The Crosstalk between Vitamin D and Pediatric Digestive Disorders
Abstract
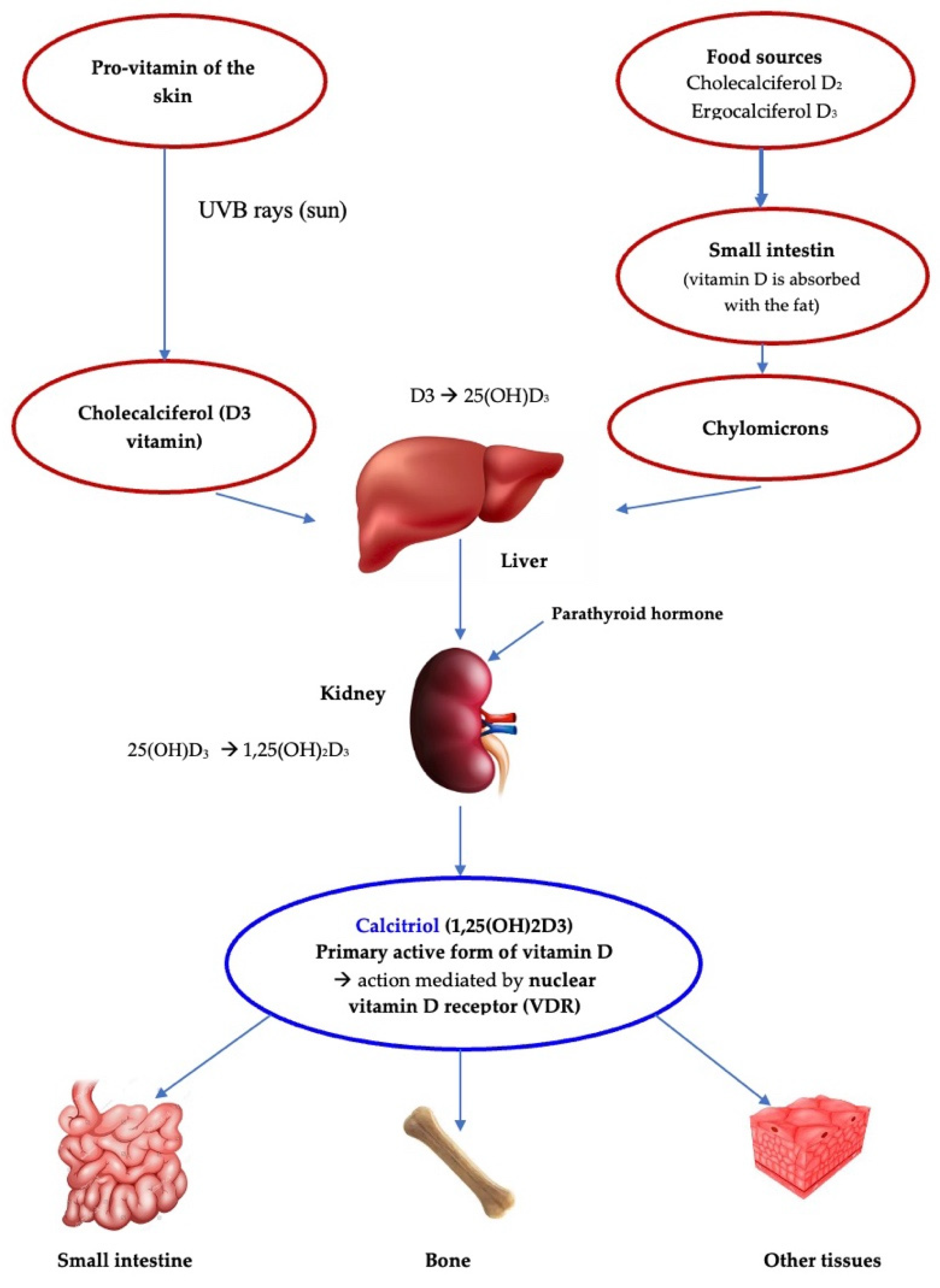
1. Introduction
2. Vitamin D and Gastrointestinal Disorders
2.1. Vitamin D, Gastritis and Gastroesophageal Reflux
2.2. Vitamin D and Celiac Disease
2.3. Vitamin D and Cystic Fibrosis (CF)
2.4. Vitamin D and Inflammatory Bowel Disease (IBD)
2.5. Vitamin D and Food Allergies
Vitamin D and Cow’s Milk Allergy (CMA)
2.6. Vitamin D and Diarrhea
2.7. Vitamin D and Constipation
| Disease | Vitamin D Roles |
|---|---|
| Gastritis and gastroesophageal reflux |
|
| Celiac disease |
|
| Cystic fibrosis |
|
| Inflammatory bowel disease |
|
| Food allergy |
|
| Diarrhea | |
| Constipation |
|
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CD | celiac disease |
| CF | cystic fibrosis |
| CFTR | cystic fibrosis conductance regulator |
| ChD | Crohn’s disease |
| CMA | cow’s milk allergy |
| H. pylori | Helicobacter Pylori |
| IBD | inflammatory bowel disease |
| IL | interleukin |
| RXR | retinoic acid receptor |
| UC | ulcerative colitis |
| VDR | vitamin D receptor |
| VDRE | vitamin D response element |
References
- Holick, M.F. The Vitamin D Deficiency Pandemic: Approaches for Diagnosis, Treatment and Prevention. Rev. Endocr. Metab. Disord. 2017, 18, 153–165. [Google Scholar] [CrossRef]
- Bakke, D.; Chatterjee, I.; Agrawal, A.; Dai, Y.; Sun, J. Regulation of Microbiota by Vitamin D Receptor: A Nuclear Weapon in Metabolic Diseases. Nucl. Receptor. Res. 2018, 5, 101377. [Google Scholar] [CrossRef]
- Haussler, M.R.; Jurutka, P.W.; Mizwicki, M.; Norman, A.W. Vitamin D Receptor (VDR)-Mediated Actions of 1α,25(OH)₂vitamin D₃: Genomic and Non-Genomic Mechanisms. Best Pract. Res. Clin. Endocrinol. Metab. 2011, 25, 543–559. [Google Scholar] [CrossRef]
- Wu, S.; Zhang, Y.-G.; Lu, R.; Xia, Y.; Zhou, D.; Petrof, E.O.; Claud, E.C.; Chen, D.; Chang, E.B.; Carmeliet, G.; et al. Intestinal Epithelial Vitamin D Receptor Deletion Leads to Defective Autophagy in Colitis. Gut 2015, 64, 1082–1094. [Google Scholar] [CrossRef]
- Wang, J.; Thingholm, L.B.; Skiecevičienė, J.; Rausch, P.; Kummen, M.; Hov, J.R.; Degenhardt, F.; Heinsen, F.-A.; Rühlemann, M.C.; Szymczak, S.; et al. Genome-Wide Association Analysis Identifies Variation in Vitamin D Receptor and Other Host Factors Influencing the Gut Microbiota. Nat. Genet. 2016, 48, 1396–1406. [Google Scholar] [CrossRef]
- Zhang, Y.-G.; Lu, R.; Wu, S.; Chatterjee, I.; Zhou, D.; Xia, Y.; Sun, J. Vitamin D Receptor Protects Against Dysbiosis and Tumorigenesis via the JAK/STAT Pathway in Intestine. Cell Mol. Gastroenterol. Hepatol. 2020, 10, 729–746. [Google Scholar] [CrossRef]
- Jin, D.; Zhang, Y.-G.; Wu, S.; Lu, R.; Lin, Z.; Zheng, Y.; Chen, H.; Cs-Szabo, G.; Sun, J. Vitamin D Receptor Is a Novel Transcriptional Regulator for Axin1. J. Steroid. Biochem. Mol. Biol. 2017, 165, 430–437. [Google Scholar] [CrossRef]
- Bashir, M.; Prietl, B.; Tauschmann, M.; Mautner, S.I.; Kump, P.K.; Treiber, G.; Wurm, P.; Gorkiewicz, G.; Högenauer, C.; Pieber, T.R. Effects of High Doses of Vitamin D3 on Mucosa-Associated Gut Microbiome Vary between Regions of the Human Gastrointestinal Tract. Eur J. Nutr. 2016, 55, 1479–1489. [Google Scholar] [CrossRef]
- Battistini, C.; Ballan, R.; Herkenhoff, M.E.; Saad, S.M.I.; Sun, J. Vitamin D Modulates Intestinal Microbiota in Inflammatory Bowel Diseases. Int. J. Mol. Sci. 2020, 22, E362. [Google Scholar] [CrossRef]
- Thacher, T.D.; Fischer, P.R.; Pettifor, J.M.; Lawson, J.O.; Isichei, C.O.; Chan, G.M. Case-Control Study of Factors Associated with Nutritional Rickets in Nigerian Children. J. Pediatr. 2000, 137, 367–373. [Google Scholar] [CrossRef]
- Atapattu, N.; Shaw, N.; Högler, W. Relationship between Serum 25-Hydroxyvitamin D and Parathyroid Hormone in the Search for a Biochemical Definition of Vitamin D Deficiency in Children. Pediatr. Res. 2013, 74, 552–556. [Google Scholar] [CrossRef]
- Pettifor, J.M. Nutritional Rickets: Pathogenesis and Prevention. Pediatr. Endocrinol. Rev. 2013, 10 (Suppl. S2), 347–353. [Google Scholar]
