Classical Hodgkin Lymphoma: A Joint Clinical and PET Model to Predict Poor Responders at Interim Assessment
Abstract
1. Introduction
2. Materials and Methods
2.1. Imaging Protocol
2.2. Data Collection
2.3. Statistical Analysis
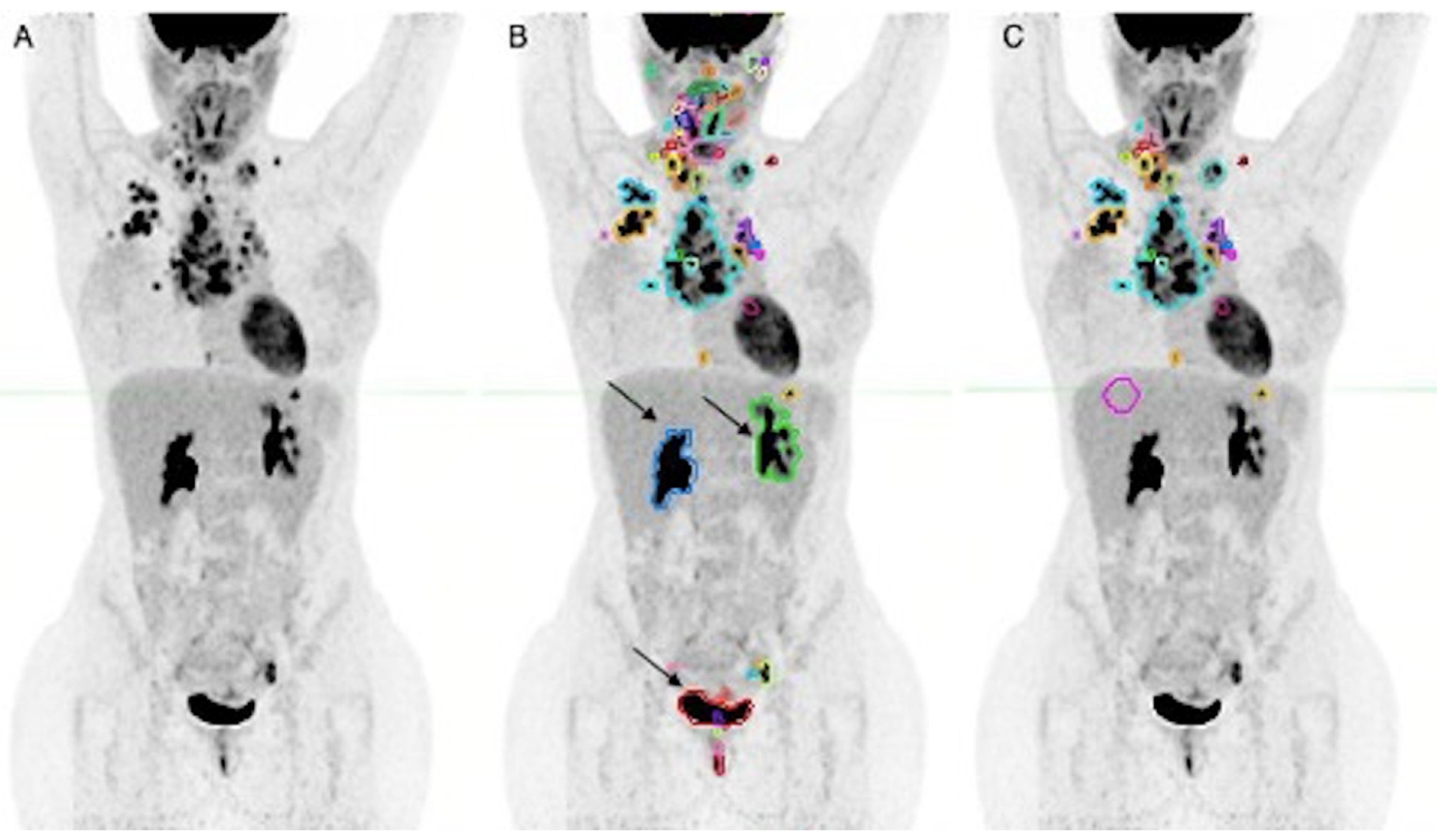
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- SEER*Explorer: An Interactive Website for SEER Cancer Statistics. Surveillance Research Program, National Cancer Institute. Available online: https://seer.cancer.gov/explorer/ (accessed on 26 May 2022).
