; Grazzini, G.; Iafrate, F.; Iussich, G.; et al. Computer-based self-training for CT colonography with and without CAD. Eur. Radiol. 2018, 28, 4783–4791. [Google Scholar] [CrossRef]
- Mascalchi, M.; Picozzi, G.; Falchini, M.; Vella, A.; Diciotti, S.; Carrozzi, L.; Pegna, A.L.; Falaschi, F. Initial LDCT appearance of incident lung cancers in the ITALUNG trial. Eur. J. Radiol. 2014, 83, 2080–2086. [Google Scholar] [CrossRef]
- Mascalchi, M.; Attinà, D.; Bertelli, E.; Falchini, M.; Vella, A.; Pegna, A.L.; Ambrosini, V.; Zompatori, M. Lung cancer associated with cystic airspaces. J. Comput. Assist. Tomogr. 2015, 39, 102–108. [Google Scholar] [CrossRef] [PubMed]
- McWilliams, A.; Tammemagi, M.C.; Mayo, J.R.; Roberts, H.; Liu, G.; Soghrati, K.; Yasufuku, K.; Martel, S.; Laberge, F.; Gingras, M.; et al. Probability of cancer in pulmonary nodules detected on first screening CT. N. Engl. J. Med. 2013, 369, 910–919. [Google Scholar] [CrossRef] [PubMed]
- González Maldonado, S.; Delorme, S.; Hüsing, A.; Motsch, E.; Kauczor, H.U.; Heussel, C.P.; Kaaks, R. Evaluation of Prediction Models for Identifying Malignancy in Pulmonary Nodules Detected via Low-Dose Computed Tomography. JAMA Netw. Open 2020, 3, e1921221. [Google Scholar] [CrossRef]
- Ciompi, F.; Chung, K.; van Riel, S.J.; Setio, A.A.A.; Gerke, P.K.; Jacobs, C.; Scholten, E.T.; Schaefer-Prokop, C.; Wille, M.M.W.; Marchianò, A.; et al. Towards automatic pulmonary nodule management in lung cancer screening with deep learning. Sci. Rep. 2017, 7, 46479. [Google Scholar] [CrossRef]
- Venkadesh, K.V.; Setio, A.A.A.; Schreuder, A.; Scholten, E.T.; Chung, K.; Wille, M.M.V.; Saghir, Z.; van Ginneken, B.; Prokop, M.; Jacobs, C. Deep Learning for Malignancy Risk Estimation of Pulmonary Nodules Detected at Low-Dose Screening CT. Radiology 2021, 300, 438–447. [Google Scholar] [CrossRef] [PubMed]
- Ricci, Z.J.; Mazzariol, F.S.; Kobi, M.; Flusberg, M.; Moses, M.; Yee, J. CT Colonography: Improving Interpretive Skill by Avoiding Pitfalls. Radiographics 2020, 40, 98–119. [Google Scholar] [CrossRef]
- Zalis, M.E.; Barish, M.A.; Choi, J.R.; Dachman, A.H.; Fenlon, H.M.; Ferrucci, J.T.; Glick, S.N.; Laghi, A.; Macari, M.; McFarland, E.G.; et al. CT colonography reporting and data system: A consensus proposal. Radiology 2005, 236, 3–9. [Google Scholar] [CrossRef]
- Camiciottoli, G.; Cavigli, E.; Grassi, L.; Diciotti, S.; Orlandi, I.; Zappa, M.; Picozzi, G.; Pegna, A.L.; Paci, E.; Falaschi, F.; et al. Prevalence and correlates of pulmonary emphysema in smokers and former smokers. A densitometric study of participants in the ITALUNG trial. Eur. Radiol. 2009, 19, 58–66. [Google Scholar] [CrossRef]
- Sverzellati, N.; Guerci, L.; Randi, G.; Calabrò, E.; La Vecchia, C.; Marchianò, A.; Pesci, A.; Zompatori, M.; Pastorino, U. Interstitial lung diseases in a lung cancer screening trial. Eur. Respir. J. 2011, 38, 392–400. [Google Scholar] [CrossRef]
- Chiles, C.; Duan, F.; Gladish, G.W.; Ravenel, J.G.; Baginski, S.G.; Snyder, B.S.; DeMello, S.; Desjardins, S.S.; Munden, R.F.; NLST Study Team. Association of Coronary Artery Calcification and Mortality in the National Lung Screening Trial: A Comparison of Three Scoring Methods. Radiology 2015, 27, 682–690. [Google Scholar] [CrossRef]
