Abstract
The role of CD47 expression as a ‘do not eat me’ signal that inhibits phagocytosis of tumor cells by macrophages is well established. Immune checkpoint therapy that targets CD47 has been successful in preclinical trials and is currently undergoing clinical investigation for various human malignancies. Here, the clinicopathological correlation with CD47 expression in clear cell renal cell carcinoma (ccRCC) was explored. CD47 expression was evaluated by immunohistochemical staining in tissue microarray sections of 235 ccRCC tissues. CD47 expression was observed in 28 (11.9%) of 235 ccRCC tissues and was significantly associated with higher WHO/ISUP grade (p = 0.001), frequent lymphovascular invasion (p = 0.036), frequent renal vein thrombus (p = 0.018), frequent sinus fat invasion (p = 0.004), frequent sarcomatous change (p = 0.001), higher pT stage (p = 0.002), higher pN stage (p = 0.002), higher pM stage (p < 0.001), and advanced American Joint Committee on Cancer stage (p = 0.002). In the survival analyses, positive CD47 expression was associated with cancer-specific survival (p = 0.003). However, positive CD47 expression was not associated with recurrence-free survival. In conclusion, CD47 expression was associated with adverse clinicopathological parameters and cancer-specific survival in patients with ccRCC.
1. Introduction
Renal cell carcinoma (RCC) is the most common cancer of the kidney and represents 2% of cancer diagnoses and deaths worldwide [1]. RCC ranks 6th in men and 10th in women among cancers globally, and it is estimated to be the 9th most common cancer in both sexes in Korea [2]. Although rates are low, the incidence has doubled in developed countries over the past half century [3]. Despite the improvement in diagnosis and therapeutic approaches for RCC during the recent two decades, it remains one of the most lethal malignancies of the urinary system [4].
Clear cell renal cell carcinoma (ccRCC) is the most common histologic subtype of RCC and accounts for more than 80% of all cases [5]. Most available treatments focus on ccRCC and RCCs with non-clear cell histologic subtypes do not have a similarly refined treatment paradigm [6]. Partial or radical nephrectomy is the first-line treatment for localized ccRCC, and systemic therapy is a recommended treatment option for advanced ccRCC or metastatic ccRCC [7,8]. The role of adjuvant systemic therapy for patients with stage I to III has been limited, although the U.S. Food and Drug Administration has approved adjuvant therapy for localized ccRCC [7]. Broadly, current systemic treatment for ccRCC is divided into targeted therapies and immunotherapies [9]. The first agent approved in the targeted therapy category was sorafenib, which targets vascular endothelial growth factor receptor (VEGFR), rapidly accelerated fibrosarcoma, and other growth factors [6]. Subsequently, similar agents including sunitinib, pazopanib, axitinib, and bevacizumab were approved [10]. Currently available immunotherapies include agents targeting the cancer immune system via inhibition of immune evasion or increase in T-lymphocyte priming [9]. Three programmed cell death protein 1 and programmed death-ligand 1 inhibitors (nivolumab, pembrolizumab, and avelumab) and one cytotoxic T-lymphocyte–associated protein 4 inhibitor (ipilimumab) have been approved [7]. However, ccRCC patients show an inconsistent prognosis and limited responses to the aforementioned treatments [11]. Therefore, the discovery of a new biomarker that better predicts ccRCC prognosis and can be a candidate for a therapeutic target is needed.