- Institute of Medicine (US) Committee to Review Dietary Reference Intakes for Vitamin D and Calcium. Dietary Reference Intakes for Calcium and Vitamin D; Ross, A.C., Taylor, C.L., Yaktine, A.L., Del Valle, H.B., Eds.; The National Academies Collection: Reports Funded by National Institutes of Health; National Academies Press (US): Washington, DC, USA, 2011. [Google Scholar]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Endocrine Society Evaluation, Treatment, and Prevention of Vitamin D Deficiency: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef]
- Winzenberg, T.; Jones, G. Vitamin D and Bone Health in Childhood and Adolescence. Calcif. Tissue Int. 2013, 92, 140–150. [Google Scholar] [CrossRef]
- Abrams, S.A.; Griffin, I.J.; Hawthorne, K.M.; Gunn, S.K.; Gundberg, C.M.; Carpenter, T.O. Relationships among Vitamin D Levels, Parathyroid Hormone, and Calcium Absorption in Young Adolescents. J. Clin. Endocrinol. Metab. 2005, 90, 5576–5581. [Google Scholar] [CrossRef]
- Abrams, S.A.; Hicks, P.D.; Hawthorne, K.M. Higher Serum 25-Hydroxyvitamin D Levels in School-Age Children Are Inconsistently Associated with Increased Calcium Absorption. J. Clin. Endocrinol. Metab. 2009, 94, 2421–2427. [Google Scholar] [CrossRef]
- Abrams, S.A.; Hawthorne, K.M.; Rogers, S.P.; Hicks, P.D.; Carpenter, T.O. Effects of Ethnicity and Vitamin D Supplementation on Vitamin D Status and Changes in Bone Mineral Content in Infants. BMC Pediatr. 2012, 12, 6. [Google Scholar] [CrossRef]
- Fu, Y.; Hu, Y.; Qin, Z.; Zhao, Y.; Yang, Z.; Li, Y.; Liang, G.; Lv, H.; Hong, H.; Song, Y.; et al. Association of Serum 25-Hydroxyvitamin D Status with Bone Mineral Density in 0–7 Year Old Children. Oncotarget 2016, 7, 80811–80819. [Google Scholar] [CrossRef]
- Kumar, J.; Muntner, P.; Kaskel, F.J.; Hailpern, S.M.; Melamed, M.L. Prevalence and Associations of 25-Hydroxyvitamin D Deficiency in US Children: NHANES 2001–2004. Pediatrics 2009, 124, e362–e370. [Google Scholar] [CrossRef]
- Mansbach, J.M.; Ginde, A.A.; Camargo, C.A. Serum 25-Hydroxyvitamin D Levels among US Children Aged 1 to 11 Years: Do Children Need More Vitamin D? Pediatrics 2009, 124, 1404–1410. [Google Scholar] [CrossRef]
- Cashman, K.D.; Dowling, K.G.; Škrabáková, Z.; Gonzalez-Gross, M.; Valtueña, J.; De Henauw, S.; Moreno, L.; Damsgaard, C.T.; Michaelsen, K.F.; Mølgaard, C.; et al. Vitamin D Deficiency in Europe: Pandemic? Am. J. Clin. Nutr. 2016, 103, 1033–1044. [Google Scholar] [CrossRef]
- Saggese, G.; Vierucci, F.; Prodam, F.; Cardinale, F.; Cetin, I.; Chiappini, E.; De’ Angelis, G.L.; Massari, M.; Miraglia Del Giudice, E.; Miraglia Del Giudice, M.; et al. Vitamin D in Pediatric Age: Consensus of the Italian Pediatric Society and the Italian Society of Preventive and Social Pediatrics, Jointly with the Italian Federation of Pediatricians. Ital. J. Pediatr. 2018, 44, 51. [Google Scholar] [CrossRef]
- Paller, A.S.; Hawk, J.L.M.; Honig, P.; Giam, Y.C.; Hoath, S.; Mack, M.C.; Stamatas, G.N. New Insights about Infant and Toddler Skin: Implications for Sun Protection. Pediatrics 2011, 128, 92–102. [Google Scholar] [CrossRef]
- Misra, M.; Pacaud, D.; Petryk, A.; Collett-Solberg, P.F.; Kappy, M. Drug and Therapeutics Committee of the Lawson Wilkins Pediatric Endocrine Society Vitamin D Deficiency in Children and Its Management: Review of Current Knowledge and Recommendations. Pediatrics 2008, 122, 398–417. [Google Scholar] [CrossRef]
- Holick, M.F. Vitamin D Deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef]
- SACN Vitamin D and Health Report. Available online: https://www.gov.uk/government/publications/sacn-vitamin-d-and-health-report (accessed on 31 July 2022).
- Dietary Reference Values for Vitamin D|EFSA. Available online: https://www.efsa.europa.eu/en/efsajournal/pub/4547 (accessed on 31 July 2022).
- Braegger, C.; Campoy, C.; Colomb, V.; Decsi, T.; Domellof, M.; Fewtrell, M.; Hojsak, I.; Mihatsch, W.; Molgaard, C.; Shamir, R.; et al. Vitamin D in the Healthy European Paediatric Population. J. Pediatr. Gastroenterol. Nutr. 2013, 56, 692–701. [Google Scholar] [CrossRef]
- Grossman, Z.; Hadjipanayis, A.; Stiris, T.; Del Torso, S.; Mercier, J.-C.; Valiulis, A.; Shamir, R. Vitamin D in European Children-Statement from the European Academy of Paediatrics (EAP). Eur. J. Pediatr. 2017, 176, 829–831. [Google Scholar] [CrossRef]
- Scientific Opinion on the Tolerable Upper Intake Level of Vitamin D|EFSA. Available online: https://www.efsa.europa.eu/en/efsajournal/pub/2813 (accessed on 31 July 2022).
