Diagnostic Value of Inflammatory Markers in Elderly Arab Women with Sarcopenia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
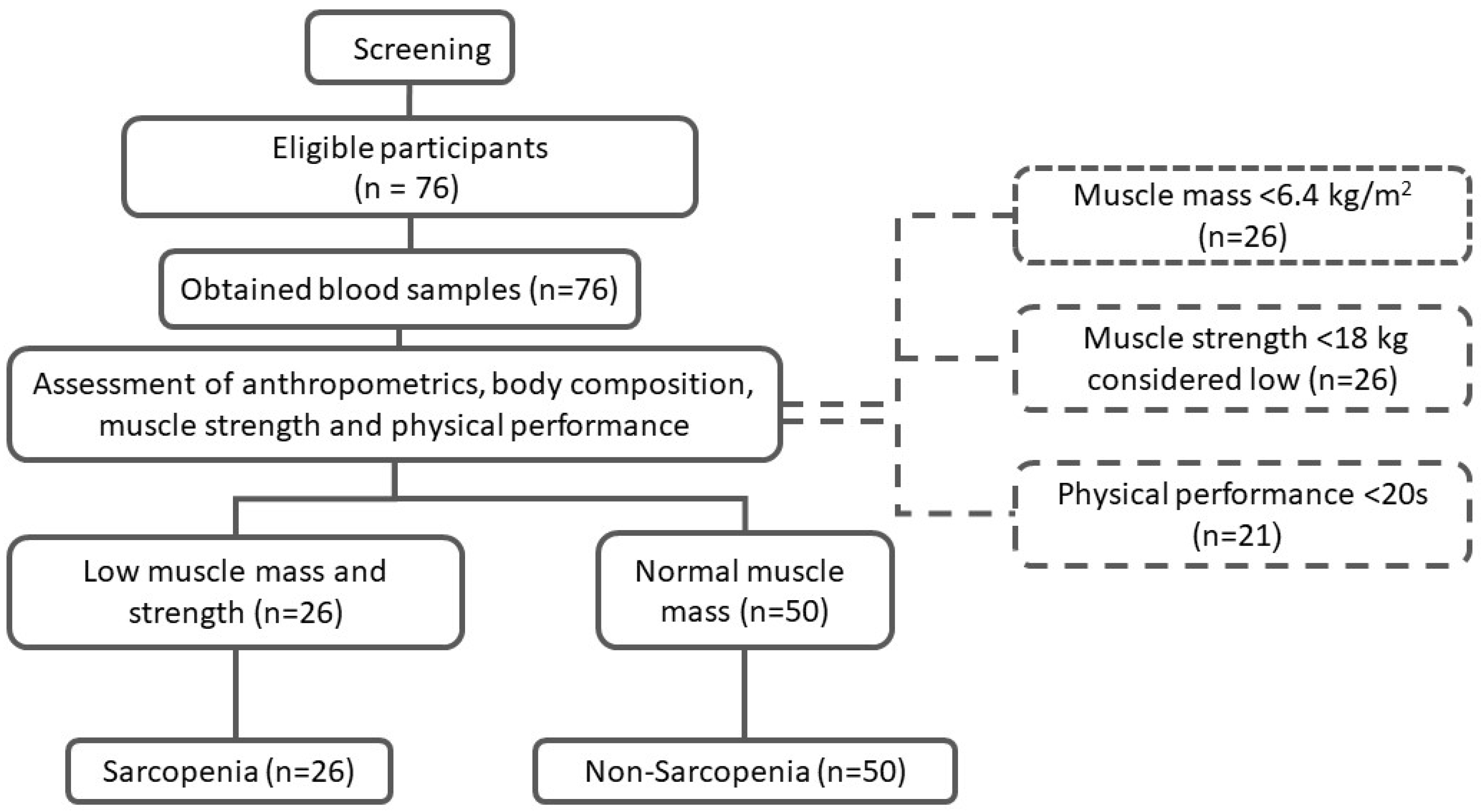
2.2. Study Participants
2.3. Demographic Data and Medical Information
2.4. Anthropometric Measurements
2.5. Muscle Mass Quantification
2.6. Muscle Strength
2.7. Physical Performance
2.8. Biochemical Analyses
2.9. Sample Size Calculation and Data Analyses
3. Results
3.1. Clinical Characteristics of Participants
3.2. Correlation between Inflammatory Markers and Study Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cruz-Jentoft, A.J.; Landi, F.; Schneider, S.M.; Zúñiga, C.; Arai, H.; Boirie, Y.; Chen, L.K.; Fielding, R.A.; Martin, F.C.; Michel, J.P.; et al. Prevalence of and interventions for sarcopenia in aging adults: A systematic review. Report of the International Sarcopenia Initiative (EWGSOP and IWGS). Age Ageing 2014, 43, 748–759. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European consensus on definition and diagnosis. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef] [PubMed]
- Salini, S.; Giovannini, S.; Covino, M.; Barillaro, C.; Acampora, N.; Gravina, E.M.; Loreti, C.; Damiano, F.P.; Franceschi, F.; Russo, A. Frailty Network in an Acute Care Setting: The New Perspective for Frail Older People. Diagnostics 2022, 12, 1228. [Google Scholar] [CrossRef]
- Chen, L.K.; Liu, L.K.; Woo, J.; Assantachai, P.; Auyeung, T.W.; Bahyah, K.S.; Chou, M.Y.; Chen, L.Y.; Hsu, P.S.; Krairit, O.; et al. Sarcopenia in Asia: Consensus report of the Asian working group for sarcopenia. J. Am. Med. Dir. Assoc. 2014, 15, 95–101. [Google Scholar] [CrossRef]
- Liang, Z.; Zhang, T.; Liu, H.; Li, Z.; Peng, L.; Wang, C.; Wang, T. Inflammaging: The ground for sarcopenia? Exp. Gerontol. 2022, 168, 111931. [Google Scholar] [CrossRef]
- Kang, M.-K.; Park, J.-G. Low Skeletal Muscle Mass Is a Risk Factor for Subclinical Atherosclerosis in Patients with Nonalcoholic Fatty Liver Disease. Diagnostics 2021, 11, 854. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Miyake, M.; Hori, S.; Ichikawa, K.; Omori, C.; Iemura, Y.; Owari, T.; Itami, Y.; Nakai, Y.; Anai, S.; et al. Clinical Impact of Sarcopenia and Inflammatory/Nutritional Markers in Patients with Unresectable Metastatic Urothelial Carcinoma Treated with Pembrolizumab. Diagnostics 2020, 10, 310. [Google Scholar] [CrossRef] [PubMed]