CD47 is a transmembrane protein that is ubiquitously expressed on the cell surface and sends a “do not eat me” signal to phagocytes, acting as an immune checkpoint [12]. CD47 initiates the signaling cascade by interacting with signal regulatory protein-α (SIRPα) expressed on macrophages [13]. This interaction activates tyrosine phosphatases SHP-1 and SHP-2 and ultimately prevents engulfment, potentially by inhibiting the accumulation of myosin-IIA at the cell membrane [13,14]. CD47 overexpression has been reported in various human malignancies and generally heralds poor prognosis [15]. As a therapeutic target, anti-CD47 antibody has shown promising results in diffuse large B-cell lymphoma, pediatric malignant brain tumor, ovarian cancer, hepatocellular carcinoma, and lung cancer [16,17,18,19,20]. Immune checkpoint agents targeting CD47 have demonstrated success in preclinical investigations and are now under clinical trials for various types of human malignancies [21]. One anti-CD47 antibody, magrolimab, is at the forefront of clinical development, and its combination treatment has been well tolerated in phase Ib/II studies with encouraging response rates [22]. However, CD47 expression in ccRCC has been rarely reported and its correlation with various clinicopathological parameters has not been well characterized [23].
To reveal the clinicopathological significance of CD47 expression in human ccRCC, CD47 protein expression was evaluated in 235 ccRCC tissue samples. Furthermore, to reveal the prognostic role of CD47 expression, survival analyses were performed.
2. Materials and Methods
2.1. Patients and Tumor Samples
A total of 241 patients diagnosed with ccRCC and who received partial or radical nephrectomy at Hanyang University Hospital (Seoul, Korea) between 2001 and 2017 was enrolled in this cohort. Patients without qualified tissue paraffin blocks were excluded, finally leaving 235 patients. Medical records and hematoxylin and eosin (H&E)-stained slides were reviewed. The 8th AJCC TNM staging system and a protocol for examining specimens from patients with invasive carcinoma of renal tubular origin (College of American Pathologists) were used to determine the pathological staging and other pathological characteristics [24,25]. Pathological parameters included tumor size, World Health Organization/International Society of Urological Pathology (WHO/ISUP) grade, lymphovascular invasion, renal vein tumor thrombus, sinus fat invasion, perirenal soft tissue invasion, tumor necrosis, sarcomatoid change, pathological T (pT) stage, pathological N (pN) stage, pathological M (pM) stage, and American Joint Committee on Cancer (AJCC) stage. Cancer-specific survival (CSS) was defined as the duration from as the date of surgery until the date of death due to the ccRCC. Recurrence-free survival (RFS) was defined as the duration from the date of surgery until the date of disease progression, relapse, or death from ccRCC.
2.2. Tissue Microarray (TMA) Construction
A manual tissue microarrayer (Unitma, Seoul, Korea) was used to construct TMAs from archival formalin-fixed, paraffin-embedded tissue blocks. The H&E-stained slides were reviewed to select the most representative area of carcinoma without necrosis and hemorrhage. A 2 mm tissue cylinder was punched from the previously marked area on each donor paraffin block and transplanted into the recipient block (Unitma). Each TMA comprised 5 × 6 samples.
2.3. Immunohistochemical Staining
CD47 immunohistochemical staining was performed with 4 µm thick sectioned slides from TMA blocks. The Ventana BenchMark XT autostainer (Ventana Medical Systems, Tucson, AZ, USA) was used according to the manufacturer’s protocol. Anti-CD47 monoclonal antibody (1:200 dilution, EPR21794; Abcam, Cambridge, UK) was used to detect CD47 expression.
2.4. Interpretation of Immunohistochemical Staining
The tumor cells with membranous staining was evaluated by the H-score method. The staining intensity was scored by a 4-tier system (0: negative, 1+: weak, 2+: moderate, 3+: strong), and the percentage of stained tumor cells was evaluated. Figure 1 shows representative microphotographs stratified by staining intensity. The H-score was determined by the following calculation method: H-score = % of weakly stained cells (1+) + 2 × % of moderately stained cells (2+) + 3 × % of strongly stained cells (3+). Positive CD47 expression was defined as H-score ≥ 5 (≥5% tumor cell membrane staining) according to reference studies [26,27]. All immunohistochemical assessments were blinded to the clinicopathological parameters and patient survival.
