A Comparison of Functional and Oncologic Outcomes between Partial Nephrectomy and Radiofrequency Ablation in Patients with Chronic Kidney Disease after Propensity Score Matching
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Patient Management
2.3. Clinical Features and Follow-Up
2.4. Statistical Analysis
3. Results
3.1. Demographic and Clinical Characteristics
3.2. Propensity Score Matching Analysis
3.3. Comparing Renal Functional Outcomes between Two Groups According to CKD Stage
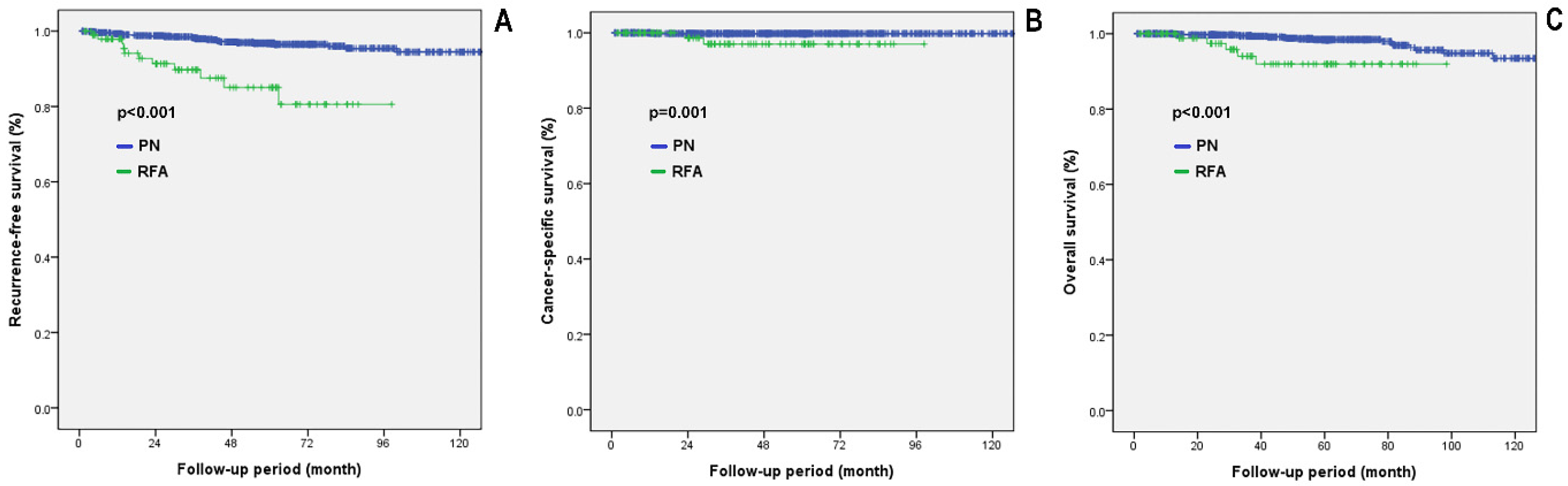
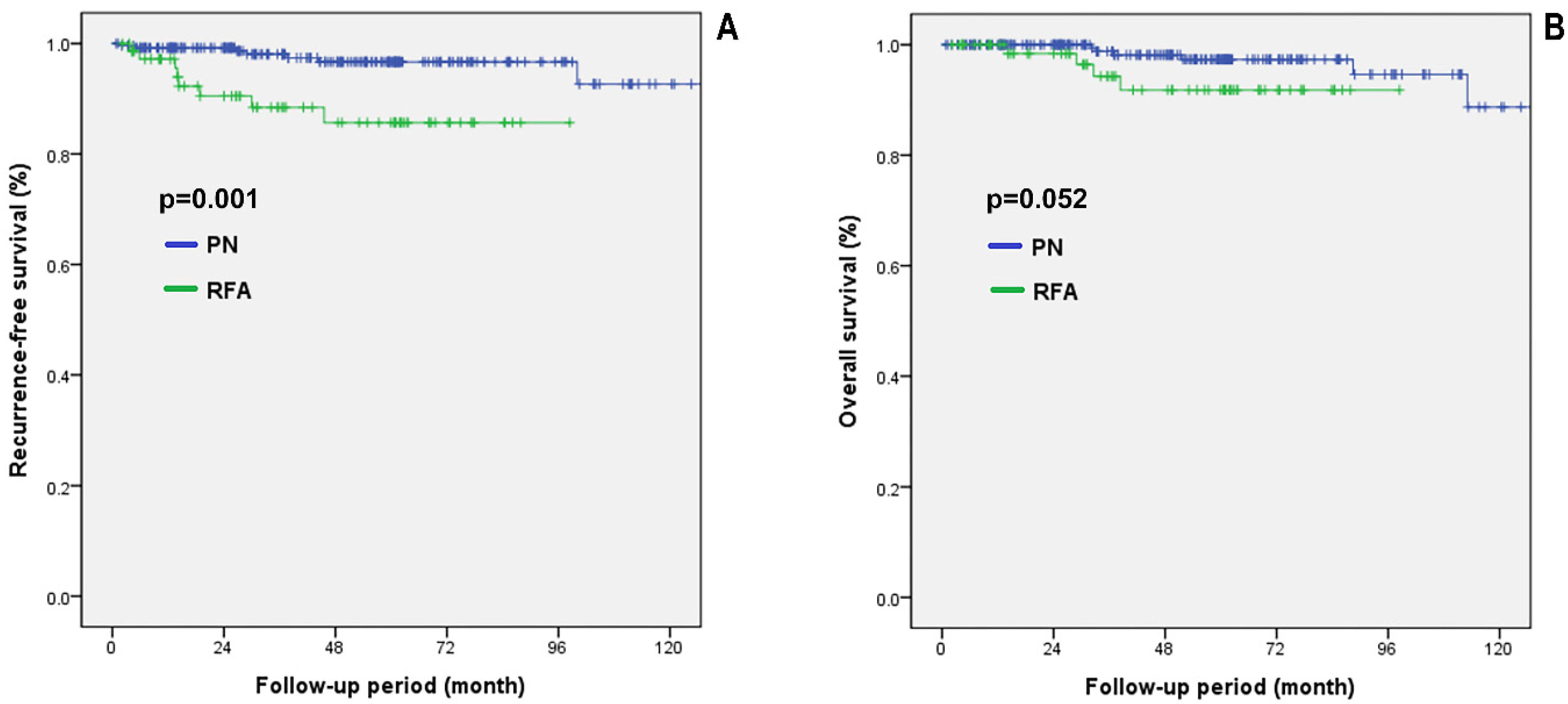
3.4. Comparing Oncological Outcomes between the PN and RFA Groups
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Miller, D.C.; Ruterbusch, J.; Colt, J.S.; Davis, F.G.; Linehan, W.M.; Chow, W.H.; Schwartz, K. Contemporary clinical epidemiology of renal cell carcinoma: Insight from a population based case-control study. J. Urol. 2010, 184, 2254–2258. [Google Scholar] [CrossRef][Green Version]
- Rini, B.I.; Campbell, S.C.; Escudier, B. Renal cell carcinoma. Lancet 2009, 373, 1119–1132. [Google Scholar] [CrossRef]
- Siekiera, J.; Jasinski, M.; Mikolajczak, W. Radiofrequency ablation of small renal masses in comorbid patients. Wideochir. Inne Tech. Maloinwazyjne 2018, 13, 212–214. [Google Scholar] [CrossRef]
- Andrews, J.R.; Atwell, T.; Schmit, G.; Lohse, C.M.; Kurup, A.N.; Weisbrod, A.; Callstrom, M.R.; Cheville, J.C.; Boorjian, S.A.; Leibovich, B.C.; et al. Oncologic Outcomes Following Partial Nephrectomy and Percutaneous Ablation for cT1 Renal Masses. Eur. Urol. 2019, 76, 244–251. [Google Scholar] [CrossRef]
- Finelli, A.; Ismaila, N.; Bro, B.; Durack, J.; Eggener, S.; Evans, A.; Gill, I.; Graham, D.; Huang, W.; Jewett, M.A.; et al. Management of Small Renal Masses: American Society of Clinical Oncology Clinical Practice Guideline. J. Clin. Oncol. 2017, 35, 668–680. [Google Scholar] [CrossRef]
- Campbell, S.; Uzzo, R.G.; Allaf, M.E.; Bass, E.B.; Cadeddu, J.A.; Chang, A.; Clark, P.E.; Davis, B.J.; Derweesh, I.H.; Giambarresi, L.; et al. Renal Mass and Localized Renal Cancer: AUA Guideline. J. Urol. 2017, 198, 520–529. [Google Scholar] [CrossRef]