- Rasmussen, T.; Køber, L.; Abdulla, J.; Pedersen, J.H.; Wille, M.M.; Dirksen, A.; Kofoed, K.F. Coronary artery calcification detected in lung cancer screening predicts cardiovascular death. Scand. Cardiovasc. J. 2015, 49, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Mascalchi, M.; Puliti, D.; Romei, C.; Picozzi, G.; De Liperi, A.; Diciotti, S.; Bartolucci, M.; Grazzini, M.; Vannucchi, L.; Falaschi, F.; et al. Moderate-severe coronary calcification predicts long-term cardiovascular death in CT lung cancer screening: The ITALUNG trial. Eur. J. Radiol. 2021, 145, 110040, Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, J.; Allison, M.; Wright, C.M. Health behavior modification after electron beam computed tomography and physician consultation. J. Behav. Med. 2011, 34, 148–155. [Google Scholar] [CrossRef]
- Kalia, N.K.; Miller, L.G.; Nasir, K.; Blumenthal, R.S.; Agrawal, N.; Budoff, M.J. Visualizing coronary calcium is associated with improvements in adherence to statin therapy. Atherosclerosis 2006, 185, 394–399. [Google Scholar] [CrossRef] [PubMed]
- Ruparel, M.; Quaife, S.L.; Dickson, J.L.; Horst, C.; Burke, S.; Taylor, M.; Ahmed, A.; Shaw, P.; Soo, M.J.; Nair, A.; et al. Evaluation of cardiovascular risk in a lung cancer screening cohort. Thorax 2019, 74, 1140–1146. [Google Scholar] [CrossRef]
- Tailor, T.D.; Chiles, C.; Yeboah, J.; Rivera, M.P.; Tong, B.C.; Schwartz, F.R.; Benefield, T.; Lane, L.M.; Stashko, I.; Thomas, S.M.; et al. Cardiovascular Risk in the Lung Cancer Screening Population: A Multicenter Study Evaluating the Association Between Coronary Artery Calcification and Preventive Statin Prescription. J. Am. Coll. Radiol. 2021, 18, 1258–1266. [Google Scholar] [CrossRef]
- Puliti, D.; Mascalchi, M.; Carozzi, F.M.; Carrozzi, L.; Falaschi, F.; Paci, E.; Lopes Pegna, A.; Aquilini, F.; Barchielli, A.; Bartolucci, M.; et al. Decreased cardiovascular mortality in the ITALUNG lung cancer screening trial: Analysis of underlying factors. Lung Cancer 2019, 138, 72–78. [Google Scholar] [CrossRef]
- Baldwin, D.; O’Dowd, E.; Ten Haaf, K. Targeted screening for lung cancer is here but who do we target and how? Thorax 2020, 75, 617–618. [Google Scholar] [CrossRef]
- Pooler, B.D.; Kim, D.H.; Pickhardt, P.J. Extracolonic Findings at Screening CT Colonography: Prevalence, Benefits, Challenges, and Opportunities. AJR Am. J. Roentgenol. 2017, 209, 94–102. [Google Scholar] [CrossRef]
- Pickhardt, P.J.; Graffy, P.M.; Zea, R.; Lee, S.J.; Liu, J.; Sandfort, V.; Summers, R.M. Automated CT biomarkers for opportunistic prediction of future cardiovascular events and mortality in an asymptomatic screening population: A retrospective cohort study. Lancet Digit. Health 2020, 2, e192–e200. [Google Scholar] [CrossRef]
- Jonas, D.E.; Reuland, D.S.; Reddy, S.M.; Nagle, M.; Clark, S.D.; Weber, R.P.; Enyioha, C.; Malo, T.L.; Brenner, A.T.; Armstrong, C.; et al. Screening for Lung Cancer With Low-Dose Computed Tomography: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA 2021, 325, 971–987. [Google Scholar] [CrossRef] [PubMed]