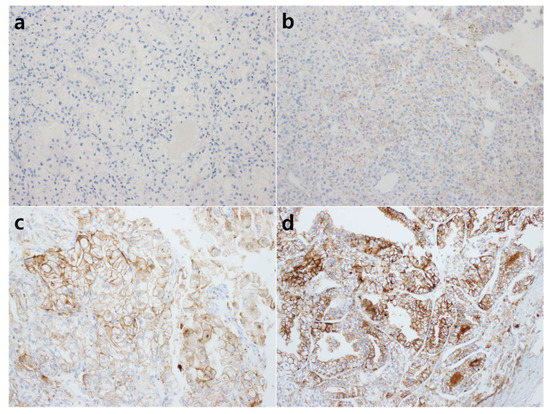
Figure 1.
Representative microphotographs of CD47 immunohistochemical staining. The intensity of nuclear staining was determined as 0: negative (a); 1+: weak (b); 2+: moderate (c); 3+: strong (d) ((a–d), original magnification ×200).
2.5. Statistical Analysis
Statistical analysis was performed with SPSS software version 21 (IBM Corp., Armonk, NY, USA). The correlation between CD47 expression and clinicopathological factors including sex, tumor size, WHO/ISUP grade, lymphovascular invasion, renal vein tumor thrombus, sinus fat invasion, perirenal soft tissue invasion, tumor necrosis, sarcomatoid change, pT stage, pN stage, pM stage, and AJCC stage was assessed using the chi-square test. The survival analyses were performed using the Kaplan–Meier method with a log-rank test and the Cox proportional hazard regression model. A p-value less than 0.05 was considered as statistically significant.
3. Results
3.1. Patient Characteristics
The patient characteristics are summarized in Table 1. The median patient age was 57 years (13–84 years), and the male to female ratio was 2.3:1. According to pathological evaluation, the mean tumor size was 4.14 cm (±2.69). In histologic grade, 25 cases (10.6%) were WHO/ISUP grade 1, 118 (50.2%) were WHO/ISUP grade 2, 75 (31.9%) were WHO/ISUP grade 3, and 17 (7.2%) were WHO/ISUP grade 4. In the AJCC staging system, 170 cases (72.3%) were pT1, 10 (4.3%) were pT2, 52 (22.1%) were pT3, and 3 (1.3%) were pT4.

Table 1.
Baseline characteristics of the patients (n = 235).
3.2. Correlations between CD47 Expression and Clinicopathological Parameters
Positive CD47 expression was observed in 28 (11.9%) of 235 ccRCC tissues. Positive CD47 expression was significantly associated with higher WHO/ISUP histologic grade (p = 0.001), frequent lymphovascular invasion (p = 0.036), frequent renal vein thrombus (p = 0.018), frequent sinus fat invasion (p = 0.004), frequent sarcomatoid change (p = 0.001), higher pT stage (p = 0.002), higher pN stage (p = 0.002), higher pM stage (p < 0.001), and higher AJCC stage (p = 0.002) (Table 2).

Table 2.
Correlation between CD47 expression and clinicopathologic factors in clear cell renal cell carcinoma.
3.3. Prognostic Role of CD47 Expression in ccRCC
Of the total 235 patients, 18 died of ccRCC and 15 experienced cancer recurrence. Patients with positive CD47 expression had a poorer prognosis for cancer-specific survival (CSS) than patients with negative CD47 expression (p = 0.003) (Figure 2a). However, there was no significant difference between the positive and negative CD47 expression groups for recurrence-free survival (p = 0.827) (Figure 2b). Univariable Cox regression analysis demonstrated that positive CD47 expression was a poor prognostic factor (p = 0.006) along with other known pathological parameters of WHO/ISUP grade, lymphovascular invasion, sinus fat invasion, sarcomatoid invasion, and pathologic TNM stage in CSS (Table 3). However, positive CD47 expression was not an independent prognostic factor in the multivariable analysis (Table 3).