- Moccia, A.A.; Donaldson, J.; Chhanabhai, M.; Hoskins, P.J.; Klasa, R.J.; Savage, K.J.; Shenkier, T.N.; Slack, G.W.; Skinnider, B.; Gascoyne, R.D.; et al. International Prognostic Score in Advanced-Stage Hodgkin’s Lymphoma: Altered Utility in the Modern Era. JCO 2012, 30, 3383–3388. [Google Scholar] [CrossRef]
- Cuccaro, A.; Bellesi, S.; Galli, E.; Zangrilli, I.; Corrente, F.; Cupelli, E.; Fatone, F.; Maiolo, E.; Alma, E.; Viscovo, M.; et al. PD-L1 Expression in Peripheral Blood Granulocytes at Diagnosis as Prognostic Factor in Classical Hodgkin Lymphoma. J. Leukoc. Biol. 2022, 112, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Meignan, M.; Gallamini, A.; Meignan, M.; Gallamini, A.; Haioun, C. Report on the First International Workshop on Interim-PET Scan in Lymphoma. Leuk. Lymphoma 2009, 50, 1257–1260. [Google Scholar] [CrossRef] [PubMed]
- Barrington, S.F.; Mikhaeel, N.G.; Kostakoglu, L.; Meignan, M.; Hutchings, M.; Müeller, S.P.; Schwartz, L.H.; Zucca, E.; Fisher, R.I.; Trotman, J.; et al. Role of Imaging in the Staging and Response Assessment of Lymphoma: Consensus of the International Conference on Malignant Lymphomas Imaging Working Group. JCO 2014, 32, 3048–3058. [Google Scholar] [CrossRef] [PubMed]
- Mohty, R.; Dulery, R.; Bazarbachi, A.H.; Savani, M.; Hamed, R.A.; Bazarbachi, A.; Mohty, M. Latest Advances in the Management of Classical Hodgkin Lymphoma: The Era of Novel Therapies. Blood Cancer J. 2021, 11, 126. [Google Scholar] [CrossRef]
- Kallergi, M.; Georgakopoulos, A.; Lyra, V.; Chatziioannou, S. Tumor Size Measurements for Predicting Hodgkin’s and Non-Hodgkin’s Lymphoma Response to Treatment. Metabolites 2022, 12, 285. [Google Scholar] [CrossRef]
- Ucar, E.; Yalcin, H.; Kavvasoglu, G.H.; Ilhan, G. Correlations between the Maximum Standard Uptake Value of Positron Emission Tomography/Computed Tomography and Laboratory Parameters before and after Treatment in Patients with Lymphoma. Chin. Med. J. 2018, 131, 1776–1779. [Google Scholar] [CrossRef]
- Herraez, I.; Bento, L.; Daumal, J.; Repetto, A.; Del Campo, R.; Perez, S.; Ramos, R.; Ibarra, J.; Mestre, F.; Bargay, J.; et al. Total Lesion Glycolysis Improves Tumor Burden Evaluation and Risk Assessment at Diagnosis in Hodgkin Lymphoma. JCM 2021, 10, 4396. [Google Scholar] [CrossRef]
- Milgrom, S.A.; Rechner, L.; Berthelsen, A. The Optimal Use of PET/CT in the Management of Lymphoma Patients. BJR 2021, 94, 20210470. [Google Scholar] [CrossRef]
- Milgrom, S.A.; Elhalawani, H.; Lee, J.; Wang, Q.; Mohamed, A.S.R.; Dabaja, B.S.; Pinnix, C.C.; Gunther, J.R.; Court, L.; Rao, A.; et al. A PET Radiomics Model to Predict Refractory Mediastinal Hodgkin Lymphoma. Sci. Rep. 2019, 9, 1322. [Google Scholar] [CrossRef]
- Lu, J.; Wu, Y.; Li, B.; Luo, X.; Zhang, W.; Zeng, Y.; Fu, J.; Liang, A.; Xiu, B. Predictive Value of Serological Factors, Maximal Standardized Uptake Value and Ratio of Ki67 in Patients Diagnosed with Non-Hodgkin’s Lymphoma. Oncol. Lett. 2020, 20, 47. [Google Scholar] [CrossRef] [PubMed]
- Albano, D.; Mazzoletti, A.; Spallino, M.; Muzi, C.; Zilioli, V.R.; Pagani, C.; Tucci, A.; Rossetti, C.; Giubbini, R.; Bertagna, F. Prognostic Role of Baseline 18F-FDG PET/CT Metabolic Parameters in Elderly HL: A Two-Center Experience in 123 Patients. Ann. Hematol. 2020, 99, 1321–1330. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Qiu, L.; Huang, X.; Wang, Q.; Lu, J. The Prognostic Significance of ΔSUVmax Assessed by PET/CT Scan after 2 Cycles of Chemotherapy in Patients with Classic Hodgkin’s Lymphoma. Ann. Hematol. 2020, 99, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, A.; Triumbari, E.K.A.; Gatta, R.; Boldrini, L.; Racca, M.; Mayerhoefer, M.; Annunziata, S. The Role of 18F-FDG PET/CT Radiomics in Lymphoma. Clin. Transl. Imaging 2021, 9, 589–598. [Google Scholar] [CrossRef]