- Assa, A.; Vong, L.; Pinnell, L.J.; Avitzur, N.; Johnson-Henry, K.C.; Sherman, P.M. Vitamin D Deficiency Promotes Epithelial Barrier Dysfunction and Intestinal Inflammation. J. Infect. Dis. 2014, 210, 1296–1305. [Google Scholar] [CrossRef]
- Kong, J.; Zhang, Z.; Musch, M.W.; Ning, G.; Sun, J.; Hart, J.; Bissonnette, M.; Li, Y.C. Novel Role of the Vitamin D Receptor in Maintaining the Integrity of the Intestinal Mucosal Barrier. Am. J. Physiol. Gastrointest Liver Physiol. 2008, 294, G208–G216. [Google Scholar] [CrossRef]
- Torki, M.; Gholamrezaei, A.; Mirbagher, L.; Danesh, M.; Kheiri, S.; Emami, M.H. Vitamin D Deficiency Associated with Disease Activity in Patients with Inflammatory Bowel Diseases. Dig. Dis. Sci. 2015, 60, 3085–3091. [Google Scholar] [CrossRef]
- Lu, R.; Wu, S.; Xia, Y.; Sun, J. The Vitamin D Receptor, Inflammatory Bowel Diseases, and Colon Cancer. Curr. Colorectal. Cancer Rep. 2012, 8, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Shang, M.; Sun, J. Vitamin D/VDR, Probiotics, and Gastrointestinal Diseases. Curr. Med. Chem. 2017, 24, 876–887. [Google Scholar] [CrossRef] [PubMed]
- White, J.H. Vitamin D Metabolism and Signaling in the Immune System. Rev. Endocr. Metab. Disord. 2012, 13, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.-J.; Wang, X.-H.; Liu, Z.-D.; Cao, W.-L.; Han, Y.; Ma, A.-G.; Xu, S.-F. Vitamin D Deficiency and the Risk of Tuberculosis: A Meta-Analysis. Drug Des. Devel Ther. 2017, 11, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Coussens, A.K. The Role of UV Radiation and Vitamin D in the Seasonality and Outcomes of Infectious Disease. Photochem. Photobiol. Sci. 2017, 16, 314–338. [Google Scholar] [CrossRef]
- Lang, P.O.; Samaras, N.; Samaras, D.; Aspinall, R. How Important Is Vitamin D in Preventing Infections? Osteoporos Int. 2013, 24, 1537–1553. [Google Scholar] [CrossRef]
- Khajavi, A.; Amirhakimi, G.H. The Rachitic Lung. Pulmonary Findings in 30 Infants and Children with Malnutritional Rickets. Clin. Pediatr. 1977, 16, 36–38. [Google Scholar] [CrossRef]
- El Shahawy, M.S.; Shady, Z.M.; Gaafar, A. Influence of Adding Vitamin D3 to Standard Clarithromycin-Based Triple Therapy on the Eradication Rates of Helicobacter Pylori Infection. Arab J. Gastroenterol. 2021, 209–214. [Google Scholar] [CrossRef]
- Yildirim, O.; Yildirim, T.; Seckin, Y.; Osanmaz, P.; Bilgic, Y.; Mete, R. The Influence of Vitamin D Deficiency on Eradication Rates of Helicobacter Pylori. Adv. Clin. Exp. Med. 2017, 26, 1377–1381. [Google Scholar] [CrossRef]
- Antico, A.; Tozzoli, R.; Giavarina, D.; Tonutti, E.; Bizzaro, N. Hypovitaminosis D as Predisposing Factor for Atrophic Type A Gastritis: A Case-Control Study and Review of the Literature on the Interaction of Vitamin D with the Immune System. Clin. Rev. Allergy Immunol. 2012, 42, 355–364. [Google Scholar] [CrossRef]
- Guo, L.; Chen, W.; Zhu, H.; Chen, Y.; Wan, X.; Yang, N.; Xu, S.; Yu, C.; Chen, L. Helicobacter Pylori Induces Increased Expression of the Vitamin d Receptor in Immune Responses. Helicobacter 2014, 19, 37–47. [Google Scholar] [CrossRef]
- Danai, P.A.; Sinha, S.; Moss, M.; Haber, M.J.; Martin, G.S. Seasonal Variation in the Epidemiology of Sepsis. Crit. Care Med. 2007, 35, 410–415. [Google Scholar] [CrossRef] [PubMed]
- Hosoda, K.; Shimomura, H.; Wanibuchi, K.; Masui, H.; Amgalanbaatar, A.; Hayashi, S.; Takahashi, T.; Hirai, Y. Identification and Characterization of a Vitamin D₃ Decomposition Product Bactericidal against Helicobacter Pylori. Sci. Rep. 2015, 5, 8860. [Google Scholar] [CrossRef]
- Bharwani, S.S.; Shaukat, Q.; Balhaj, G.; Ashari, M. A Failing to Thrive 18 Month Old with Vitamin D Deficiency Rickets and Helicobacter Pylori Gastritis. BMJ Case Rep. 2011, 2011, bcr0420114160. [Google Scholar] [CrossRef]
- Bao, A.; Li, Y.; Tong, Y.; Zheng, H.; Wu, W.; Wei, C. Tumor-Suppressive Effects of 1, 25-Dihydroxyvitamin D3 in Gastric Cancer Cells. Hepatogastroenterology 2013, 60, 943–948. [Google Scholar] [CrossRef] [PubMed]
- Zhou, A.; Li, L.; Zhao, G.; Min, L.; Liu, S.; Zhu, S.; Guo, Q.; Liu, C.; Zhang, S.; Li, P. Vitamin D3 Inhibits Helicobacter Pylori Infection by Activating the VitD3/VDR-CAMP Pathway in Mice. Front. Cell Infect. Microbiol. 2020, 10, 566730. [Google Scholar] [CrossRef] [PubMed]
- Cavalcoli, F.; Zilli, A.; Conte, D.; Massironi, S. Micronutrient Deficiencies in Patients with Chronic Atrophic Autoimmune Gastritis: A Review. World J. Gastroenterol. 2017, 23, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Mehta, P.; Furuta, G.T.; Brennan, T.; Henry, M.L.; Maune, N.C.; Sundaram, S.S.; Menard-Katcher, C.; Atkins, D.; Takurukura, F.; Giffen, S.; et al. Nutritional State and Feeding Behaviors of Children With Eosinophilic Esophagitis and Gastroesophageal Reflux Disease. J. Pediatr. Gastroenterol. Nutr. 2018, 66, 603–608. [Google Scholar] [CrossRef]
- Giustina, A.; Adler, R.A.; Binkley, N.; Bollerslev, J.; Bouillon, R.; Dawson-Hughes, B.; Ebeling, P.R.; Feldman, D.; Formenti, A.M.; Lazaretti-Castro, M.; et al. Consensus Statement from 2nd International Conference on Controversies in Vitamin D. Rev. Endocr. Metab. Disord. 2020, 21, 89–116. [Google Scholar] [CrossRef]
- Hill, I.D. Celiac Disease--a Never-Ending Story? J. Pediatr. 2003, 143, 289–291. [Google Scholar] [CrossRef]
- Tanpowpong, P.; Camargo, C.A. Early-Life Vitamin D Deficiency and Childhood-Onset Coeliac Disease. Public Health Nutr. 2014, 17, 823–826. [Google Scholar] [CrossRef] [PubMed]
- Ahlawat, R.; Weinstein, T.; Pettei, M.J. Vitamin D in Pediatric Gastrointestinal Disease. Curr. Opin Pediatr. 2017, 29, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Magnus, M.C.; Stene, L.C.; Håberg, S.E.; Nafstad, P.; Stigum, H.; London, S.J.; Nystad, W. Prospective Study of Maternal Mid-Pregnancy 25-Hydroxyvitamin D Level and Early Childhood Respiratory Disorders. Paediatr. Perinat. Epidemiol. 2013, 27, 532–541. [Google Scholar] [CrossRef]
- Aghajafari, F.; Nagulesapillai, T.; Ronksley, P.E.; Tough, S.C.; O’Beirne, M.; Rabi, D.M. Association between Maternal Serum 25-Hydroxyvitamin D Level and Pregnancy and Neonatal Outcomes: Systematic Review and Meta-Analysis of Observational Studies. BMJ 2013, 346, f1169. [Google Scholar] [CrossRef] [PubMed]
- Munger, K.L.; Åivo, J.; Hongell, K.; Soilu-Hänninen, M.; Surcel, H.-M.; Ascherio, A. Vitamin D Status During Pregnancy and Risk of Multiple Sclerosis in Offspring of Women in the Finnish Maternity Cohort. JAMA Neurol. 2016, 73, 515–519. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, I.M.; Joner, G.; Jenum, P.A.; Eskild, A.; Torjesen, P.A.; Stene, L.C. Maternal Serum Levels of 25-Hydroxy-Vitamin D during Pregnancy and Risk of Type 1 Diabetes in the Offspring. Diabetes 2012, 61, 175–178. [Google Scholar] [CrossRef] [PubMed]