- Bano, G.; Trevisan, C.; Carraro, S.; Solmi, M.; Luchini, C.; Stubbs, B.; Manzato, E.; Sergi, G.; Veronese, N. Inflammation and sarcopenia: A systematic review and meta-analysis. Maturitas 2017, 96, 10–15. [Google Scholar] [CrossRef]
- Bellanti, F.; Romano, A.D.; Buglio, A.; Lo-Castriotta, V.; Guglielmi, G.; Greco, A.; Serviddio, G.; Vendemiale, G. Oxidative stress is increased in sarcopenia and associated with cardiovascular disease risk in sarcopenic obesity. Maturitas 2018, 109, 6–12. [Google Scholar] [CrossRef]
- Zacarías-Flores, M.; Sánchez-Rodríguez, M.A.; García-Anaya, O.D.; Correa-Muñoz, E.; Mendoza-Núñez, V.M. Relationship between oxidative stress and muscle mass loss in early postmenopause: An exploratory study. Endocrinol. Diabetes Nutr. 2018, 65, 328–334. [Google Scholar] [CrossRef]
- Alsaawi, T.A.; Aldisi, D.; Abulmeaty, M.M.A.; Khattak, M.N.K.; Alnaami, A.M.; Sabico, S.; Al-Daghri, N.M. Screening for Sarcopenia among Elderly Arab Females: Influence of Body Composition, Lifestyle, Irisin, and Vitamin D. Nutrients 2022, 14, 1855. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.L.; de Sá, C.M.A.T.; Brito, D.C.; Batista, C.L.; da Costa, M.K.M.E.; de Lima, K.B.A.G.; Souza, J.M.; Ramos, I.L.T. Accuracy parameters as indicators of anthropometric adiposity visceral scheduled for two-dimensional equation. Nutr. Hosp. 2015, 32, 2046–2053. [Google Scholar]
- Teo, B.W.; Toh, Q.C.; Chan, X.W.; Xu, H.; Li, J.L.; Lee, E.J. Assessment of muscle mass and its association with protein intake in a multi-ethnic Asian population: Relevance in chronic kidney disease. Asia Pac. J. Clin. Nutr. 2014, 23, 619–625. [Google Scholar] [PubMed]
- Ezeukwu, A.O.; Agwubike, E.O. Anthropometric measures of adiposity as correlates of the atherogenic index of plasma in non-obese sedentary Nigerian males. Libyan J. Med. 2014, 9, 23798. [Google Scholar] [CrossRef]
- Janssen, I.; Heymsfield, S.B.; Baumgartner, R.N.; Ross, R. Estimation of skeletal muscle mass by bioelectrical impedance analysis. J. Appl. Physiol. 2000, 89, 465–471. [Google Scholar] [CrossRef]
- Podsiadlo, D.; Richardson, S. The timed “Up & Go”: A test of basic functional mobility for frail elderly persons. J. Am. Geriatr. Soc. 1991, 39, 142–148. [Google Scholar]
- Al-Daghri, N.M.; Al-Shuwaie, A.Y.A.; Alghamdi, A.; Amer, O.E.; Khattak, M.N.K.; Ansari, M.G.A.; Alnaami, A.M.; Sabico, S. Tristetraprolin, Inflammation, and Metabolic Syndrome in Arab Adults: A Case Control Study. Biology 2021, 10, 550. [Google Scholar] [CrossRef]