- Frood, R.; Burton, C.; Tsoumpas, C.; Frangi, A.F.; Gleeson, F.; Patel, C.; Scarsbrook, A. Baseline PET/CT Imaging Parameters for Prediction of Treatment Outcome in Hodgkin and Diffuse Large B Cell Lymphoma: A Systematic Review. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 3198–3220. [Google Scholar] [CrossRef]
- Cheson, B.D.; Fisher, R.I.; Barrington, S.F.; Cavalli, F.; Schwartz, L.H.; Zucca, E.; Lister, T.A. Recommendations for Initial Evaluation, Staging, and Response Assessment of Hodgkin and Non-Hodgkin Lymphoma: The Lugano Classification. JCO 2014, 32, 3059–3067. [Google Scholar] [CrossRef]
- Bouallègue, F.B.; Tabaa, Y.A.; Kafrouni, M.; Cartron, G.; Vauchot, F.; Mariano-Goulart, D. Association between Textural and Morphological Tumor Indices on Baseline PET-CT and Early Metabolic Response on Interim PET-CT in Bulky Malignant Lymphomas. Med. Phys. 2017, 44, 4608–4619. [Google Scholar] [CrossRef]
- Rogasch, J.M.M.; Hundsdoerfer, P.; Hofheinz, F.; Wedel, F.; Schatka, I.; Amthauer, H.; Furth, C. Pretherapeutic FDG-PET Total Metabolic Tumor Volume Predicts Response to Induction Therapy in Pediatric Hodgkin’s Lymphoma. BMC Cancer 2018, 18, 521. [Google Scholar] [CrossRef]
- Pike, L.C.; Kirkwood, A.A.; Patrick, P.; Radford, J.; Burton, C.; Stevens, L.; Clifton-Hadley, L.; Johnson, P.W.; Barrington, S.F. Can baseline PET-CT features predict outcomes in advanced hodgkin lymphoma? A prospective evaluation of UK patients in the rathl trial (CRUK/07/033). Hematol. Oncol. 2017, 35, 37–38. [Google Scholar] [CrossRef]
- Boellaard, R.; Delgado-Bolton, R.; Oyen, W.J.G.; Giammarile, F.; Tatsch, K.; Eschner, W.; Verzijlbergen, F.J.; Barrington, S.F.; Pike, L.C.; Weber, W.A.; et al. FDG PET/CT: EANM Procedure Guidelines for Tumour Imaging: Version 2.0. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 328–354. [Google Scholar] [CrossRef]
- Hasenclever, D.; Diehl, V.; Armitage, J.O.; Assouline, D.; Björkholm, M.; Brusamolino, E.; Canellos, G.P.; Carde, P.; Crowther, D.; Cunningham, D.; et al. A Prognostic Score for Advanced Hodgkin’s Disease. N. Engl. J. Med. 1998, 339, 1506–1514. [Google Scholar] [CrossRef] [PubMed]
- Dean, E.A.; Mhaskar, R.S.; Lu, H.; Mousa, M.S.; Krivenko, G.S.; Lazaryan, A.; Bachmeier, C.A.; Chavez, J.C.; Nishihori, T.; Davila, M.L.; et al. High Metabolic Tumor Volume Is Associated with Decreased Efficacy of Axicabtagene Ciloleucel in Large B-Cell Lymphoma. Blood Adv. 2020, 4, 3268–3276. [Google Scholar] [CrossRef] [PubMed]
- Kanoun, S.; Rossi, C.; Berriolo-Riedinger, A.; Dygai-Cochet, I.; Cochet, A.; Humbert, O.; Toubeau, M.; Ferrant, E.; Brunotte, F.; Casasnovas, R.-O. Baseline Metabolic Tumour Volume Is an Independent Prognostic Factor in Hodgkin Lymphoma. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 1735–1743. [Google Scholar] [CrossRef] [PubMed]
- Bari, A.; Marcheselli, R.; Sacchi, S.; Re, A.; Pagani, C.; Tucci, A.; Botto, B.; Vitolo, U.; Molinari, A.L.; Puccini, B.; et al. The Classic Prognostic Factors in Advanced Hodgkin’s Lymphoma Patients Are Losing Their Meaning at the Time of Pet-Guided Treatments. Ann. Hematol. 2020, 99, 277–282. [Google Scholar] [CrossRef]