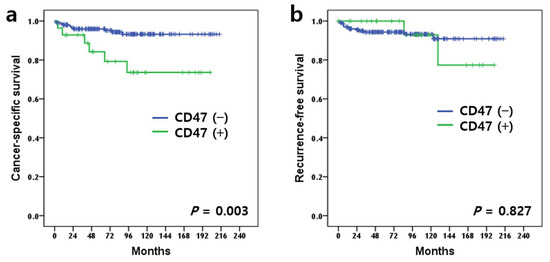
Figure 2.
Kaplan–Meier survival curves stratified by CD47 expression. Cancer-specific survival (a) (p = 0.003, log-rank test) and recurrence-free survival (b) (p = 0.827, log-rank test).

Table 3.
Univariable and multivariable Cox regression analysis of pathological parameters for cancer-specific survival in clear cell renal cell carcinoma. (n = 235).
4. Discussion
CD47, a highly glycosylated protein, normally acts as a “do not eat me” signal in healthy cells to prevent phagocytosis by macrophages, whereas CD47 is downregulated in redundant cells, promoting macrophages to clear them [12]. CD47 is also overexpressed in various types of cancer cells to avoid the innate immune system [12]. CD47 overexpression has been reported in various human malignancies including non-Hodgkin lymphoma, stomach cancer, oral squamous cell carcinoma, lung cancer, breast cancer, and hepatocellular carcinoma and has been correlated with adverse prognosis [28,29,30,31,32,33]. After the discovery of its role in tumor evasion of immune surveillance, CD47 has become a novel biomarker for cancer immunotherapy, and several anti-CD47 antibodies have been studied, including B6H12, Hu5F9-G4, and ZF1 [34,35,36]. Immune checkpoint agents targeting CD47 have demonstrated success in preclinical investigations and are now under clinical trials [21]. However, CD47, as a prognostic biomarker and potential therapeutic target, lacks research focusing on its significance in ccRCC.
To date, there are two studies investigating CD47 expression in RCC and ccRCC, a subtype of RCC. In Gazel et al., the intensity of CD47 immunohistochemical staining in RCC was analyzed and correlated with histopathological features [37]. A significant correlation was not found between CD47 expression and tumor size, capsular invasion, vascular invasion, or distant metastasis [37]. However, strong CD47 expression was frequently identified in patient groups with non-clear cell RCC subtypes, higher Fuhrman nuclear grades, lymph node metastasis, and advanced stages [37]. We additionally subdivided the patients into higher and lower CD47 expression using intensity scores. Higher CD47 expression tends to correlate with aggressive phenotypes; however, the number of higher expression group was too small to be statistically significant. Establishing a standard cut-off for CD47 expression requires additional studies using different cut-off values and validation studies in clinical trials. Jiang et al. explored the significance of CD47 expression in ccRCC and correlated it with CD8+ T lymphocyte infiltration, molecular features, and response to combination therapy [23]. The patient group with high CD47 expression was prone to dismal prognosis in both overall survival and recurrence-free survival and was more likely to be classified as ccB molecular subtype [23]. Additionally, high CD47 expression correlated with an immunosuppressed tumor microenvironment of exhausted CD8+ T cells [23]. Interestingly, high CD47 expression was associated with improved response to VEGFR tyrosine kinase inhibitor + immune checkpoint inhibitor combination therapies, and the authors suggested CD47 as a potential target for immunotherapy in ccRCC [23].
In this study, positive CD47 expression was identified in a minority (11.9%) of ccRCC samples. It correlated with aggressive pathological phenotypes, including higher histologic grade, the presence of lymphovascular invasion, renal vein thrombus, sinus fat invasion, frequent sarcomatoid change, higher pathological stages, and poor patient survival. These findings suggest that CD47 protein expression in ccRCC demonstrates an adverse role in tumor immunity and prognosis, consistent with the results from previous studies. The aggressive phenotypes attributes to the fact that CD47 promotes tumor cell growth and motility [38,39]. A preclinical study demonstrated that the downregulation of CD47 inhibits the growth and metastasis of human cancer cells [40]. Recent clinical data revealed that anti-CD47 antibody induced encouraging anti-cancer effects, suggesting that CD47 is also a potential therapeutic target in ccRCC [22]. However, several limitations exist in this study. First, we performed a retrospective analysis of limited ethnicity (mostly Korean) in a single medical center. Second, a detailed mechanism of CD47 expression involving tumor immune evasion in ccRCC has not been mentioned. We also applied only a single marker, which requires other biomarkers relevant to the tumor immune environment, such as macrophages and anti-tumor T cells [41]. Further molecular study is needed to identify the cancer-specific immune evasion mechanism of CD47 in ccRCC. Third, the therapeutic benefit of CD47 expression according to type of adjuvant therapy has not been determined because patients enrolled in this study did not receive immunotherapy or targeted therapy. A large-scale cohort study that can predict response to current treatment according to CD47 expression is needed.