- Mascalchi, M.; Mazzoni, L.N.; Falchini, M.; Belli, G.; Picozzi, G.; Merlini, V.; Vella, A.; Diciotti, S.; Falaschi, F.; Lopes Pegna, A.; et al. Dose exposure in the ITALUNG trial of lung cancer screening with low-dose CT. Br. J. Radiol. 2012, 85, 1134–1139. [Google Scholar] [CrossRef] [PubMed]
- Yamamura, S.; Oda, S.; Imuta, M.; Utsunomiya, D.; Yoshida, M.; Namimoto, T.; Yuki, H.; Kidoh, M.; Funama, Y.; Baba, H.; et al. Reducing the Radiation Dose for CT Colonography: Effect of Low Tube Voltage and Iterative Reconstruction. Acad. Radiol. 2016, 23, 155–162. [Google Scholar] [CrossRef]
- Kang, H.J.; Kim, S.H.; Shin, C.I.; Joo, I.; Ryu, H.; Kim, S.G.; Im, J.P.; Han, J.K. Sub-millisievert CT colonography: Effect of knowledge-based iterative reconstruction on the detection of colonic polyps. Eur. Radiol. 2018, 28, 5258–5266. [Google Scholar] [CrossRef] [PubMed]
- Cianci, R.; Delli Pizzi, A.; Esposito, G.; Timpani, M.; Tavoletta, A.; Pulsone, P.; Basilico, R.; Cotroneo, A.R.; Filippone, A. Ultra-low dose CT colonography with automatic tube current modulation and sinogram-affirmed iterative reconstruction: Effects on radiation exposure and image quality. J. Appl. Clin. Med. Phys. 2019, 20, 321–330. [Google Scholar] [CrossRef]
- Liu, J.J.; Xue, H.D.; Liu, W.; Yan, J.; Pan, W.D.; Li, B.; Xu, K.; Wang, Y.; Li, P.; Xiao, Y.; et al. CT colonography with spectral filtration and advanced modeled iterative reconstruction in the third-generation dual-source CT: Image quality, radiation dose and performance in clinical utility. Acad. Radiol. 2021, 28, e127–e136. [Google Scholar] [CrossRef]
- Martini, K.; Higashigaito, K.; Barth, B.K.; Baumueller, S.; Alkadhi, H.; Frauenfelder, T. Ultralow-dose CT with tin filtration for detection of solid and sub solid pulmonary nodules: A phantom study. Br. J. Radiol. 2015, 88, 20150389. [Google Scholar] [CrossRef]
- Schwyzer, M.; Messerli, M.; Eberhard, M.; Skawran, S.; Martini, K.; Frauenfelder, T. Impact of dose reduction and iterative reconstruction algorithm on the detectability of pulmonary nodules by artificial intelligence. Diagn. Interv. Imaging 2022, 103, 273–280. [Google Scholar] [CrossRef]
- Nagatani, Y.; Takahashi, M.; Murata, K.; Ikeda, M.; Yamashiro, T.; Miyara, T.; Koyama, H.; Koyama, M.; Sato, Y.; Moriya, H.; et al. Lung nodule detection performance in five observers on computed tomography (CT) with adaptive iterative dose reduction using three-dimensional processing (AIDR 3D) in a Japanese multicenter study: Comparison between ultra-low-dose CT and low-dose CT by receiver-operating characteristic analysis. Eur. J. Radiol. 2015, 84, 1401–1412. [Google Scholar] [CrossRef]
- Fujita, M.; Higaki, T.; Awaya, Y.; Nakanishi, T.; Nakamura, Y.; Tatsugami, F.; Baba, Y.; Iida, M.; Awai, K. Lung cancer screening with ultra-low dose CT using full iterative reconstruction. Jpn. J. Radiol. 2017, 35, 179–189. [Google Scholar] [CrossRef]
- Nomura, Y.; Higaki, T.; Fujita, M.; Miki, S.; Awaya, Y.; Nakanishi, T.; Yoshikawa, T.; Hayashi, N.; Awai, K. Effects of Iterative Reconstruction Algorithms on Computer-assisted Detection (CAD) Software for Lung Nodules in Ultra-low-dose CT for Lung Cancer Screening. Acad. Radiol. 2017, 24, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Qi, W.; Sun, Y.; Jiang, Y.; Liu, X.; Hong, N. Screening for lung cancer using sub-millisievert chest CT with iterative reconstruction algorithm: Image quality and nodule detectability. Br. J. Radiol. 2018, 91, 20170658. [Google Scholar] [CrossRef] [PubMed]