- Lebwohl, B.; Green, P.H.R.; Murray, J.A.; Ludvigsson, J.F. Season of Birth in a Nationwide Cohort of Coeliac Disease Patients. Arch. Dis. Child. 2013, 98, 48–51. [Google Scholar] [CrossRef]
- Tanpowpong, P.; Obuch, J.C.; Jiang, H.; McCarty, C.E.; Katz, A.J.; Leffler, D.A.; Kelly, C.P.; Weir, D.C.; Leichtner, A.M.; Camargo, C.A. Multicenter Study on Season of Birth and Celiac Disease: Evidence for a New Theoretical Model of Pathogenesis. J. Pediatr. 2013, 162, 501–504. [Google Scholar] [CrossRef]
- Mårild, K.; Tapia, G.; Haugen, M.; Dahl, S.R.; Cohen, A.S.; Lundqvist, M.; Lie, B.A.; Stene, L.C.; Størdal, K. Maternal and Neonatal Vitamin D Status, Genotype and Childhood Celiac Disease. PLoS ONE 2017, 12, e0179080. [Google Scholar] [CrossRef]
- Vici, G.; Camilletti, D.; Polzonetti, V. Possible Role of Vitamin D in Celiac Disease Onset. Nutrients 2020, 12, E1051. [Google Scholar] [CrossRef]
- Cukrowska, B.; Sowińska, A.; Bierła, J.B.; Czarnowska, E.; Rybak, A.; Grzybowska-Chlebowczyk, U. Intestinal Epithelium, Intraepithelial Lymphocytes and the Gut Microbiota—Key Players in the Pathogenesis of Celiac Disease. World J. Gastroenterol. 2017, 23, 7505–7518. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Singh, T.P.; Wei, X.; Yao, H.; Wang, H. Protective Effect of 1,25-Dihydroxy Vitamin D3 on Pepsin-Trypsin-Resistant Gliadin-Induced Tight Junction Injuries. Dig. Dis. Sci. 2018, 63, 92–104. [Google Scholar] [CrossRef]
- Fasano, A. All Disease Begins in the (Leaky) Gut: Role of Zonulin-Mediated Gut Permeability in the Pathogenesis of Some Chronic Inflammatory Diseases. F1000Res 2020, 9, F1000 Faculty Rev-69. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhu, J.; Chen, G.; Zuo, S.; Zhang, J.; Chen, Z.; Wang, X.; Li, J.; Liu, Y.; Wang, P. 1,25-Dihydroxyvitamin D3 Preserves Intestinal Epithelial Barrier Function from TNF-α Induced Injury via Suppression of NF-KB P65 Mediated MLCK-P-MLC Signaling Pathway. Biochem. Biophys. Res. Commun. 2015, 460, 873–878. [Google Scholar] [CrossRef] [PubMed]
- Aydemir, Y.; Erdogan, B.; Türkeli, A. Vitamin D Deficiency Negatively Affects Both the Intestinal Epithelial Integrity and Bone Metabolism in Children with Celiac Disease. Clin. Res. Hepatol. Gastroenterol. 2021, 45, 101523. [Google Scholar] [CrossRef]
- Zanchi, C.; Di Leo, G.; Ronfani, L.; Martelossi, S.; Not, T.; Ventura, A. Bone Metabolism in Celiac Disease. J. Pediatr. 2008, 153, 262–265. [Google Scholar] [CrossRef]
- Villanueva, J.; Maranda, L.; Nwosu, B.U. Is Vitamin D Deficiency a Feature of Pediatric Celiac Disease? J. Pediatr. Endocrinol. Metab 2012, 25, 607–610. [Google Scholar] [CrossRef]
- Caruso, R.; Pallone, F.; Stasi, E.; Romeo, S.; Monteleone, G. Appropriate Nutrient Supplementation in Celiac Disease. Ann. Med. 2013, 45, 522–531. [Google Scholar] [CrossRef]
- Sdepanian, V.L.; de Miranda Carvalho, C.N.; de Morais, M.B.; Colugnati, F.A.B.; Fagundes-Neto, U. Bone Mineral Density of the Lumbar Spine in Children and Adolescents with Celiac Disease on a Gluten-Free Diet in São Paulo, Brazil. J. Pediatr. Gastroenterol. Nutr. 2003, 37, 571–576. [Google Scholar] [CrossRef]
- Shepherd, S.J.; Gibson, P.R. Nutritional Inadequacies of the Gluten-Free Diet in Both Recently-Diagnosed and Long-Term Patients with Coeliac Disease. J. Hum. Nutr. Diet. 2013, 26, 349–358. [Google Scholar] [CrossRef]
- Jansen, M.A.E.; Kiefte-de Jong, J.C.; Gaillard, R.; Escher, J.C.; Hofman, A.; Jaddoe, V.W.V.; Hooijkaas, H.; Moll, H.A. Growth Trajectories and Bone Mineral Density in Anti-Tissue Transglutaminase Antibody-Positive Children: The Generation R Study. Clin. Gastroenterol. Hepatol. 2015, 13, 913–920.e5. [Google Scholar] [CrossRef] [PubMed]
- Mager, D.R.; Qiao, J.; Turner, J. Vitamin D and K Status Influences Bone Mineral Density and Bone Accrual in Children and Adolescents with Celiac Disease. Eur. J. Clin. Nutr. 2012, 66, 488–495. [Google Scholar] [CrossRef] [PubMed]
- Hill, I.D.; Fasano, A.; Guandalini, S.; Hoffenberg, E.; Levy, J.; Reilly, N.; Verma, R. NASPGHAN Clinical Report on the Diagnosis and Treatment of Gluten-Related Disorders. J. Pediatr. Gastroenterol. Nutr. 2016, 63, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Tapia, A.; Hill, I.D.; Kelly, C.P.; Calderwood, A.H.; Murray, J.A. American College of Gastroenterology ACG Clinical Guidelines: Diagnosis and Management of Celiac Disease. Am. J. Gastroenterol. 2013, 108, 656–676, quiz 677. [Google Scholar] [CrossRef]
- Ludvigsson, J.F.; Bai, J.C.; Biagi, F.; Card, T.R.; Ciacci, C.; Ciclitira, P.J.; Green, P.H.R.; Hadjivassiliou, M.; Holdoway, A.; van Heel, D.A.; et al. Diagnosis and Management of Adult Coeliac Disease: Guidelines from the British Society of Gastroenterology. Gut 2014, 63, 1210–1228. [Google Scholar] [CrossRef]
- Ahlawat, R.; Weinstein, T.; Markowitz, J.; Kohn, N.; Pettei, M.J. Should We Assess Vitamin D Status in Pediatric Patients With Celiac Disease? J. Pediatr. Gastroenterol. Nutr. 2019, 69, 449–454. [Google Scholar] [CrossRef]
- Strausbaugh, S.D.; Davis, P.B. Cystic Fibrosis: A Review of Epidemiology and Pathobiology. Clin. Chest Med. 2007, 28, 279–288. [Google Scholar] [CrossRef]
- Salvatore, D.; Buzzetti, R.; Baldo, E.; Forneris, M.P.; Lucidi, V.; Manunza, D.; Marinelli, I.; Messore, B.; Neri, A.S.; Raia, V.; et al. An Overview of International Literature from Cystic Fibrosis Registries. Part 3. Disease Incidence, Genotype/Phenotype Correlation, Microbiology, Pregnancy, Clinical Complications, Lung Transplantation, and Miscellanea. J. Cyst. Fibros 2011, 10, 71–85. [Google Scholar] [CrossRef]
- Collawn, J.F.; Matalon, S. CFTR and Lung Homeostasis. Am. J. Physiol Lung Cell Mol. Physiol. 2014, 307, L917–L923. [Google Scholar] [CrossRef]
- Wolfenden, L.L.; Judd, S.E.; Shah, R.; Sanyal, R.; Ziegler, T.R.; Tangpricha, V. Vitamin D and Bone Health in Adults with Cystic Fibrosis. Clin. Endocrinol. (Oxf) 2008, 69, 374–381. [Google Scholar] [CrossRef]
- Rovner, A.J.; Stallings, V.A.; Schall, J.I.; Leonard, M.B.; Zemel, B.S. Vitamin D Insufficiency in Children, Adolescents, and Young Adults with Cystic Fibrosis despite Routine Oral Supplementation. Am. J. Clin. Nutr. 2007, 86, 1694–1699. [Google Scholar] [CrossRef] [PubMed]