- Milgrom, S.A.; Dong, W.; Akhtari, M.; Smith, G.L.; Pinnix, C.C.; Mawlawi, O.; Rohren, E.; Garg, N.; Chuang, H.; Yehia, Z.A.; et al. Chemotherapy Response Assessment by FDG-PET-CT in Early-Stage Classical Hodgkin Lymphoma: Moving Beyond the Five-Point Deauville Score. Int. J. Radiat. Oncol. Biol. Phys. 2017 97, 333–338. [CrossRef]


| Patients’ Characteristics | n (Range) | % |
|---|---|---|
| Sex | ||
| Female | 82 | 56 |
| Male | 64 | 44 |
| Age | ||
| Mean (range) | 39 (16–82) | |
| <45 years old | 106 | 73 |
| ≥45 years old | 40 | 27 |
| B symptoms | ||
| No | 86 | 59 |
| Yes | 60 | 41 |
| Disease stage | ||
| Limited (I—IIB with no bulk) | 70 | 48 |
| Advanced (IIB with bulk—IV) | 76 | 52 |
| White blood cell count | ||
| <15.000/mm3 | 124 | 85 |
| ≥15.000/mm3 | 22 | 15 |
| Lymphocyte count | ||
| <600/mm3 and/or <8% of white blood cell count | 3 | 2 |
| ≥600/mm3 | 143 | 98 |
| Hemoglobinemia | ||
| <10.5 g/dL | 29 | 20 |
| ≥10.5 g/dL | 117 | 80 |
| Albuminemia | ||
| ≥4 g/dL | 73 | 50 |
| <4 g/dL | 73 | 50 |
| Erythrocyte sedimentation rate | ||
| >30 mm/h, with B symptoms | 86 | 59 |
| >50 mm/h, without B symptoms | 60 | 41 |
| Fibrinogen | ||
| <400 mg/dL | 42 | 29 |
| ≥400 mg/dL | 104 | 71 |
| Lactate dehydrogenase | ||
| <Normal range | 122 | 84 |
| >Normal range | 24 | 16 |
| PET Parameter | Values |
|---|---|
| TMTV (bPET) | |
| Mean value ± DS | 218.80 ± 249.37 |
| Median (1st–3rd Quartile) | 116.91 (47.52–312.10) |
| Range | 0.73–1145.68 |
| SUVmax (bPET) | |
| Mean value ± DS | 16.40 ± 8.31 |
| Median (1st–3rd quartile) | 15.33 (10.88–20.16) |
| Range | 2.55–66.0 |
| Deauville Score (iPET) | |
| <4 | |
| No. of patients (%) | 120 (82) |
| ≥4 | |
| No. of patients (%) | 26 (18) |
| Variable | Univariate p-Value | Multivariate p-Value |
|---|---|---|
| TMTV > 177.02 mL | 0.002 * | 0.013 * |
| SUVmax > 14.67 | 0.002 * | 0.002 * |
| Stage (advanced) | 0.022 * | 0.067 |
| Age ≥ 45 years | 0.045 * | 0.05 * |
| Sex | 0.478 | Excluded |
| WBC ≥ 15,000/mm3 | 0.222 | Excluded |
| Symptoms (present) | 0.942 | Excluded |
| Albuminemia ≥ 4 g/dL | 0.287 | Excluded |
| Fibrinogen ≥ 400 mg/dL | 0.718 | Excluded |
| Erythrocyte sedimentation rate (>30 mm/h with B symptoms vs. >50 mm/h without) | 0.714 | Excluded |
| Hemoglobinemia < 10.5 g/dL | 0.899 | Excluded |
| Lymphocyte count < 600/mm3 or <8% of WBC | 0.896 | Excluded |
| LDH > normal range | 0.846 | Excluded |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Triumbari, E.K.A.; Morland, D.; Cuccaro, A.; Maiolo, E.; Hohaus, S.; Annunziata, S. Classical Hodgkin Lymphoma: A Joint Clinical and PET Model to Predict Poor Responders at Interim Assessment. Diagnostics 2022, 12, 2325. https://doi.org/10.3390/diagnostics12102325
Triumbari EKA, Morland D, Cuccaro A, Maiolo E, Hohaus S, Annunziata S. Classical Hodgkin Lymphoma: A Joint Clinical and PET Model to Predict Poor Responders at Interim Assessment. Diagnostics. 2022; 12(10):2325. https://doi.org/10.3390/diagnostics12102325
Chicago/Turabian StyleTriumbari, Elizabeth Katherine Anna, David Morland, Annarosa Cuccaro, Elena Maiolo, Stefan Hohaus, and Salvatore Annunziata. 2022. "Classical Hodgkin Lymphoma: A Joint Clinical and PET Model to Predict Poor Responders at Interim Assessment" Diagnostics 12, no. 10: 2325. https://doi.org/10.3390/diagnostics12102325
APA StyleTriumbari, E. K. A., Morland, D., Cuccaro, A., Maiolo, E., Hohaus, S., & Annunziata, S. (2022). Classical Hodgkin Lymphoma: A Joint Clinical and PET Model to Predict Poor Responders at Interim Assessment. Diagnostics, 12(10), 2325. https://doi.org/10.3390/diagnostics12102325