- Asoudeh, F.; Dashti, F.; Raeesi, S.; Heshmat, R.; Bidkhori, M.; Jalilian, Z.; Hashemi, R. Inflammatory cytokines and sarcopenia in Iranian adults-results from SARIR study. Sci. Rep. 2022, 12, 5471. [Google Scholar] [CrossRef]
- Kamper, R.S.; Alcazar, J.; Andersen, L.L.; Haddock, B.; Jørgensen, N.R.; Hovind, P.; Suetta, C. Associations between inflammatory markers, body-composition, and physical function: The CopenhagenSarcopenia Study. J. Cachexia Sarcopenia Muscle 2021, 12, 1641–1652. [Google Scholar] [CrossRef]
- Rong, Y.-D.; Bian, A.-L.; Hu, H.-Y.; Ma, Y.; Zhou, X.-Z. Study on the relationship between elderly sarcopenia and inflammatory cytokine IL-6, anti-inflammatory cytokine IL-10. BMC Geriatr. 2018, 18, 1–6. [Google Scholar] [CrossRef]
- Can, B.; Kara, O.; Kizilarslanoglu, M.C.; Arik, G.; Aycicek, G.S.; Sumer, F.; Civelek, R.; Demirtas, C.; Ulger, Z. Serum markers of inflammation and oxidative stress in sarcopenia. Aging Clin. Exp. Res. 2017, 29, 745–752. [Google Scholar] [CrossRef] [PubMed]
- Dupont, J.; Antonio, L.; Dedeyne, L.; O’Neill, T.W.; Vanderschueren, D.; Rastrelli, G.; Maggi, M.; Bártfai, G.; Casanueva, F.F.; Giwercman, A.; et al. Inflammatory markers are associated with quality of life, physical activity, and gait speed but not sarcopenia in aged men (40–79 years). J. Cachexia Sarcopenia Muscle 2021, 12, 1818–1831. [Google Scholar] [CrossRef]
- Dalle, S.; Rossmeislova, L.; Koppo, K. The Role of Inflammation in Age-Related Sarcopenia. Front. Physiol. 2017, 12, 1045. [Google Scholar] [CrossRef] [PubMed]
- Schaap, L.A.; Pluijm, S.M.F.; Deeg, D.J.H.; Visser, M. Inflammatory markers and loss of muscle mass (sarcopenia) and strength. Am. J. Med. 2006, 119, 526.e9–526.e17. [Google Scholar] [CrossRef] [PubMed]
- Calvani, R.; Picca, A.; Marini, F.; Biancolillo, A.; Gervasoni, J.; Persichilli, S.; Primiano, A.; Coelho-Junior, H.J.; Cesari, M.; Bossola, M.; et al. Identification of biomarkers for physical frailty and sarcopenia through a new multi-marker approach: Results from the BIOSPHERE study. Geroscience 2021, 43, 727–740. [Google Scholar] [CrossRef]
- Sabico, S.; Alguwaihes, A.M. Emerging Markers for Sarcopenia. In Sarcopenia; Veronese, N., Beaudart, C., Sabico, S., Eds.; Practical Issues in Geriatrics; Springer: Cham, Switzerland, 2021. [Google Scholar]
| All (n = 76) | Non-Sarcopenia (n = 50) | Sarcopenia (n = 26) | p-Value | |
|---|---|---|---|---|
| Education | 39 (51.3) | 25 (50.0) | 14 (53.8) | 0.93 |