5. Conclusions
CD47 expression was investigated in 235 ccRCC patients. A positive CD47 expression was identified in 11.9% of ccRCC tissues and was significantly correlated with aggressive phenotypes and poor patient survival. The expression of CD47 in ccRCC is deemed a valuable therapeutic target for immunotherapy.
Author Contributions
Conceptualization, S.P. and H.K.; Data curation, H.P., J.S. and Y.K.; Formal analysis, H.P.; Funding acquisition, H.K.; Investigation, H.P., S.J., S.B., H.S. and H.C.; Writing—original draft, H.P. and H.K.; Writing—review and editing, J.M. and S.P. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by a research fund of Hanyang University (HY-202200000001036).
Institutional Review Board Statement
This study was approved by the Institutional Review Board of Hanyang University Hospital, and the requirement for informed consent was waived (HYUH 2018-05-005, approved on 22 May 2018).
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.W.; Won, Y.J.; Kong, H.J.; Lee, E.S. Prediction of Cancer Incidence and Mortality in Korea, 2018. Cancer Res. Treat. 2018, 50, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Padala, S.A.; Barsouk, A.; Thandra, K.C.; Saginala, K.; Mohammed, A.; Vakiti, A.; Rawla, P.; Barsouk, A. Epidemiology of Renal Cell Carcinoma. World J. Oncol. 2020, 11, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Capitanio, U.; Bensalah, K.; Bex, A.; Boorjian, S.A.; Bray, F.; Coleman, J.; Gore, J.L.; Sun, M.; Wood, C.; Russo, P. Epidemiology of Renal Cell Carcinoma. Eur. Urol. 2019, 75, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Ljungberg, B.; Bensalah, K.; Canfield, S.; Dabestani, S.; Hofmann, F.; Hora, M.; Kuczyk, M.A.; Lam, T.; Marconi, L.; Merseburger, A.S.; et al. EAU guidelines on renal cell carcinoma: 2014 update. Eur. Urol. 2015, 67, 913–924. [Google Scholar] [CrossRef]
- Dizman, N.; Arslan, Z.E.; Feng, M.; Pal, S.K. Sequencing Therapies for Metastatic Renal Cell Carcinoma. Urol. Clin. N. Am. 2020, 47, 305–318. [Google Scholar] [CrossRef]
- Motzer, R.J.; Jonasch, E.; Agarwal, N.; Alva, A.; Baine, M.; Beckermann, K.; Carlo, M.I.; Choueiri, T.K.; Costello, B.A.; Derweesh, I.H.; et al. Kidney Cancer, Version 3.2022, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2022, 20, 71–90. [Google Scholar] [CrossRef]
- Escudier, B.; Porta, C.; Schmidinger, M.; Rioux-Leclercq, N.; Bex, A.; Khoo, V.; Grünwald, V.; Gillessen, S.; Horwich, A. Renal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up†. Ann. Oncol. 2019, 30, 706–720. [Google Scholar] [CrossRef]
- Castro, D.V.; Malhotra, J.; Meza, L.; Govindarajan, A.; Philip, E.J.; Pal, S.K. How to Treat Renal Cell Carcinoma: The Current Treatment Landscape and Cardiovascular Toxicities. JACC Cardio Oncol. 2022, 4, 271–275. [Google Scholar] [CrossRef]