- Hall, W.B.; Sparks, A.A.; Aris, R.M. Vitamin d Deficiency in Cystic Fibrosis. Int. J. Endocrinol 2010, 2010, 218691. [Google Scholar] [CrossRef] [PubMed]
- Tangpricha, V.; Kelly, A.; Stephenson, A.; Maguiness, K.; Enders, J.; Robinson, K.A.; Marshall, B.C.; Borowitz, D. Cystic Fibrosis Foundation Vitamin D Evidence-Based Review Committee An Update on the Screening, Diagnosis, Management, and Treatment of Vitamin D Deficiency in Individuals with Cystic Fibrosis: Evidence-Based Recommendations from the Cystic Fibrosis Foundation. J. Clin. Endocrinol. Metab. 2012, 97, 1082–1093. [Google Scholar] [CrossRef]
- Cemlyn-Jones, J.; Gamboa, F.; Teixeira, L.; Bernardo, J.; Robalo Cordeiro, C. Sarcoidosis: A Less Common Presentation. Rev. Port. Pneumol. 2009, 15, 543–552. [Google Scholar] [CrossRef]
- Douros, K.; Loukou, I.; Nicolaidou, P.; Tzonou, A.; Doudounakis, S. Bone Mass Density and Associated Factors in Cystic Fibrosis Patients of Young Age. J. Paediatr. Child. Health 2008, 44, 681–685. [Google Scholar] [CrossRef] [PubMed]
- Finklea, J.D.; Grossmann, R.E.; Tangpricha, V. Vitamin D and Chronic Lung Disease: A Review of Molecular Mechanisms and Clinical Studies. Adv. Nutr. 2011, 2, 244–253. [Google Scholar] [CrossRef]
- Black, P.N.; Scragg, R. Relationship between Serum 25-Hydroxyvitamin d and Pulmonary Function in the Third National Health and Nutrition Examination Survey. Chest 2005, 128, 3792–3798. [Google Scholar] [CrossRef]
- Chesdachai, S.; Tangpricha, V. Treatment of Vitamin D Deficiency in Cystic Fibrosis. J. Steroid. Biochem. Mol. Biol. 2016, 164, 36–39. [Google Scholar] [CrossRef]
- Yim, S.; Dhawan, P.; Ragunath, C.; Christakos, S.; Diamond, G. Induction of Cathelicidin in Normal and CF Bronchial Epithelial Cells by 1,25-Dihydroxyvitamin D(3). J. Cyst. Fibros 2007, 6, 403–410. [Google Scholar] [CrossRef]
- Herscovitch, K.; Dauletbaev, N.; Lands, L.C. Vitamin D as an Anti-Microbial and Anti-Inflammatory Therapy for Cystic Fibrosis. Paediatr. Respir. Rev. 2014, 15, 154–162. [Google Scholar] [CrossRef]
- Korf, H.; Decallonne, B.; Mathieu, C. Vitamin D for Infections. Curr. Opin. Endocrinol. Diabetes Obes. 2014, 21, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Grossmann, R.E.; Zughaier, S.M.; Kumari, M.; Seydafkan, S.; Lyles, R.H.; Liu, S.; Sueblinvong, V.; Schechter, M.S.; Stecenko, A.A.; Ziegler, T.R.; et al. Pilot Study of Vitamin D Supplementation in Adults with Cystic Fibrosis Pulmonary Exacerbation: A Randomized, Controlled Trial. Dermatoendocrinol 2012, 4, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.J.; Alvarez, J.A.; Smith, E.M.; Killilea, D.W.; Chmiel, J.F.; Joseph, P.M.; Grossmann, R.E.; Gaggar, A.; Ziegler, T.R.; Tangpricha, V.; et al. Changes in Mineral Micronutrient Status During and After Pulmonary Exacerbation in Adults With Cystic Fibrosis. Nutr. Clin. Pract. 2015, 30, 838–843. [Google Scholar] [CrossRef] [PubMed]
- Dorsey, J.; Gonska, T. Bacterial Overgrowth, Dysbiosis, Inflammation, and Dysmotility in the Cystic Fibrosis Intestine. J. Cyst. Fibros 2017, 16 (Suppl. S2), S14–S23. [Google Scholar] [CrossRef]
- Morin, G.; Orlando, V.; St-Martin Crites, K.; Patey, N.; Mailhot, G. Vitamin D Attenuates Inflammation in CFTR Knockdown Intestinal Epithelial Cells but Has No Effect in Cells with Intact CFTR. Am. J. Physiol. Gastrointest Liver Physiol. 2016, 310, G539–G549. [Google Scholar] [CrossRef]
- Le, T.N. Updates in Vitamin D Therapy in Cystic Fibrosis. Curr. Opin. Endocrinol. Diabetes Obes. 2018, 25, 361–365. [Google Scholar] [CrossRef]
- Kanhere, M.; He, J.; Chassaing, B.; Ziegler, T.R.; Alvarez, J.A.; Ivie, E.A.; Hao, L.; Hanfelt, J.; Gewirtz, A.T.; Tangpricha, V. Bolus Weekly Vitamin D3 Supplementation Impacts Gut and Airway Microbiota in Adults With Cystic Fibrosis: A Double-Blind, Randomized, Placebo-Controlled Clinical Trial. J. Clin. Endocrinol. Metab. 2018, 103, 564–574. [Google Scholar] [CrossRef]
- Alvarez, J.A.; Chong, E.Y.; Walker, D.I.; Chandler, J.D.; Michalski, E.S.; Grossmann, R.E.; Uppal, K.; Li, S.; Frediani, J.K.; Tirouvanziam, R.; et al. Plasma Metabolomics in Adults with Cystic Fibrosis during a Pulmonary Exacerbation: A Pilot Randomized Study of High-Dose Vitamin D3 Administration. Metabolism 2017, 70, 31–41. [Google Scholar] [CrossRef]
- Li, X.-X.; Liu, Y.; Luo, J.; Huang, Z.-D.; Zhang, C.; Fu, Y. Vitamin D Deficiency Associated with Crohn’s Disease and Ulcerative Colitis: A Meta-Analysis of 55 Observational Studies. J. Transl. Med. 2019, 17, 323. [Google Scholar] [CrossRef]
- Garg, M.; Lubel, J.S.; Sparrow, M.P.; Holt, S.G.; Gibson, P.R. Review Article: Vitamin D and Inflammatory Bowel Disease--Established Concepts and Future Directions. Aliment. Pharmacol. Ther. 2012, 36, 324–344. [Google Scholar] [CrossRef]
- Zhao, H.; Zhang, H.; Wu, H.; Li, H.; Liu, L.; Guo, J.; Li, C.; Shih, D.Q.; Zhang, X. Protective Role of 1,25(OH)2 Vitamin D3 in the Mucosal Injury and Epithelial Barrier Disruption in DSS-Induced Acute Colitis in Mice. BMC Gastroenterol. 2012, 12, 57. [Google Scholar] [CrossRef] [PubMed]
- Gombart, A.F.; Borregaard, N.; Koeffler, H.P. Human Cathelicidin Antimicrobial Peptide (CAMP) Gene Is a Direct Target of the Vitamin D Receptor and Is Strongly up-Regulated in Myeloid Cells by 1,25-Dihydroxyvitamin D3. FASEB J. 2005, 19, 1067–1077. [Google Scholar] [CrossRef] [PubMed]
- Bora, S.; Cantorna, M.T. The Role of UVR and Vitamin D on T Cells and Inflammatory Bowel Disease. Photochem. Photobiol. Sci. 2017, 16, 347–353. [Google Scholar] [CrossRef]
- Barbalho, S.M.; Goulart, R.d.A.; Gasparini, R.G. Associations between Inflammatory Bowel Diseases and Vitamin D. Crit. Rev. Food Sci Nutr 2019, 59, 1347–1356. [Google Scholar] [CrossRef] [PubMed]
- Ananthakrishnan, A.N. Epidemiology and Risk Factors for IBD. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Lim, W.-C.; Hanauer, S.B.; Li, Y.C. Mechanisms of Disease: Vitamin D and Inflammatory Bowel Disease. Nat. Clin. Pract. Gastroenterol Hepatol 2005, 2, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Farraye, F.A.; Nimitphong, H.; Stucchi, A.; Dendrinos, K.; Boulanger, A.B.; Vijjeswarapu, A.; Tanennbaum, A.; Biancuzzo, R.; Chen, T.C.; Holick, M.F. Use of a Novel Vitamin D Bioavailability Test Demonstrates That Vitamin D Absorption Is Decreased in Patients with Quiescent Crohn’s Disease. Inflamm. Bowel. Dis. 2011, 17, 2116–2121. [Google Scholar] [CrossRef]
- Gorham, E.D.; Garland, C.F.; Garland, F.C.; Grant, W.B.; Mohr, S.B.; Lipkin, M.; Newmark, H.L.; Giovannucci, E.; Wei, M.; Holick, M.F. Vitamin D and Prevention of Colorectal Cancer. J. Steroid. Biochem. Mol. Biol. 2005, 97, 179–194. [Google Scholar] [CrossRef]