| Illiterate | ||||
| Elementary | 20 (26.3) | 13 (26.0) | 7 (26.9) | |
| Middle school | 7 (9.2) | 5 (10.0) | 2 (7.7) | |
| High School | 3 (3.9) | 2 (4.0) | 1 (3.8) | |
| College degree | 7 (9.2) | 5 (10.0) | 2 (7.7) | |
| Marital Status Married | 50 (65.8) | 34 (68.0) | 16 (61.5) | 0.42 |
| Widowed | 24 (31.6) | 14 (28.0) | 10 (38.5) | |
| Divorced | 2 (2.6) | 2 (4.0) | 0 | |
| Employment Unemployed | 66 (86.8) | 43 (86.0) | 23 (88.5) | 0.58 |
| Retired | 8 (31.6) | 5 (10.0) | 3 (11.5) | |
| Home Business | 2 (2.6) | 2 (4.0) | 0 | |
| Medical history DM | 46 (60.5) | 29 (58.0) | 17 (65.4) | 0.36 |
| Hypertension | 54 (71.1) | 36 (72.0) | 18 (69.2) | 0.50 |
| High Cholesterol | 35 (46.1) | 25 (50.0) | 10 (38.5) | 0.24 |
| Osteoporosis | 6 (7.9) | 4 (8.0) | 2 (7.7) | 0.67 |
| Rheumatoid | 2 (2.6) | 1 (2.0) | 1 (3.8) | 0.57 |
| Asthma | 6 (7.9) | 5 (10.0) | 1 (3.8) | 0.32 |
| Hypothyroidism | 6 (7.9) | 6 (12.0) | 0 | 0.07 |
| Comorbidity | 54 (71.1) | 37 (74.0) | 17 (65.4) | 0.30 |
| One health condition | 69 (90.8) | 47 (94.0) | 22 (84.6) | 0.18 |
| Parameters | All | Non-Sarcopenia | Sarcopenia | p-Value |
|---|---|---|---|---|
| n | 76 | 50 | 26 | |
| Age (years) | 66.8 ± 5.7 | 66.5 ± 5.7 | 67.5 ± 5.7 | 0.11 |
| BMI (kg/m2) | 30.7 ± 5.6 | 32.2 ± 5.8 | 27.8 ± 2.7 | 0.001 |
| Waist (cm) | 92.8 ± 11.3 | 95.8 ± 11.2 | 87.5 ± 9.7 | 0.003 |
| Hips (cm) | 106.8 ± 10.4 | 109.0 ± 11.4 | 102.7 ± 6.6 | 0.02 |
| WHR (cm) | 0.87 ± 0.07 | 0.87 ± 0.08 | 0.86 ± 0.07 | 0.54 |
| Parameters | All | Non-Sarcopenia | Sarcopenia | p-Value |
|---|---|---|---|---|
| n | 76 | 50 | 26 | |
| Midarm Circumference | 28.0 ± 3.7 | 29.0 ± 3.6 | 26.2 ± 3.1 | 0.002 |
| Triceps Skinfold Thickness | 17.3 ± 3.4 | 17.5 ± 3.2 | 16.8 ± 3.2 | 0.43 |
| Conicity Index | 1.24± 0.11 | 1.24 ± 0.11 | 1.23 ± 0.12 | 0.77 |
| Midarm Muscle Area | 41.4 ± 11.5 | 44.5 ± 11.7 | 35.3 ± 8.5 | 0.001 |
| Abdominal Volume Index | 17.3 ± 4.4 | 18.3 ± 4.5 | 15.5 ± 3.4 | 0.009 |
| Handgrip Strength | 15.7 ± 4.3 | 16.9 ± 4.3 | 13.4 ± 3.4 | 0.001 |
| TUG | 16.2 ± 3.9 | 16.3 ± 4.1 | 16.0 ± 3.4 | 0.75 |
| Muscle Mass | 39.1 ± 4.7 | 40.8 ± 4.7 | 35.9 ± 2.8 | <0.001 |
| Right Leg Muscle | 6.6 ± 1.0 | 6.8 ± 1.0 | 6.1 ± 0.7 | 0.001 |
| Left Leg Muscle | 6.7 ± 1.2 | 6.9 ± 1.0 | 6.4 ± 1.4 | 0.11 |
| Right Arm Muscle | 1.9 ± 0.3 | 2.0 ± 0.3 | 1.7 ± 0.2 | <0.001 |
| Left Arm Muscle | 2.0 ± 0.3 | 2.1 ± 0.3 | 1.8 ± 0.2 | <0.001 |