- Ye, K.; Chen, M.; Li, J.; Zhu, Q.; Lu, Y.; Yuan, H. Ultra-low-dose CT reconstructed with ASiR-V using SmartmA for pulmonary nodule detection and Lung-RADS classifications compared with low-dose CT. Clin. Radiol. 2021, 76, 156.e1–156.e8. [Google Scholar] [CrossRef]
- Jiang, B.; Li, N.; Shi, X.; Zhang, S.; Li, J.; de Bock, G.H.; Vliegenthart, R.; Xie, X. Deep Learning Reconstruction Shows Better Lung Nodule Detection for Ultra-Low-Dose Chest CT. Radiology 2022, 303, 202–212. [Google Scholar] [CrossRef]
- van der Meulen, M.P.; Lansdorp-Vogelaar, I.; Goede, S.L.; Kuipers, E.J.; Dekker, E.; Stoker, J.; van Ballegooijen, M. Colorectal Cancer: Cost-effectiveness of Colonoscopy versus CT Colonography Screening with Participation Rates and Costs. Radiology 2018, 287, 901–911. [Google Scholar] [CrossRef] [PubMed]
- Mantellini, P.; Lippi, G.; Sali, L.; Grazzini, G.; Delsanto, S.; Mallardi, B.; Falchini, M.; Castiglione, G.; Carozzi, F.M.; Mascalchi, M.; et al. Cost analysis of colorectal cancer screening with CT colonography in Italy. Eur. J. Health Econ. 2018, 19, 735–746. [Google Scholar] [CrossRef] [PubMed]
- Sverzellati, N.; Silva, M.; Calareso, G.; Galeone, C.; Marchianò, A.; Sestini, S.; Sozzi, G.; Pastorino, U. Low-dose computed tomography for lung cancer screening: Comparison of performance between annual and biennial screen. Eur. Radiol. 2016, 26, 3821–3829. [Google Scholar] [CrossRef]
- Field, J.K.; Duffy, S.W.; Baldwin, D.R.; Brain, K.E.; Devaraj, A.; Eisen, T.; Green, B.A.; Holemans, J.A.; Kavanagh, T.; Kerr, K.M.; et al. The UK Lung Cancer Screening Trial: A pilot randomised controlled trial of low-dose computed tomography screening for the early detection of lung cancer. Health Technol. Assess 2016, 20, 1–146. [Google Scholar] [CrossRef]
- Heerink, W.J.; de Bock, G.H.; de Jonge, G.J.; Groen, H.J.; Vliegenthart, R.; Oudkerk, M. Complication rates of CT-guided transthoracic lung biopsy: Meta-analysis. Eur. Radiol. 2017, 27, 138–148. [Google Scholar] [CrossRef]
- Zhang, J.; Feng, Q.; Huang, Y.; Ouyang, L.; Luo, F. Updated Evaluation of Robotic- and Video-Assisted Thoracoscopic Lobectomy or Segmentectomy for Lung Cancer: A Systematic Review and Meta-Analysis. Front. Oncol. 2022, 12, 853530. [Google Scholar] [CrossRef]
- Rutter, M.D.; Nickerson, C.; Rees, C.J.; Patnick, J.; Blanks, R.G. Risk factors for adverse events related to polypectomy in the English Bowel Cancer Screening Programme. Endoscopy 2014, 46, 90–97. [Google Scholar] [CrossRef]
- Brenner, D.J. Radiation risks potentially associated with low-dose CT screening of adult smokers for lung cancer. Radiology 2004, 231, 440–445. [Google Scholar] [CrossRef] [PubMed]
- Mascalchi, M.; Belli, G.; Zappa, M.; Picozzi, G.; Falchini, M.; Della Nave, R.; Allescia, G.; Masi, A.; Pegna, A.L.; Villari, N.; et al. Risk-benefit analysis of X-ray exposure associated with lung cancer screening in the Italung-CT trial. AJR Am. J. Roentgenol. 2006, 187, 421–429. [Google Scholar] [CrossRef] [PubMed]