- Dabestani, S.; Beisland, C.; Stewart, G.D.; Bensalah, K.; Gudmundsson, E.; Lam, T.B.; Gietzmann, W.; Zakikhani, P.; Marconi, L.; Fernandez-Pello, S.; et al. Intensive Imaging-based Follow-up of Surgically Treated Localised Renal Cell Carcinoma Does Not Improve Post-recurrence Survival: Results from a European Multicentre Database (RECUR). Eur. Urol. 2019, 75, 261–264. [Google Scholar] [CrossRef]
- Chang, X.; Liu, T.; Zhang, F.; Ji, C.; Zhao, X.; Wang, W.; Guo, H. Radiofrequency ablation versus partial nephrectomy for clinical T1a renal-cell carcinoma: Long-term clinical and oncologic outcomes based on a propensity score analysis. J. Endourol. 2015, 29, 518–525. [Google Scholar] [CrossRef]
- Yin, X.; Cui, L.; Li, F.; Qi, S.; Yin, Z.; Gao, J. Radiofrequency Ablation Versus Partial Nephrectomy in Treating Small Renal Tumors: A Systematic Review and Meta-Analysis. Medicine 2015, 94, e2255. [Google Scholar] [CrossRef]
- Pan, X.W.; Cui, X.M.; Huang, H.; Huang, Y.; Li, L.; Wang, Z.J.; Qu, F.J.; Gao, Y.; Cui, X.G.; Xu, D.F. Radiofrequency ablation versus partial nephrectomy for treatment of renal masses: A systematic review and meta-analysis. Kaohsiung J. Med. Sci. 2015, 31, 649–658. [Google Scholar] [CrossRef]
- Sonmez, M.G.; Kara, C. The effect of zero-ischaemia laparoscopic minimally invasive partial nephrectomy using the modified sequential preplaced suture renorrhaphy technique on long-term renal functions. Wideochir. Inne Tech. Maloinwazyjne 2017, 12, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Castro, A., Jr.; Jenkins, L.C.; Salas, N.; Lorber, G.; Leveillee, R.J. Ablative therapies for small renal tumours. Nat. Rev. Urol. 2013, 10, 284–291. [Google Scholar] [CrossRef]
- Thompson, R.H.; Atwell, T.; Schmit, G.; Lohse, C.M.; Kurup, A.N.; Weisbrod, A.; Psutka, S.P.; Stewart, S.B.; Callstrom, M.R.; Cheville, J.C.; et al. Comparison of partial nephrectomy and percutaneous ablation for cT1 renal masses. Eur. Urol. 2015, 67, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Lucas, S.M.; Stern, J.M.; Adibi, M.; Zeltser, I.S.; Cadeddu, J.A.; Raj, G.V. Renal function outcomes in patients treated for renal masses smaller than 4 cm by ablative and extirpative techniques. J. Urol. 2008, 179, 75–79; discussion 79–80. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Bedir, S.; Cadeddu, J.A.; Gahan, J.C. Long-term outcomes in healthy adults after radiofrequency ablation of T1a renal tumours. BJU Int. 2014, 113, 51–55. [Google Scholar] [CrossRef]