| Trunk | 21.9 ± 2.7 | 23.0 ± 2.5 | 19.9 ± 1.9 | <0.001 |
| Predictive equation (kg/m2) | 6.5 ± 0.9 | 6.9 ± 0.9 | 5.9 ± 0.3 | <0.001 |
| Parameters | All | Non-Sarcopenia | Sarcopenia | p-Value |
|---|---|---|---|---|
| n | 76 | 50 | 26 | |
| Glucose (mmol/L) | 10.8 ± 4.0 | 10.6 ± 3.8 | 11.0 ± 4.6 | 0.75 |
| Total Cholesterol (mmol/L) | 5.2 ± 1.0 | 5.1 ± 1.1 | 5.3 ± 1.1 | 0.70 |
| HDL-Cholesterol (mmol/L) | 1.5 ± 0.4 | 1.5 ± 0.4 | 1.4 ± 0.4 | 0.70 |
| CRP | 3.0 (1.5–5.2) | 2.7 (1.4–5.0) | 3.6 (1.8–5.6) | 0.19 |
| IL-6 | 9.9 ± 2.5 | 10.1 ± 2.6 | 9.72 ± 2.1 | 0.59 |
| TNF-α | 30.3 (5.6–75.5) | 27.5 (3.4–73.4) | 50.8 (17.4–78) | 0.13 |
| Parameters | IL-6 | Log-TNF-α | Log-CRP | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Non-Sarc | Sarcopenia | Non-Sarc | Sarcopenia | Non-Sarc | Sarcopenia | |||||||
| r | p | r | p | r | p | r | p | r | p | r | p | |
| Age (years) | 0.17 | 0.26 | 0.19 | 0.36 | −0.20 | 0.18 | −0.40 | 0.06 | 0.03 | 0.86 | 0.27 | 0.18 |
| Body Composition | ||||||||||||
| BMI (kg/m2) | −0.12 | 0.42 | −0.15 | 0.47 | 0.19 | 0.21 | 0.17 | 0.44 | 0.15 | 0.28 | 0.13 | 0.53 |
| Waist (cm) | −0.17 | 0.28 | −0.01 | 0.98 | 0.29 | 0.06 | −0.12 | 0.61 | 0.04 | 0.79 | 0.26 | 0.23 |
| WHR | −0.20 | 0.20 | 0.31 | 0.15 | −0.05 | 0.74 | −0.29 | 0.20 | −0.02 | 0.91 | 0.28 | 0.19 |
| Glucose and Lipids | ||||||||||||
| Glucose (mmol/L) | −0.06 | 0.80 | 0.18 | 0.44 | 0.06 | 0.60 | 0.12 | 0.44 | 0.05 | 0.93 | 0.03 | 0.96 |
| Total Chol (mmol/L) | 0.05 | 0.76 | 0.08 | 0.73 | −0.18 | 0.28 | 0.02 | 0.92 | 0.07 | 0.66 | 0.10 | 0.67 |
| HDL-Chol (mmol/L) | −0.10 | 0.55 | −0.05 | 0.81 | 0.00 | 0.98 | −0.16 | 0.51 | −0.07 | 0.68 | −0.38 | 0.08 |
| Muscle Strength and Function | ||||||||||||
| MAC | −0.02 | 0.90 | −0.34 | 0.09 | 0.26 | 0.09 | −0.06 | 0.80 | 0.13 | 0.37 | 0.01 | 0.97 |
| TSF | −0.02 | 0.89 | −0.21 | 0.31 | 0.03 | 0.84 | −0.13 | 0.57 | 0.03 | 0.84 | 0.42 | 0.04 |
| CI | −0.07 | 0.68 | 0.16 | 0.46 | 0.07 | 0.67 | −0.21 | 0.35 | 0.01 | 0.95 | 0.22 | 0.31 |
| MAMA | −0.01 | 0.65 | −0.34 | 0.10 | 0.30 | 0.04 | −0.03 | 0.90 | 0.14 | 0.33 | −0.15 | 0.48 |
| AVI | −0.15 | 0.32 | −0.01 | 0.97 | 0.21 | 0.19 | −0.10 | 0.66 | 0.09 | 0.56 | 0.26 | 0.22 |
| Handgrip Strength | 0.02 | 0.87 | 0.01 | 0.96 | 0.13 | 0.37 | 0.01 | 0.97 | −0.13 | 0.37 | −0.06 | 0.78 |
| TUG | −0.10 | 0.52 | 0.48 | 0.02 | 0.30 | 0.04 | −0.17 | 0.44 | −0.14 | 0.34 | −0.11 | 0.60 |
| Muscle Mass | −0.11 | 0.48 | −0.08 | 0.69 | 0.23 | 0.12 | −0.20 | 0.35 | 0.08 | 0.59 | −0.34 | 0.09 |