- National Research Council. Health Risks from Exposure to Low Levels of Ionizing Radiation: BEIR VII—Phase 2; National Academies Press: Washington, DC, USA, 2006. [Google Scholar]
- International Commission on Radiological Protection. The 2007 Recommendations of the International Commission on Radiological Protection. ICRP publication 103. Ann. ICRP 2007, 37, 1–332. [Google Scholar]
- International Commission on Radiological Protection. Radiation dose to patients from radiopharmaceuticals. Addendum 3 to ICRP Publication 53. ICRP Publication 106.Approved by the Commission in October 2007. Ann. ICRP 2008, 38, 1–197. [Google Scholar]
- McCunney, R.J.; Li, J. Radiation risks in lung cancer screening programs: A comparison with nuclear industry workers and atomic bomb survivors. Chest 2014, 145, 618–624. [Google Scholar] [CrossRef]
- Perisinakis, K.; Seimenis, I.; Tzedakis, A.; Karantanas, A.; Damilakis, J. Radiation burden and associated cancer risk for a typical population to be screened for lung cancer with low-dose CT: A phantom study. Eur. Radiol. 2018, 28, 4370–4378. [Google Scholar] [CrossRef] [PubMed]
- Rampinelli, C.; De Marco, P.; Origgi, D.; Maisonneuve, P.; Casiraghi, M.; Veronesi, G.; Spaggiari, L.; Bellomi, M. Exposure to low dose computed tomography for lung cancer screening and risk of cancer: Secondary analysis of trial data and risk-benefit analysis. BMJ 2017, 356, j347. [Google Scholar] [CrossRef]
- van Klaveren, R.J.; Oudkerk, M.; Prokop, M.; Scholten, E.T.; Nackaerts, K.; Vernhout, R.; van Iersel, C.A.; van den Bergh, K.A.; van ‘t Westeinde, S.; van der Aalst, C.; et al. Management of lung nodules detected by volume CT scanning. N. Engl. J. Med. 2009, 361, 2221–2229. [Google Scholar] [CrossRef]
- Yousaf-Khan, U.; van der Aalst, C.; de Jong, P.A.; Heuvelmans, M.; Scholten, E.; Lammers, J.W.; van Ooijen, P.; Nackaerts, K.; Weenink, C.; Groen, H.; et al. Final screening round of the NELSON lung cancer screening trial: The effect of a 2.5-year screening interval. Thorax 2017, 72, 48–56. [Google Scholar] [CrossRef]
- Pastorino, U.; Sverzellati, N.; Sestini, S.; Silva, M.; Sabia, F.; Boeri, M.; Cantarutti, A.; Sozzi, G.; Corrao, G.; Marchianò, A. Ten-year results of the Multicentric Italian Lung Detection trial demonstrate the safety and efficacy of biennial lung cancer screening. Eur. J. Cancer 2019, 118, 142–148. [Google Scholar] [CrossRef]
- Grover, H.; King, W.; Bhattarai, N.; Moloney, E.; Sharp, L.; Fuller, L. Systematic review of the cost-effectiveness of screening for lung cancer with low dose computed tomography. Lung Cancer 2022, 170, 20–33. [Google Scholar] [CrossRef] [PubMed]
- Hassan, C.; Pickhardt, P.J. Cost-effectiveness of CT colonography. Radiol. Clin. N. Am. 2013, 51, 89–97. [Google Scholar] [CrossRef]
- Cadham, C.J.; Cao, P.; Jayasekera, J.; Taylor, K.L.; Levy, D.T.; Jeon, J.; Elkin, E.B.; Foley, K.L.; Joseph, A.; Kong, C.Y.; et al. Cost-Effectiveness of Smoking Cessation Interventions in the Lung Cancer Screening Setting: A Simulation Study. J. Natl. Cancer Inst. 2021, 113, 1065–1073. [Google Scholar] [CrossRef]