- Nor Azhari, M.Z.; Tan, Y.H.; Sunga, P.A.; Yip, S.K.; Cheng, C.W. Laparoscopic partial nephrectomy for renal tumours: Early experience in Singapore general hospital. Ann. Acad. Med. Singap. 2009, 38, 576-5. [Google Scholar]
- Kaouk, J.H.; Khalifeh, A.; Hillyer, S.; Haber, G.P.; Stein, R.J.; Autorino, R. Robot-assisted laparoscopic partial nephrectomy: Step-by-step contemporary technique and surgical outcomes at a single high-volume institution. Eur. Urol. 2012, 62, 553–561. [Google Scholar] [CrossRef]
- Sukumar, S.; Rogers, C.G. Robotic partial nephrectomy: Surgical technique. BJU Int. 2011, 108, 942–947. [Google Scholar] [CrossRef]
- Xiaobing, W.; Wentao, G.; Guangxiang, L.; Fan, Z.; Weidong, G.; Hongqian, G.; Gutian, Z. Comparison of radiofrequency ablation and partial nephrectomy for tumor in a solitary kidney. BMC Urol. 2017, 17, 79. [Google Scholar] [CrossRef]
- Campbell, S.C.; Novick, A.C.; Belldegrun, A.; Blute, M.L.; Chow, G.K.; Derweesh, I.H.; Faraday, M.M.; Kaouk, J.H.; Leveillee, R.J.; Matin, S.F.; et al. Guideline for management of the clinical T1 renal mass. J. Urol. 2009, 182, 1271–1279. [Google Scholar] [CrossRef]
- Ljungberg, B.; Cowan, N.C.; Hanbury, D.C.; Hora, M.; Kuczyk, M.A.; Merseburger, A.S.; Patard, J.J.; Mulders, P.F.; Sinescu, I.C.; European Association of Urology Guideline, G. EAU guidelines on renal cell carcinoma: The 2010 update. Eur. Urol. 2010, 58, 398–406. [Google Scholar] [CrossRef]
- Katsanos, K.; Mailli, L.; Krokidis, M.; McGrath, A.; Sabharwal, T.; Adam, A. Systematic review and meta-analysis of thermal ablation versus surgical nephrectomy for small renal tumours. Cardiovasc. Intervent. Radiol. 2014, 37, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Pantelidou, M.; Challacombe, B.; McGrath, A.; Brown, M.; Ilyas, S.; Katsanos, K.; Adam, A. Percutaneous Radiofrequency Ablation Versus Robotic-Assisted Partial Nephrectomy for the Treatment of Small Renal Cell Carcinoma. Cardiovasc. Intervent. Radiol. 2016, 39, 1595–1603. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.H.; Park, B.K.; Kim, C.K.; Choi, H.Y.; Lee, H.M. Comparison of percutaneous radiofrequency ablation and open partial nephrectomy for the treatment of size- and location-matched renal masses. Int. J. Hyperth. 2012, 28, 227–234. [Google Scholar] [CrossRef]