- Wilkins, C.H.; Sheline, Y.I.; Roe, C.M.; Birge, S.J.; Morris, J.C. Vitamin D Deficiency Is Associated with Low Mood and Worse Cognitive Performance in Older Adults. Am. J. Geriatr. Psychiatry 2006, 14, 1032–1040. [Google Scholar] [CrossRef]
- Raffner Basson, A.; Swart, R.; Jordaan, E.; Mazinu, M.; Watermeyer, G. Vitamin D Deficiency Increases the Risk for Moderate to Severe Disease Activity in Crohn’s Disease Patients in South Africa, Measured by the Harvey Bradshaw Index. J. Am. Coll Nutr. 2016, 35, 163–174. [Google Scholar] [CrossRef]
- El-Matary, W.; Sikora, S.; Spady, D. Bone Mineral Density, Vitamin D, and Disease Activity in Children Newly Diagnosed with Inflammatory Bowel Disease. Dig. Dis. Sci. 2011, 56, 825–829. [Google Scholar] [CrossRef] [PubMed]
- Gubatan, J.; Moss, A.C. Vitamin D in Inflammatory Bowel Disease: More than Just a Supplement. Curr. Opin. Gastroenterol. 2018, 34, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Del Pinto, R.; Pietropaoli, D.; Chandar, A.K.; Ferri, C.; Cominelli, F. Association between Inflammatory Bowel Disease and Vitamin D Deficiency: A Systematic Review and Meta-Analysis. Inflamm. Bowel. Dis. 2015, 21, 2708–2717. [Google Scholar] [CrossRef] [PubMed]
- Chatu, S.; Chhaya, V.; Holmes, R.; Neild, P.; Kang, J.-Y.; Pollok, R.C.; Poullis, A. Factors Associated with Vitamin D Deficiency in a Multicultural Inflammatory Bowel Disease Cohort. Frontline Gastroenterol. 2013, 4, 51–56. [Google Scholar] [CrossRef]
- Zullow, S.; Jambaulikar, G.; Rustgi, A.; Quezada, S.; Cross, R.K. Risk Factors for Vitamin D Deficiency and Impact of Repletion in a Tertiary Care Inflammatory Bowel Disease Population. Dig. Dis. Sci. 2017, 62, 2072–2078. [Google Scholar] [CrossRef]
- Lee, S.; Metcalfe, A.; Raman, M.; Leung, Y.; Aghajafari, F.; Letourneau, N.; Panaccione, R.; Kaplan, G.G.; Seow, C.H. Pregnant Women with Inflammatory Bowel Disease Are at Increased Risk of Vitamin D Insufficiency: A Cross-Sectional Study. J. Crohns Colitis 2018, 12, 702–709. [Google Scholar] [CrossRef]
- Blanck, S.; Aberra, F. Vitamin d Deficiency Is Associated with Ulcerative Colitis Disease Activity. Dig. Dis. Sci. 2013, 58, 1698–1702. [Google Scholar] [CrossRef]
- Ulitsky, A.; Ananthakrishnan, A.N.; Naik, A.; Skaros, S.; Zadvornova, Y.; Binion, D.G.; Issa, M. Vitamin D Deficiency in Patients with Inflammatory Bowel Disease: Association with Disease Activity and Quality of Life. JPEN J. Parenter. Enteral. Nutr. 2011, 35, 308–316. [Google Scholar] [CrossRef]
- Dolatshahi, S.; Pishgar, E.; Jamali, R. Does Serum 25 Hydroxy Vitamin D Level Predict Disease Activity in Ulcerative Colitis Patients? Acta Clin. Belg. 2016, 71, 46–50. [Google Scholar] [CrossRef]
- Scolaro, B.L.; Barretta, C.; Matos, C.H.; Malluta, E.F.; de Almeida, I.B.T.; Braggio, L.D.; Bobato, S.; Specht, C.M. Deficiency of Vitamin D and Its Relation with Clinical and Laboratory Activity of Inflammatory Bowel Diseases. J. Coloproctol. (Rio J.) 2018, 38, 99–104. [Google Scholar] [CrossRef]
- Schäffler, H.; Schmidt, M.; Huth, A.; Reiner, J.; Glass, Ä.; Lamprecht, G. Clinical Factors Are Associated with Vitamin D Levels in IBD Patients: A Retrospective Analysis. J. Dig. Dis. 2018, 19, 24–32. [Google Scholar] [CrossRef]
- Frigstad, S.O.; Høivik, M.; Jahnsen, J.; Dahl, S.R.; Cvancarova, M.; Grimstad, T.; Berset, I.P.; Huppertz-Hauss, G.; Hovde, Ø.; Torp, R.; et al. Vitamin D Deficiency in Inflammatory Bowel Disease: Prevalence and Predictors in a Norwegian Outpatient Population. Scand. J. Gastroenterol. 2017, 52, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Alrefai, D.; Jones, J.; El-Matary, W.; Whiting, S.J.; Aljebreen, A.; Mirhosseini, N.; Vatanparast, H. The Association of Vitamin D Status with Disease Activity in a Cohort of Crohn’s Disease Patients in Canada. Nutrients 2017, 9, E1112. [Google Scholar] [CrossRef] [PubMed]
- Meckel, K.; Li, Y.C.; Lim, J.; Kocherginsky, M.; Weber, C.; Almoghrabi, A.; Chen, X.; Kaboff, A.; Sadiq, F.; Hanauer, S.B.; et al. Serum 25-Hydroxyvitamin D Concentration Is Inversely Associated with Mucosal Inflammation in Patients with Ulcerative Colitis. Am. J. Clin. Nutr. 2016, 104, 113–120. [Google Scholar] [CrossRef]
- Ye, L.; Lin, Z.; Liu, J.; Cao, Q. Vitamin D Deficiency Is Associated with Endoscopic Severity in Patients with Crohn’s Disease. Gastroenterol. Res. Pract. 2017, 2017, 4869718. [Google Scholar] [CrossRef] [PubMed]
- Ko, K.H.; Kim, Y.S.; Lee, B.K.; Choi, J.H.; Woo, Y.M.; Kim, J.Y.; Moon, J.S. Vitamin D Deficiency Is Associated with Disease Activity in Patients with Crohn’s Disease. Intest. Res. 2019, 17, 70–77. [Google Scholar] [CrossRef]
- Ananthakrishnan, A.N.; Cagan, A.; Gainer, V.S.; Cai, T.; Cheng, S.-C.; Savova, G.; Chen, P.; Szolovits, P.; Xia, Z.; De Jager, P.L.; et al. Normalization of Plasma 25-Hydroxy Vitamin D Is Associated with Reduced Risk of Surgery in Crohn’s Disease. Inflamm. Bowel Dis. 2013, 19, 1921–1927. [Google Scholar] [CrossRef]
- Kabbani, T.A.; Koutroubakis, I.E.; Schoen, R.E.; Ramos-Rivers, C.; Shah, N.; Swoger, J.; Regueiro, M.; Barrie, A.; Schwartz, M.; Hashash, J.G.; et al. Association of Vitamin D Level With Clinical Status in Inflammatory Bowel Disease: A 5-Year Longitudinal Study. Am. J. Gastroenterol. 2016, 111, 712–719. [Google Scholar] [CrossRef]
- Ryan, F.J.; Ahern, A.M.; Fitzgerald, R.S.; Laserna-Mendieta, E.J.; Power, E.M.; Clooney, A.G.; O’Donoghue, K.W.; McMurdie, P.J.; Iwai, S.; Crits-Christoph, A.; et al. Colonic Microbiota Is Associated with Inflammation and Host Epigenomic Alterations in Inflammatory Bowel Disease. Nat. Commun. 2020, 11, 1512. [Google Scholar] [CrossRef]
- Schirmer, M.; Garner, A.; Vlamakis, H.; Xavier, R.J. Microbial Genes and Pathways in Inflammatory Bowel Disease. Nat. Rev. Microbiol. 2019, 17, 497–511. [Google Scholar] [CrossRef]
- Stange, E.F.; Schroeder, B.O. Microbiota and Mucosal Defense in IBD: An Update. Expert Rev. Gastroenterol. Hepatol. 2019, 13, 963–976. [Google Scholar] [CrossRef]
- Gubatan, J.; Chou, N.D.; Nielsen, O.H.; Moss, A.C. Systematic Review with Meta-Analysis: Association of Vitamin D Status with Clinical Outcomes in Adult Patients with Inflammatory Bowel Disease. Aliment. Pharmacol. Ther. 2019, 50, 1146–1158. [Google Scholar] [CrossRef]
- Garg, M.; Hendy, P.; Ding, J.N.; Shaw, S.; Hold, G.; Hart, A. The Effect of Vitamin D on Intestinal Inflammation and Faecal Microbiota in Patients with Ulcerative Colitis. J. Crohns Colitis 2018, 12, 963–972. [Google Scholar] [CrossRef]
