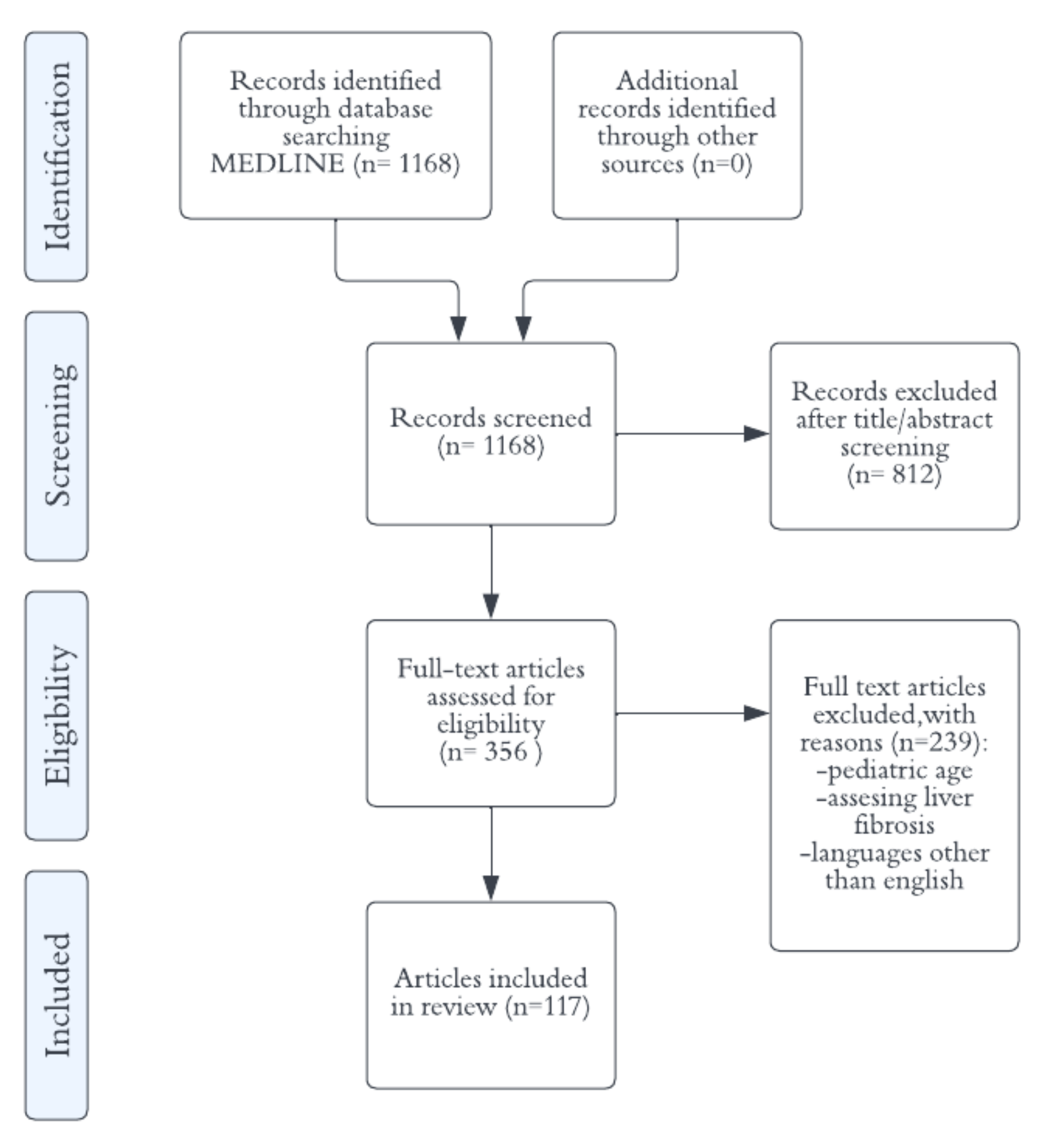
Ultrasound Methods for the Assessment of Liver Steatosis: A Critical Appraisal
Abstract
1. Introduction
2. B-Mode Ultrasound
Semi-Quantitative Ultrasound Scores
3. Colour Doppler Imaging
4. Quantitative Ultrasound-Based Methods
4.1. Spectral Based Techniques
4.1.1. Controlled Attenuation Parameter (CAP)
4.1.2. Attenuation Imaging (ATI)
4.1.3. Attenuation Measurement Function (ATT)
4.1.4. Attenuation Plane-Wave Ultrasound (Att.PLUS)
4.1.5. Ultrasound-Guided Attenuation Parameter (UGAP)
4.1.6. Tissue Attenuation Imaging (TAI) and Tissue Scatter Distribution Imaging (TSI)
4.1.7. Ultrasound-Derived Fat Fraction (UDFF) and Backscatter Coefficient (BSC)
4.1.8. Liver Fat Quantification
4.2. Techniques Based on the Envelope Statistics of the Backscattered Ultrasound
4.2.1. Acoustic Structure Quantification (ASQ) and Normalized Local Variance (NLV)
| Authors/Reference | No | Etiology | Reference Method | Method | Cut-Off | AUROC | Cut-Off | AUROC | Cut-Off | AUROC | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| S1 | S2 | S3 | |||||||||
| Bae et al. | [53] | 108 | CLD | LB | ATI1 | 0.635 | 0.843 | 0.7 | 0.886 | 0.745 | 0.926 |
| Tada et al. | [56] | 148 | CLD | LB | ATI 1 | 0.66 | 0.85 | 0.67 | 0.91 | 0.68 | 0.91 |
| Jeon et al. | [57] | 87 | CLD | MRI-PDFF | ATI 1 | 0.59 | 0.76 | ||||
| Ferraioli et al. | [12] | 129 | NAFLD and controls | MRI-PDFF | ATI 1 | 0.63 | 0.91 | 0.72 | 0.95 | ||
| Ferraioli et al. | [11] | 72 | NAFLD risk | MRI-PDFF | ATI-PEN 1 ATI-GEN 1 | >0.69 >0.62 | 0.90 0.92 | ||||
| Dioguardi et al. | [58] | 101 | CLD | LB | ATI 1 | 0.69 | 0.80 | 0.72 | 0.89 | ||
| Sugimoto et al. | [59] | 111 | NAFLD | LB | ATI 1 | 0.67 | 0.88 | 0.72 | 0.86 | 0.86 | 0.79 |
| Tada et al. | [60] | 119 | CLD | MRI-PDFF | ATI 1 | 0.63 | 0.81 | 0.72 | 0.87 | 0.75 | 0.91 |
| Bae et al. | [10] | 120 | LR for susp. mlg | LB | ATI 1 | 0.66 | 0.914 | 0.66 | 0.914 | ||
| Lee at al. | [61] | 102 | NAFLD | LB | ATI 1 | 0.64 | 0.93 | 0.7 | 0.9 | 0.73 | 0.83 |
| Hsu et al. | [62] | 28 | CLD | LB | ATI 1 | 0.69 | 0.97 | 0.78 | 0.99 | 0.82 | 0.97 |
| Kwon et al. | [63] | 100 | CLD | MRI-PDFF | ATI 1 | 0.62 | 0.91 | 0.72 | 0.94 | ||
| Jang et al. | [64] | 57 | LT donors | LB | ATI 1 | 0.62 | 0.808 | ||||
| Tamaki et al. | [69] | 351 | CLD | LB | ATT 1 | 0.62 | 0.79 | 0.67 | 0.87 | 0.73 | 0.96 |