- Park, B.K.; Gong, I.H.; Kang, M.Y.; Sung, H.H.; Jeon, H.G.; Jeong, B.C.; Jeon, S.S.; Lee, H.M.; Seo, S.I. RFA versus robotic partial nephrectomy for T1a renal cell carcinoma: A propensity score-matched comparison of mid-term outcome. Eur. Radiol. 2018, 28, 2979–2985. [Google Scholar] [CrossRef]
- Park, S.Y.; Park, B.K.; Kim, C.K. Thermal ablation in renal cell carcinoma: What affects renal function? Int. J. Hyperth. 2012, 28, 729–734. [Google Scholar] [CrossRef]
- Olweny, E.O.; Park, S.K.; Tan, Y.K.; Best, S.L.; Trimmer, C.; Cadeddu, J.A. Radiofrequency ablation versus partial nephrectomy in patients with solitary clinical T1a renal cell carcinoma: Comparable oncologic outcomes at a minimum of 5 years of follow-up. Eur. Urol. 2012, 61, 1156–1161. [Google Scholar] [CrossRef]
- Pierorazio, P.M.; Johnson, M.H.; Patel, H.D.; Sozio, S.M.; Sharma, R.; Iyoha, E.; Bass, E.B.; Allaf, M.E. Management of Renal Masses and Localized Renal Cancer: Systematic Review and Meta-Analysis. J. Urol. 2016, 196, 989–999. [Google Scholar] [CrossRef]
- Khalifeh, A.; Kaouk, J.H.; Bhayani, S.; Rogers, C.; Stifelman, M.; Tanagho, Y.S.; Kumar, R.; Gorin, M.A.; Sivarajan, G.; Samarasekera, D.; et al. Positive surgical margins in robot-assisted partial nephrectomy: A multi-institutional analysis of oncologic outcomes (leave no tumor behind). J. Urol. 2013, 190, 1674–1679. [Google Scholar] [CrossRef]


| Variables | CKD Stage 2 (60 ≤ eGFR < 90) | CKD Stage 3 or Higher (eGFR < 60) | ||||
|---|---|---|---|---|---|---|
| PN (n = 1060) | RFA (n = 135) | p | PN (n = 84) | RFA (n = 53) | p | |
| Age, mean ± SD | 56.0 ± 11.1 | 63.0 ± 12.4 | <0.001 | 64.7 ± 9.7 | 66.5 ± 11.7 | 0.348 |
| Sex, male, n (%) | 803 (75.8) | 100 (74.1) | 0.669 | 62 (73.8) | 41 (77.4) | 0.640 |
| HTN, n (%) | 416 (39.2) | 62 (45.9) | 0.223 | 57 (67.9) | 43 (81.1) | 0.088 |
| DM, n (%) | 150 (14.2) | 22 (16.3) | 0.607 | 30 (35.7) | 18 (34.0) | 0.817 |
| Pre-treatment eGFR | 78.6 ± 7.6 | 75.9 ± 7.9 | <0.001 | 51.3 ± 9.3 | 33.2 ± 18.8 | <0.001 |
| Clinical tumor size (cm) | 2.37 ± 0.80 | 2.13 ± 0.83 | 0.001 | 2.52 ± 0.75 | 2.20 ± 0.85 | 0.022 |
| Tumor histology, n (%) | <0.001 | <0.001 | ||||
| Unknown (not biopsied) | 0 (0) | 47 (34.8) | 0 (0) | 19 (35.8) | ||
| Benign | 35 (3.3) | 12 (8.9) | 2 (2.4) | 9 (17.0) | ||
| Clear cell RCC | 845 (79.7) | 62 (45.9) | 69 (82.1) | 22 (41.5) | ||