- Comandone, A.; Vana, F.; Comandone, T.; Tucci, M. Antiangiogenic Therapy in Clear Cell Renal Carcinoma (CCRC): Pharmacological Basis and Clinical Results. Cancers 2021, 13, 5896. [Google Scholar] [CrossRef]
- Xu, W.; Atkins, M.B.; McDermott, D.F. Checkpoint inhibitor immunotherapy in kidney cancer. Nat. Rev. Urol. 2020, 17, 137–150. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Liu, F.; Li, C.; Liang, X.; Li, C.; Liu, Y.; Yi, Z.; Zhang, L.; Fu, S.; Zeng, Y. Role of CD47 in tumor immunity: A potential target for combination therapy. Sci. Rep. 2022, 12, 9803. [Google Scholar] [CrossRef] [PubMed]
- Logtenberg, M.E.W.; Scheeren, F.A.; Schumacher, T.N. The CD47-SIRPα Immune Checkpoint. Immunity 2020, 52, 742–752. [Google Scholar] [CrossRef]
- Tsai, R.K.; Discher, D.E. Inhibition of “self” engulfment through deactivation of myosin-II at the phagocytic synapse between human cells. J. Cell Biol. 2008, 180, 989–1003. [Google Scholar] [CrossRef] [PubMed]
- Takimoto, C.H.; Chao, M.P.; Gibbs, C.; McCamish, M.A.; Liu, J.; Chen, J.Y.; Majeti, R.; Weissman, I.L. The Macrophage ‘Do not eat me’ signal, CD47, is a clinically validated cancer immunotherapy target. Ann. Oncol. 2019, 30, 486–489. [Google Scholar] [CrossRef] [PubMed]
- Sikic, B.I.; Lakhani, N.; Patnaik, A.; Shah, S.A.; Chandana, S.R.; Rasco, D.; Colevas, A.D.; O’Rourke, T.; Narayanan, S.; Papadopoulos, K.; et al. First-in-Human, First-in-Class Phase I Trial of the Anti-CD47 Antibody Hu5F9-G4 in Patients with Advanced Cancers. J. Clin. Oncol. 2019, 37, 946–953. [Google Scholar] [CrossRef] [PubMed]
- Gholamin, S.; Mitra, S.S.; Feroze, A.H.; Liu, J.; Kahn, S.A.; Zhang, M.; Esparza, R.; Richard, C.; Ramaswamy, V.; Remke, M.; et al. Disrupting the CD47-SIRPα anti-phagocytic axis by a humanized anti-CD47 antibody is an efficacious treatment for malignant pediatric brain tumors. Sci. Transl. Med. 2017, 9, eaaf2968. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Xu, B.; Teng, K.Y.; Song, M.; Zhu, Z.; Chen, Y.; Wang, J.; Zhang, J.; Feng, M.; Kaur, B.; et al. Targeting Fc Receptor-Mediated Effects and the “Don’t Eat Me” Signal with an Oncolytic Virus Expressing an Anti-CD47 Antibody to Treat Metastatic Ovarian Cancer. Clin. Cancer Res. 2022, 28, 201–214. [Google Scholar] [CrossRef]
- Lo, J.; Lau, E.Y.; So, F.T.; Lu, P.; Chan, V.S.; Cheung, V.C.; Ching, R.H.; Cheng, B.Y.; Ma, M.K.; Ng, I.O.; et al. Anti-CD47 antibody suppresses tumour growth and augments the effect of chemotherapy treatment in hepatocellular carcinoma. Liver Int. 2016, 36, 737–745. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, L.; Yang, L.; Li, H.; Li, R.; Yu, J.; Yang, L.; Wei, F.; Yan, C.; Sun, Q.; et al. Anti-CD47 Antibody as a Targeted Therapeutic Agent for Human Lung Cancer and Cancer Stem Cells. Front. Immunol. 2017, 8, 404. [Google Scholar] [CrossRef]