- Toumazis, I.; de Nijs, K.; Cao, P.; Bastani, M.; Munshi, V.; Ten Haaf, K.; Jeon, J.; Gazelle, G.S.; Feuer, E.J.; de Koning, H.J.; et al. Cost-effectiveness Evaluation of the 2021 US Preventive Services Task Force Recommendation for Lung Cancer Screening. JAMA Oncol. 2021, 7, 1833–1842. [Google Scholar] [CrossRef]
- Ten Haaf, K.; van der Aalst, C.M.; de Koning, H.J.; Kaaks, R.; Tammemägi, M.C. Personalising lung cancer screening: An overview of risk-stratification opportunities and challenges. Int. J. Cancer 2021, 149, 250–263. [Google Scholar] [CrossRef] [PubMed]
- Kowada, A. Cost-effectiveness and health impact of lung cancer screening with low-dose computed tomography for never smokers in Japan and the United States: A modelling study. BMC Pulm. Med. 2022, 22, 19. [Google Scholar] [CrossRef] [PubMed]
| Never Smokers | Smoker/Ex-Smoker * | Smoker/Ex-Smoker * | Never Smoker | |
| Age (Years) | Average CRC risk ° | Average CRC Risk ° | High CRC Risk | High CRC Risk |
| 50 | SBM ^ FS § CTC $ OC & | chest CT and CTC | chest CT OC | OC |
| 51 | SBM | chest CT | chest CT | |
| 52 | SBM | chest CT | chest CT OC | OC |
| 53 | SBM | chest CT | chest CT | |
| 54 | SBM | chest CT | chest CT OC | OC |
| 55 | SBM FS CTC | chest CT and CTC | chest CT | |
| 56 | SBM | chest CT | chest CT OC | OC |
| 57 | SBM | chest CT | chest CT | |
| 58 | SBM | chest CT | chest CT OC | OC |
| 59 | SBM | chest CT | chest CT | |
| 60 | SBM FS CTC OC | chest CT and CTC | chest CT OC | OC |
| 51 | SBM | chest CT | chest CT | |
| 61 | SBM | chest CT | chest CT OC | OC |
| 62 | SBM | chest CT | chest CT | |
| 63 | SBM | chest CT | chest CT OC | OC |
| 64 | SBM | chest CT | chest CT | |
| 65 | SBM FS CTC | chest CT and CTC | chest CT OC | OC |
| 66 | SBM | chest CT | chest CT | |
| 67 | SBM | chest CT | chest CT OC | OC |
| 68 | SBM | chest CT | chest CT | |
| 69 | SBM | chest CT | chest CT OC | OC |
| 70 | SBM FS CTC OC | chest CT and CTC | chest CT | |
| 71 | SBM | chest CT | chest CT OC | OC |
| 72 | SBM | chest CT | chest CT | |
| 73 | SBM | chest CT | chest CT OC | OC |
| 74 | SBM | chest CT | chest CT | |
| 75 | SBM FS CTC | chest CT and CTC | chest CT OC | OC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mascalchi, M.; Picozzi, G.; Puliti, D.; Gorini, G.; Mantellini, P.; Sali, L. Single CT Appointment for Double Lung and Colorectal Cancer Screening: Is the Time Ripe? Diagnostics 2022, 12, 2326. https://doi.org/10.3390/diagnostics12102326
Mascalchi M, Picozzi G, Puliti D, Gorini G, Mantellini P, Sali L. Single CT Appointment for Double Lung and Colorectal Cancer Screening: Is the Time Ripe? Diagnostics. 2022; 12(10):2326. https://doi.org/10.3390/diagnostics12102326
Chicago/Turabian StyleMascalchi, Mario, Giulia Picozzi, Donella Puliti, Giuseppe Gorini, Paola Mantellini, and Lapo Sali. 2022. "Single CT Appointment for Double Lung and Colorectal Cancer Screening: Is the Time Ripe?" Diagnostics 12, no. 10: 2326. https://doi.org/10.3390/diagnostics12102326
APA StyleMascalchi, M., Picozzi, G., Puliti, D., Gorini, G., Mantellini, P., & Sali, L. (2022). Single CT Appointment for Double Lung and Colorectal Cancer Screening: Is the Time Ripe? Diagnostics, 12(10), 2326. https://doi.org/10.3390/diagnostics12102326