| Non-clear cell RCC | 177 (16.7) | 14 (10.4) | 13 (15.5) | 3 (5.7) | ||
| Other type malignancy | 3 (0.3) | 0 (0) | 0 (0) | 0 (0) | ||
| Follow-up period (in months), mean ± SD | 52.4 ± 33.1 | 51.0 ± 36.0 | 0.649 | 52.4 ± 35.7 | 43.3 ± 39.0 | 0.166 |
| Variables | CKD Stage 2 (60 ≤ eGFR < 90) | CKD stage 3 or Higher (eGFR < 60) | ||||
|---|---|---|---|---|---|---|
| PN (n = 270) | RFA (n = 135) | p | PN (n = 53) | RFA (n = 53) | p | |
| Age, mean ± SD | 62.8 ± 10.9 | 63.0 ± 12.4 | 0.861 | 66.6 ± 8.8 | 66.5 ± 11.7 | 0.940 |
| Sex, male, n (%) | 195 (72.2) | 100 (74.1) | 0.693 | 38 (71.7) | 41 (77.4) | 0.504 |
| HTN, n (%) | 124 (45.9) | 62 (45.9) | 0.795 | 38 (71.7) | 43 (81.1) | 0.237 |
| DM, n (%) | 50 (18.5) | 22 (16.3) | 0.495 | 20 (37.7) | 18 (34.0) | 0.684 |
| Pre-treatment eGFR | 76.3 ± 8.2 | 75.9 ± 7.9 | 0.664 | 48.6 ± 10.6 | 33.2 ± 18.8 | <0.001 |
| Clinical tumor size (cm) | 2.19 ± 0.74 | 2.13 ± 0.83 | 0.513 | 2.27 ± 0.70 | 2.20 ± 0.85 | 0.668 |
| Tumor histology, n (%) | 0.001 | 0.002 | ||||
| Unknown (not biopsied) | 0 (0) | 47 (34.8) | 0 (0) | 19 (35.8) | ||
| Benign | 8 (3.0) | 12 (8.9) | 2 (3.8) | 9 (17.0) | ||
| Clear cell RCC | 213 (78.9) | 62 (45.9) | 46 (86.8) | 22 (41.5) | ||
| Non-clear cell RCC | 49 (18.1) | 14 (10.4) | 5 (9.4) | 3 (5.7) | ||
| Follow-up period (in months), mean ± SD | 51.4 ± 34.2 | 51.0 ± 36.0 | 0.914 | 54.3 ± 40.3 | 43.3 ± 39.0 | 0.158 |
| Variables | CKD Stage 2 (60 ≤ eGFR < 90) All Patients | CKD stage 2 (60 ≤ eGFR < 90) Propensity Score Matched Cohorts | ||||
|---|---|---|---|---|---|---|
| PN (n = 1060) | RFA (n = 135) | p | PN (n = 270) | RFA (n = 135) | p | |
| eGFR Pre-treatment | 78.6 ± 7.6 | 75.9 ± 7.9 | <0.001 | 76.3 ± 8.2 | 75.9 ± 7.9 | 0.668 |
| eGFR at 1 year post-treatment | 58.8 ± 32.9 | 49.6 ± 34.8 | 0.002 | 56.4 ± 31.7 | 49.6 ± 34.8 | 0.057 |
| % Change eGFR at 1 year post-treatment | −4.3 ± 14.4 | −5.5 ± 15.7 | 0.458 | −6.1 ± 14.1 | −5.5 ± 15.7 | 0.713 |
| CKD upstaging at 1 year post-treatment, n, (%) | 85 (8.0) | 19 (14.1) | 0.019 | 35 (13.0) | 19 (14.1) | 0.756 |
| Upstaging to stage 3 | 84 (7.9) | 19 (14.1) | 35 (13.0) | 19 (14.1) | ||
| Upstaging to stage 4 | 1 (0.1) | 0 (0.0) | 0 (0) | 0 (0.0) | ||
| Upstaging to stage 5 | 0 (0) | 0 (0.0) | 0 (0) | 0 (0.0) | ||
| Variables | CKD Stage 3 or Higher (eGFR < 60) | ||