- Maute, R.; Xu, J.; Weissman, I.L. CD47-SIRPα-targeted therapeutics: Status and prospects. Immuno-Oncol. Technol. 2022, 13, 100070. [Google Scholar] [CrossRef] [PubMed]
- Advani, R.; Flinn, I.; Popplewell, L.; Forero, A.; Bartlett, N.L.; Ghosh, N.; Kline, J.; Roschewski, M.; LaCasce, A.; Collins, G.P.; et al. CD47 Blockade by Hu5F9-G4 and Rituximab in Non-Hodgkin’s Lymphoma. N. Engl. J. Med. 2018, 379, 1711–1721. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Zeng, H.; Liu, Z.; Jin, K.; Hu, B.; Chang, Y.; Liu, L.; Zhu, Y.; Xu, L.; Wang, Z.; et al. Immune inactivation by CD47 expression predicts clinical outcomes and therapeutic responses in clear cell renal cell carcinoma patients. Urol. Oncol. 2022, 40, 166.e115–166.e125. [Google Scholar] [CrossRef] [PubMed]
- Srigley, J.R.; Amin, M.B.; Delahunt, B.; Campbell, S.C.; Chang, A.; Grignon, D.J.; Humphrey, P.A.; Leibovich, B.C.; Montironi, R.; Renshaw, A.A.; et al. Protocol for the examination of specimens from patients with invasive carcinoma of renal tubular origin. Arch. Pathol. Lab. Med. 2010, 134, e25–e30. [Google Scholar] [CrossRef]
- Amin, M.B.; Edge, S.B.; Greene, F.L.; Byrd, D.R.; Brookland, R.K.; Washington, M.K.; Gershenwald, J.E.; Compton, C.C.; Hess, K.R.; Sullivan, D.C. AJCC Cancer Staging Manual; Springer International Publishing: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Choueiri, T.K.; Fay, A.P.; Gray, K.P.; Callea, M.; Ho, T.H.; Albiges, L.; Bellmunt, J.; Song, J.; Carvo, I.; Lampron, M.; et al. PD-L1 expression in nonclear-cell renal cell carcinoma. Ann. Oncol. 2014, 25, 2178–2184. [Google Scholar] [CrossRef]
- Xiao, W.J.; Xu, F.J.; Zhang, X.; Zhou, S.X.; Zhang, H.L.; Dai, B.; Zhu, Y.; Shi, G.H.; Shen, Y.J.; Zhu, Y.P.; et al. The Prognostic Value of Programmed Death-Ligand 1 in a Chinese Cohort with Clear Cell Renal Cell Carcinoma. Front. Oncol. 2019, 9, 879. [Google Scholar] [CrossRef]
- Chao, M.P.; Tang, C.; Pachynski, R.K.; Chin, R.; Majeti, R.; Weissman, I.L. Extranodal dissemination of non-Hodgkin lymphoma requires CD47 and is inhibited by anti-CD47 antibody therapy. Blood 2011, 118, 4890–4901. [Google Scholar] [CrossRef]
- Yoshida, K.; Tsujimoto, H.; Matsumura, K.; Kinoshita, M.; Takahata, R.; Matsumoto, Y.; Hiraki, S.; Ono, S.; Seki, S.; Yamamoto, J.; et al. CD47 is an adverse prognostic factor and a therapeutic target in gastric cancer. Cancer Med. 2015, 4, 1322–1333. [Google Scholar] [CrossRef]
- Sakakura, K.; Takahashi, H.; Kaira, K.; Toyoda, M.; Murata, T.; Ohnishi, H.; Oyama, T.; Chikamatsu, K. Relationship between tumor-associated macrophage subsets and CD47 expression in squamous cell carcinoma of the head and neck in the tumor microenvironment. Lab. Investig. 2016, 96, 994–1003. [Google Scholar] [CrossRef]
- Barrera, L.; Montes-Servín, E.; Hernandez-Martinez, J.M.; García-Vicente, M.; Montes-Servín, E.; Herrera-Martínez, M.; Crispín, J.C.; Borbolla-Escoboza, J.R.; Arrieta, O. CD47 overexpression is associated with decreased neutrophil apoptosis/phagocytosis and poor prognosis in non-small-cell lung cancer patients. Br. J. Cancer 2017, 117, 385–397. [Google Scholar] [CrossRef]