| R-leg-M | −0.13 | 0.39 | −0.12 | 0.55 | 0.30 | 0.04 | −0.22 | 0.31 | 0.12 | 0.42 | −0.41 | 0.04 |
| L-leg-M | −0.10 | 0.52 | −0.14 | 0.50 | 0.11 | 0.46 | 0.09 | 0.67 | 0.13 | 0.37 | 0.10 | 0.64 |
| R-arm-M | −0.05 | 0.75 | −0.14 | 0.48 | 0.21 | 0.17 | −0.29 | 0.16 | 0.06 | 0.66 | −0.23 | 0.26 |
| L-arm-M | −0.12 | 0.41 | −0.14 | 0.51 | 0.22 | 0.14 | −0.25 | 0.24 | 0.14 | 0.32 | −0.20 | 0.32 |
| Trunk | −0.09 | 0.53 | 0.05 | 0.80 | 0.23 | 0.12 | −0.25 | 0.24 | 0.03 | 0.86 | −0.35 | 0.08 |
| Predictive equation (kg/m2) | −0.19 | 0.20 | 0.04 | 0.83 | 0.25 | 0.10 | 0.12 | 0.58 | 0.09 | 0.54 | −0.01 | 0.98 |
| Parameters | OR (95% CI) | p-Value |
|---|---|---|
| IL-6 | ||
| T1 (<8.5) | 1.0 | |
| T2 (8.51–10.42) | 0.65 (0.19–2.21) | 0.50 |
| T3 (>10.42) | 0.27 (0.06–1.18) | 0.08 |
| TNF-α | ||
| T1 (<23.4) | 1.0 | |
| T2 (23.41–71.2) | 3.56 (0.63–20.16) | 0.15 |
| T3 (>71.2) | 5.85 (1.07–32.08) | 0.04 |
| CRP | ||
| T1 (<1.6) | 1.0 | |
| T2 (1.61–4.86) | 1.90 (0.53–6.76) | 0.32 |
| T3 (>4.86) | 0.95 (0.24–3.81) | 0.94 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aldisi, D.; Abulmeaty, M.M.A.; Alsaawi, T.A.; Alorf, A.S.; Mujlli, G.; Alshahrani, A.M.; Alahmari, R.M.; Alquraishi, M.; Al-Daghri, N.M.; Alruwaili, N.W.; et al. Diagnostic Value of Inflammatory Markers in Elderly Arab Women with Sarcopenia. Diagnostics 2022, 12, 2293. https://doi.org/10.3390/diagnostics12102293
Aldisi D, Abulmeaty MMA, Alsaawi TA, Alorf AS, Mujlli G, Alshahrani AM, Alahmari RM, Alquraishi M, Al-Daghri NM, Alruwaili NW, et al. Diagnostic Value of Inflammatory Markers in Elderly Arab Women with Sarcopenia. Diagnostics. 2022; 12(10):2293. https://doi.org/10.3390/diagnostics12102293
Chicago/Turabian StyleAldisi, Dara, Mahmoud M. A. Abulmeaty, Tafany A. Alsaawi, Abeer S. Alorf, Gadah Mujlli, Atheer M. Alshahrani, Rajwa M. Alahmari, Mohammed Alquraishi, Nasser M. Al-Daghri, Nawaf W. Alruwaili, and et al. 2022. "Diagnostic Value of Inflammatory Markers in Elderly Arab Women with Sarcopenia" Diagnostics 12, no. 10: 2293. https://doi.org/10.3390/diagnostics12102293
APA StyleAldisi, D., Abulmeaty, M. M. A., Alsaawi, T. A., Alorf, A. S., Mujlli, G., Alshahrani, A. M., Alahmari, R. M., Alquraishi, M., Al-Daghri, N. M., Alruwaili, N. W., & Sabico, S. (2022). Diagnostic Value of Inflammatory Markers in Elderly Arab Women with Sarcopenia. Diagnostics, 12(10), 2293. https://doi.org/10.3390/diagnostics12102293