- Garg, M.; Rosella, O.; Rosella, G.; Wu, Y.; Lubel, J.S.; Gibson, P.R. Evaluation of a 12-Week Targeted Vitamin D Supplementation Regimen in Patients with Active Inflammatory Bowel Disease. Clin. Nutr. 2018, 37, 1375–1382. [Google Scholar] [CrossRef]
- Guzman-Prado, Y.; Samson, O.; Segal, J.P.; Limdi, J.K.; Hayee, B. Vitamin D Therapy in Adults With Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis. Inflamm. Bowel Dis. 2020, 26, 1819–1830. [Google Scholar] [CrossRef]
- Mechie, N.-C.; Mavropoulou, E.; Ellenrieder, V.; Petzold, G.; Kunsch, S.; Neesse, A.; Amanzada, A. Serum Vitamin D but Not Zinc Levels Are Associated with Different Disease Activity Status in Patients with Inflammatory Bowel Disease. Medicine 2019, 98, e15172. [Google Scholar] [CrossRef]
- Mentella, M.C.; Scaldaferri, F.; Pizzoferrato, M.; Gasbarrini, A.; Miggiano, G.A.D. The Association of Disease Activity, BMI and Phase Angle with Vitamin D Deficiency in Patients with IBD. Nutrients 2019, 11, E2583. [Google Scholar] [CrossRef]
- Sharifi, A.; Hosseinzadeh-Attar, M.J.; Vahedi, H.; Nedjat, S. A Randomized Controlled Trial on the Effect of Vitamin D3 on Inflammation and Cathelicidin Gene Expression in Ulcerative Colitis Patients. Saudi J. Gastroenterol. 2016, 22, 316–323. [Google Scholar] [CrossRef]
- Sharifi, A.; Vahedi, H.; Nedjat, S.; Rafiei, H.; Hosseinzadeh-Attar, M.J. Effect of Single-Dose Injection of Vitamin D on Immune Cytokines in Ulcerative Colitis Patients: A Randomized Placebo-Controlled Trial. APMIS 2019, 127, 681–687. [Google Scholar] [CrossRef]
- Allen, K.J.; Koplin, J.J.; Ponsonby, A.-L.; Gurrin, L.C.; Wake, M.; Vuillermin, P.; Martin, P.; Matheson, M.; Lowe, A.; Robinson, M.; et al. Vitamin D Insufficiency Is Associated with Challenge-Proven Food Allergy in Infants. J. Allergy Clin. Immunol. 2013, 131, 1109–1116, 1116.e1-6. [Google Scholar] [CrossRef]
- Peroni, D.G.; Boner, A.L. Food Allergy: The Perspectives of Prevention Using Vitamin D. Curr. Opin. Allergy Clin. Immunol. 2013, 13, 287–292. [Google Scholar] [CrossRef]
- Vassallo, M.F.; Camargo, C.A. Potential Mechanisms for the Hypothesized Link between Sunshine, Vitamin D, and Food Allergy in Children. J. Allergy Clin. Immunol. 2010, 126, 217–222. [Google Scholar] [CrossRef]
- Lack, G. Clinical Practice. Food Allergy. N. Engl. J. Med. 2008, 359, 1252–1260. [Google Scholar] [CrossRef]
- Peroni, D.G.; Piacentini, G.L.; Cametti, E.; Chinellato, I.; Boner, A.L. Correlation between Serum 25-Hydroxyvitamin D Levels and Severity of Atopic Dermatitis in Children. Br. J. Dermatol. 2011, 164, 1078–1082. [Google Scholar] [CrossRef]
- Poole, A.; Song, Y.; Brown, H.; Hart, P.H.; Zhang, G.B. Cellular and Molecular Mechanisms of Vitamin D in Food Allergy. J. Cell Mol. Med. 2018, 22, 3270–3277. [Google Scholar] [CrossRef]
- Koplin, J.J.; Peters, R.L.; Allen, K.J. Prevention of Food Allergies. Immunol. Allergy Clin. N. Am. 2018, 38, 1–11. [Google Scholar] [CrossRef]
- Berry, M.J.; Adams, J.; Voutilainen, H.; Feustel, P.J.; Celestin, J.; Järvinen, K.M. Impact of Elimination Diets on Growth and Nutritional Status in Children with Multiple Food Allergies. Pediatr. Allergy Immunol. 2015, 26, 133–138. [Google Scholar] [CrossRef]
- Miraglia Del Giudice, M.; Allegorico, A. The Role of Vitamin D in Allergic Diseases in Children. J. Clin. Gastroenterol. 2016, 50 (Suppl. S2), S133–S135. [Google Scholar] [CrossRef]
- Allen, K.J.; Koplin, J.J. Why Does Australia Appear to Have the Highest Rates of Food Allergy? Pediatr. Clin. N. Am. 2015, 62, 1441–1451. [Google Scholar] [CrossRef]
- Nowak, S.; Wang, H.; Schmidt, B.; Jarvinen, K.M. Vitamin D and Iron Status in Children with Food Allergy. Ann. Allergy Asthma Immunol 2021, 127, 57–63. [Google Scholar] [CrossRef]
- Silva, C.M.; da Silva, S.A.; Antunes, M.M.d.C.; da Silva, G.A.P.; Sarinho, E.S.C.; Brandt, K.G. Do Infants with Cow’s Milk Protein Allergy Have Inadequate Levels of Vitamin D? J. Pediatr. (Rio J.) 2017, 93, 632–638. [Google Scholar] [CrossRef] [PubMed]
- Shaker, M.; Venter, C. The Ins and Outs of Managing Avoidance Diets for Food Allergies. Curr. Opin. Pediatr. 2016, 28, 567–572. [Google Scholar] [CrossRef] [PubMed]
- Foong, R.-X.; Meyer, R.; Dziubak, R.; Lozinsky, A.C.; Godwin, H.; Reeve, K.; Hussain, S.T.; Nourzaie, R.; Shah, N. Establishing the Prevalence of Low Vitamin D in Non-Immunoglobulin-E Mediated Gastrointestinal Food Allergic Children in a Tertiary Centre. World Allergy Organ. J. 2017, 10, 4. [Google Scholar] [CrossRef] [PubMed]
- Giannetti, A.; Bernardini, L.; Cangemi, J.; Gallucci, M.; Masetti, R.; Ricci, G. Role of Vitamin D in Prevention of Food Allergy in Infants. Front. Pediatr. 2020, 8, 447. [Google Scholar] [CrossRef] [PubMed]
- Yepes-Nuñez, J.J.; Brożek, J.L.; Fiocchi, A.; Pawankar, R.; Cuello-García, C.; Zhang, Y.; Morgano, G.P.; Agarwal, A.; Gandhi, S.; Terracciano, L.; et al. Vitamin D Supplementation in Primary Allergy Prevention: Systematic Review of Randomized and Non-Randomized Studies. Allergy 2018, 73, 37–49. [Google Scholar] [CrossRef]
- Matsui, T.; Tanaka, K.; Yamashita, H.; Saneyasu, K.-I.; Tanaka, H.; Takasato, Y.; Sugiura, S.; Inagaki, N.; Ito, K. Food Allergy Is Linked to Season of Birth, Sun Exposure, and Vitamin D Deficiency. Allergol. Int. 2019, 68, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Dogan, E.; Sevinc, E. The Vitamin D Status and Serum Eosinophilic Cationic Protein Levels in Infants with Cow’s Milk Protein Allergy. Am. J. Transl Res. 2020, 12, 8208–8215. [Google Scholar]
- Venter, C.; Groetch, M.; Netting, M.; Meyer, R. A Patient-Specific Approach to Develop an Exclusion Diet to Manage Food Allergy in Infants and Children. Clin. Exp. Allergy 2018, 48, 121–137. [Google Scholar] [CrossRef]
- Rozenberg, S.; Body, J.-J.; Bruyère, O.; Bergmann, P.; Brandi, M.L.; Cooper, C.; Devogelaer, J.-P.; Gielen, E.; Goemaere, S.; Kaufman, J.-M.; et al. Effects of Dairy Products Consumption on Health: Benefits and Beliefs—A Commentary from the Belgian Bone Club and the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases. Calcif. Tissue Int. 2016, 98, 1–17. [Google Scholar] [CrossRef]
- Mehta, H.; Ramesh, M.; Feuille, E.; Groetch, M.; Wang, J. Growth Comparison in Children with and without Food Allergies in 2 Different Demographic Populations. J. Pediatr. 2014, 165, 842–848. [Google Scholar] [CrossRef]