- Yuan, J.; Shi, X.; Chen, C.; He, H.; Liu, L.; Wu, J.; Yan, H. High expression of CD47 in triple negative breast cancer is associated with epithelial-mesenchymal transition and poor prognosis. Oncol. Lett. 2019, 18, 3249–3255. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Bang, S.; Jee, S.; Paik, S.S.; Jang, K. Clinicopathological significance of CD47 expression in hepatocellular carcinoma. J. Clin. Pathol. 2021, 74, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Wang, J.; Willingham, S.B.; Martin, R.; Wernig, G.; Weissman, I.L. Anti-CD47 antibodies promote phagocytosis and inhibit the growth of human myeloma cells. Leukemia 2012, 26, 2538–2545. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, L.; Zhao, F.; Tseng, S.; Narayanan, C.; Shura, L.; Willingham, S.; Howard, M.; Prohaska, S.; Volkmer, J.; et al. Pre-Clinical Development of a Humanized Anti-CD47 Antibody with Anti-Cancer Therapeutic Potential. PLoS ONE 2015, 10, e0137345. [Google Scholar] [CrossRef] [PubMed]
- Zeng, D.; Sun, Q.; Chen, A.; Fan, J.; Yang, X.; Xu, L.; Du, P.; Qiu, W.; Zhang, W.; Wang, S.; et al. A fully human anti-CD47 blocking antibody with therapeutic potential for cancer. Oncotarget 2016, 7, 83040–83050. [Google Scholar] [CrossRef]
- Gazel, E.; Kaya, E.; Turhan, N.; Yığman, Y.M.; Şirin, M.E.; Ceylan, C.; Odabaş, O. Does Immunohistochemical CD47 Staining Intensity Predict Prognozis of Renal Cell Carcinoma. Mathews J. Urol. Nephrol. 2018, 2, 007. [Google Scholar]
- Zhao, H.; Wang, J.; Kong, X.; Li, E.; Liu, Y.; Du, X.; Kang, Z.; Tang, Y.; Kuang, Y.; Yang, Z.; et al. CD47 Promotes Tumor Invasion and Metastasis in Non-small Cell Lung Cancer. Sci. Rep. 2016, 6, 29719. [Google Scholar] [CrossRef]
- Wang, C.L.; Lin, M.J.; Hsu, C.Y.; Lin, H.Y.; Tsai, H.P.; Long, C.Y.; Tsai, E.M.; Hsieh, T.H.; Wu, C.H. CD47 promotes cell growth and motility in epithelial ovarian cancer. Biomed. Pharmacother. 2019, 119, 109105. [Google Scholar] [CrossRef]
- Willingham, S.B.; Volkmer, J.P.; Gentles, A.J.; Sahoo, D.; Dalerba, P.; Mitra, S.S.; Wang, J.; Contreras-Trujillo, H.; Martin, R.; Cohen, J.D.; et al. The CD47-signal regulatory protein alpha (SIRPa) interaction is a therapeutic target for human solid tumors. Proc. Natl. Acad. Sci. USA 2012, 109, 6662–6667. [Google Scholar] [CrossRef]
- Tseng, D.; Volkmer, J.P.; Willingham, S.B.; Contreras-Trujillo, H.; Fathman, J.W.; Fernhoff, N.B.; Seita, J.; Inlay, M.A.; Weiskopf, K.; Miyanishi, M.; et al. Anti-CD47 antibody-mediated phagocytosis of cancer by macrophages primes an effective antitumor T-cell response. Proc. Natl. Acad. Sci. USA 2013, 110, 11103–11108. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).