|---|---|---|---|
| PN (n = 84) | RFA (n = 53) | p | |
| eGFR Pre-treatment | 51.3 ± 9.3 | 33.2 ± 18.8 | <0.001 |
| eGFR at 1 year post-treatment | 38.3 ± 23.6 | 23.1 ± 23.1 | <0.001 |
| % Change eGFR at 1 year post-treatment | −5.4 ± 17.2 | −12.2 ± 23.4 | 0.132 |
| eGFR at 2 years post-treatment | 40.2 ± 22.6 | 25.0 ± 24.9 | <0.001 |
| % Change eGFR at 2 years post-treatment | −8.0 ± 24.3 | −16.8 ± 27.6 | 0.093 |
| eGFR at 3 years post-treatment | 48.5 ± 16.8 | 32.5 ± 22.5 | <0.001 |
| % Change eGFR at 3 years post-treatment | −7.3 ± 27.9 | −22.0 ± 28.4 | 0.012 |
| CKD upstaging at 3 years post-treatment, n, (%) | 6 (7.1) | 9 (17.0) | 0.072 |
| Upstaging to stage 4 | 2 (2.4) | 3 (5.7) | |
| Upstaging to stage 5 | 4 (4.7) | 6 (11.3) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ryoo, H.; Kang, M.; Sung, H.H.; Jeon, H.G.; Jeong, B.C.; Jeon, S.S.; Lee, H.M.; Park, B.K.; Seo, S.I. A Comparison of Functional and Oncologic Outcomes between Partial Nephrectomy and Radiofrequency Ablation in Patients with Chronic Kidney Disease after Propensity Score Matching. Diagnostics 2022, 12, 2292. https://doi.org/10.3390/diagnostics12102292
Ryoo H, Kang M, Sung HH, Jeon HG, Jeong BC, Jeon SS, Lee HM, Park BK, Seo SI. A Comparison of Functional and Oncologic Outcomes between Partial Nephrectomy and Radiofrequency Ablation in Patients with Chronic Kidney Disease after Propensity Score Matching. Diagnostics. 2022; 12(10):2292. https://doi.org/10.3390/diagnostics12102292
Chicago/Turabian StyleRyoo, Hyunsoo, Minyong Kang, Hyun Hwan Sung, Hwang Gyun Jeon, Byong Chang Jeong, Seong Soo Jeon, Hyun Moo Lee, Byung Kwan Park, and Seong Il Seo. 2022. "A Comparison of Functional and Oncologic Outcomes between Partial Nephrectomy and Radiofrequency Ablation in Patients with Chronic Kidney Disease after Propensity Score Matching" Diagnostics 12, no. 10: 2292. https://doi.org/10.3390/diagnostics12102292
APA StyleRyoo, H., Kang, M., Sung, H. H., Jeon, H. G., Jeong, B. C., Jeon, S. S., Lee, H. M., Park, B. K., & Seo, S. I. (2022). A Comparison of Functional and Oncologic Outcomes between Partial Nephrectomy and Radiofrequency Ablation in Patients with Chronic Kidney Disease after Propensity Score Matching. Diagnostics, 12(10), 2292. https://doi.org/10.3390/diagnostics12102292