- Sardecka-Milewska, I.; Łoś-Rycharska, E.; Gawryjołek, J.; Toporowska-Kowalska, E.; Krogulska, A. Role of FOXP3 Expression and Serum Vitamin D and C Concentrations When Predicting Acquisition of Tolerance in Infants With Cow’s Milk Allergy. J. Investig. Allergol. Clin. Immunol. 2020, 30, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Mei, X.; Cai, X.; Zhuo, Y.; Zhang, L.; Guo, H.; Yang, H.; Yang, G. Association of Blood Eosinophilia and Vitamin D Insufficiency in Young Infants with Cow Milk Allergy. Asia Pac. J. Clin. Nutr. 2019, 28, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Ercan, N.; Bostanci, İ.B.; Ozmen, S.; Tekindal, M.A. Is There an Association between Vitamin D Levels and Cow’s Milk Protein Allergy at Infancy? Arch. Argent. Pediatr. 2019, 117, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Kosek, M.; Bern, C.; Guerrant, R.L. The Global Burden of Diarrhoeal Disease, as Estimated from Studies Published between 1992 and 2000. Bull. World Health Organ. 2003, 81, 197–204. [Google Scholar] [PubMed]
- Liu, L.; Johnson, H.L.; Cousens, S.; Perin, J.; Scott, S.; Lawn, J.E.; Rudan, I.; Campbell, H.; Cibulskis, R.; Li, M.; et al. Global, Regional, and National Causes of Child Mortality: An Updated Systematic Analysis for 2010 with Time Trends since 2000. Lancet 2012, 379, 2151–2161. [Google Scholar] [CrossRef]
- Yin, K.; Agrawal, D.K. Vitamin D and Inflammatory Diseases. J. Inflamm. Res. 2014, 7, 69–87. [Google Scholar] [CrossRef]
- Thornton, K.A.; Marín, C.; Mora-Plazas, M.; Villamor, E. Vitamin D Deficiency Associated with Increased Incidence of Gastrointestinal and Ear Infections in School-Age Children. Pediatr. Infect. Dis. J. 2013, 32, 585–593. [Google Scholar] [CrossRef]
- Abed, N.; Shaban, N.; Aly, M.; Abdel-gawad, E. Vitamin D Status in Children with Re-Current Acute Diarrhea. Int. J. Curr. Microbiol. Appl. Sci. 2014, 3, 856–868. [Google Scholar]
- Bahijri, S.M. Serum 25-Hydroxy Cholecalciferol in Infants and Preschool Children in the Western Region of Saudi Arabia. Etiological Factors. Saudi Med. J. 2001, 22, 973–979. [Google Scholar]
- Siddiqui, T.S.; Rai, M.I. Presentation and Predisposing Factors of Nutritional Rickets in Children of Hazara Division. J. Ayub Med. Coll Abbottabad 2005, 17, 29–32. [Google Scholar]
- Hassam, I.; Kisenge, R.; Aboud, S.; Manji, K. Association of Vitamin D and Diarrhoea in Children Aged Less than Five Years at Muhimbili National Hospital, Dar Es Salaam: An Unmatched Case Control Study. BMC Pediatr. 2019, 19, 237. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.M.S.; Magalhaes, R.J.S.; Ahmed, T.; Long, K.Z.; Hossain, M.; Islam, M.M.; Mahfuz, M.; Gaffar, S.M.A.; Sharmeen, A.; Haque, R.; et al. Vitamin-D Status Is Not a Confounder of the Relationship between Zinc and Diarrhoea: A Study in 6-24-Month-Old Underweight and Normal-Weight Children of Urban Bangladesh. Eur. J. Clin. Nutr. 2016, 70, 620–628. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.J.; Gray, S.; Sison, C.; Arramraju, S.; John, B.K.; Hussain, S.A.; Kim, S.H.; Mehta, P.; Rubin, M. Low Vitamin D Level Is an Independent Predictor of Poor Outcomes in Clostridium Difficile-Associated Diarrhea. Therap. Adv. Gastroenterol. 2014, 7, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Bucak, I.H.; Ozturk, A.B.; Almis, H.; Cevik, M.Ö.; Tekin, M.; Konca, Ç.; Turgut, M.; Bulbul, M. Is There a Relationship between Low Vitamin D and Rotaviral Diarrhea? Pediatr. Int. 2016, 58, 270–273. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.; Mach, N. Role of Vitamin D in the Hygiene Hypothesis: The Interplay between Vitamin D, Vitamin D Receptors, Gut Microbiota, and Immune Response. Front. Immunol. 2016, 7, 627. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Liu, T.; Shi, Y.; Tian, F.; Hu, H.; Deb, D.K.; Chen, Y.; Bissonnette, M.; Li, Y.C. Gut Epithelial Vitamin D Receptor Regulates Microbiota-Dependent Mucosal Inflammation by Suppressing Intestinal Epithelial Cell Apoptosis. Endocrinology 2018, 159, 967–979. [Google Scholar] [CrossRef]
- Shahini, E.; Iannone, A.; Romagno, D.; Armandi, A.; Carparelli, S.; Principi, M.; Viggiani, M.T.; Ierardi, E.; Di Leo, A.; Barone, M. Clinical Relevance of Serum Non-Organ-Specific Antibodies in Patients with HCV Infection Receiving Direct-Acting Antiviral Therapy. Aliment. Pharmacol. Ther. 2018, 48, 1138–1145. [Google Scholar] [CrossRef]
- Thompson, A.J.; Baranzini, S.E.; Geurts, J.; Hemmer, B.; Ciccarelli, O. Multiple Sclerosis. Lancet 2018, 391, 1622–1636. [Google Scholar] [CrossRef]
- Spear, E.T.; Holt, E.A.; Joyce, E.J.; Haag, M.M.; Mawe, S.M.; Hennig, G.W.; Lavoie, B.; Applebee, A.M.; Teuscher, C.; Mawe, G.M. Altered Gastrointestinal Motility Involving Autoantibodies in the Experimental Autoimmune Encephalomyelitis Model of Multiple Sclerosis. Neurogastroenterol. Motil. 2018, 30, e13349. [Google Scholar] [CrossRef]
- Chia, Y.W.; Gill, K.P.; Jameson, J.S.; Forti, A.D.; Henry, M.M.; Swash, M.; Shorvon, P.J. Paradoxical Puborectalis Contraction Is a Feature of Constipation in Patients with Multiple Sclerosis. J. Neurol. Neurosurg. Psychiatry 1996, 60, 31–35. [Google Scholar] [CrossRef]
- Li, Q.; Michel, K.; Annahazi, A.; Demir, I.E.; Ceyhan, G.O.; Zeller, F.; Komorowski, L.; Stöcker, W.; Beyak, M.J.; Grundy, D.; et al. Anti-Hu Antibodies Activate Enteric and Sensory Neurons. Sci. Rep. 2016, 6, 38216. [Google Scholar] [CrossRef] [PubMed]
- Panarese, A.; Pesce, F.; Porcelli, P.; Riezzo, G.; Iacovazzi, P.A.; Leone, C.M.; De Carne, M.; Rinaldi, C.M.; Shahini, E. Chronic Functional Constipation Is Strongly Linked to Vitamin D Deficiency. World J. Gastroenterol. 2019, 25, 1729–1740. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mărginean, C.O.; Meliț, L.E.; Borka Balas, R.; Văsieșiu, A.M.; Fleșeriu, T. The Crosstalk between Vitamin D and Pediatric Digestive Disorders. Diagnostics 2022, 12, 2328. https://doi.org/10.3390/diagnostics12102328
Mărginean CO, Meliț LE, Borka Balas R, Văsieșiu AM, Fleșeriu T. The Crosstalk between Vitamin D and Pediatric Digestive Disorders. Diagnostics. 2022; 12(10):2328. https://doi.org/10.3390/diagnostics12102328
Chicago/Turabian StyleMărginean, Cristina Oana, Lorena Elena Meliț, Reka Borka Balas, Anca Meda Văsieșiu, and Tudor Fleșeriu. 2022. "The Crosstalk between Vitamin D and Pediatric Digestive Disorders" Diagnostics 12, no. 10: 2328. https://doi.org/10.3390/diagnostics12102328
APA StyleMărginean, C. O., Meliț, L. E., Borka Balas, R., Văsieșiu, A. M., & Fleșeriu, T. (2022). The Crosstalk between Vitamin D and Pediatric Digestive Disorders. Diagnostics, 12(10), 2328. https://doi.org/10.3390/diagnostics12102328