| Fujiwara et al. | [75] | 163 | CLD | LB | UGAP 1 | 0.53 | 0.9 | 0.60 | 0.95 | 0.65 | 0.96 |
| Tada et al. | [73] | 126 | CLD | MRI-PDFF | UGAP 1 | 0.60 | 0.92 | 0.69 | 0.87 | 0.69 | 0.89 |
| Ogino et al. | [76] | 84 | NAFLD | LB | UGAP 1 | 0.6 | 0.94 | 0.71 | 0.95 | 0.72 | 0.88 |
| Kuroda et al. | [77] | 202 | NAFLD | LB | UGAP 1 | 0.49 | 0.89 | 0.65 | 0.91 | 0.69 | 0.92 |
| Kuroda et al. | [65] | 105 | NAFLD | LB | UGAP 1 ATI 1 | 0.62 0.64 | 0.89 0.876 | 0.72 0.71 | 0.90 0.88 | 0.75 0.75 | 0.91 0.91 |
| Imajo et al. | [80] | 1010 | CLD | MRI-PDFF | UGAP 1 | 0.65 | 0.910 | 0.71 | 0.912 | 0.77 | 0.894 |
| Jeon et al. | [81] | 120 | NAFLD | MRI-PDFF | TAI 1 TSI | >0.884 >91.2 | 0.861 0.964 | ||||
| Lin et sl. | [84] | 204 | NAFLD and controls | MRI-PDFF | BSC 2 | 0.0038 | 0.98 | ||||
| Labyed et al. | [36] | 101 | NAFLD | LB MRI-PDFF | UDFF 3 | 8.1 6.34 | 0.94 0.97 | 15.9 / | 0.88 / | 16.1 / | 0.83 / |
| Bae et al. | [91] | 194 | CLD or post-OLT | LB | NLV | 1.095 | 0.911 | 1.055 | 0.974 | 1.025 | 0.954 |
| Zhao et al. | [97] | 34 | MAFLD | LB | NLV | 1.145 | 0.875 | 1.1 | 0.735 | 1.1 | 0.583 |
| Imbault et al. | [100] | 17 | NAFLD risk | MRI-PDFF LB | SSE 4 | 1.541 1.555 | 0.942 0.952 | ||||
| Dioguardi et al. | [99] | 100 | CLD | MRI-PDFF | SSE 4 | ≤1.537 | 0.882 | 1.511 | 0.989 | 1.511 | 0.989 |
4.2.2. Speed of Sound Estimation (SSE) and Sound Speed Plane-Wave Ultrasound (SSp.PLUS)
5. Clinical Significance of Detecting and Grading Liver Steatosis
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global Epidemiology of Nonalcoholic Fatty Liver Disease-Meta-Analytic Assessment of Prevalence, Incidence, and Outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, C.F.; Shi, L.; Löwe, A.; Dong, Y.; Potthoff, A.; Sparchez, Z.; Teufel, A.; Guth, S.; Koch, J.; Barr, R.G.; et al. Conventional Ultrasound for Diagnosis of Hepatic Steatosis is Better than Believed. Z. Für Gastroenterol. 2021, 60, 1235–1248. [Google Scholar] [CrossRef] [PubMed]
- Ferraioli, G.; Berzigotti, A.; Barr, R.G.; Choi, B.I.; Cui, X.W.; Dong, Y.; Gilja, O.H.; Lee, J.Y.; Lee, D.H.; Moriyasu, F.; et al. Quantification of Liver Fat Content with Ultrasound: A WFUMB Position Paper. Ultrasound Med. Biol. 2021, 47, 2803–2820. [Google Scholar] [CrossRef] [PubMed]
- Kleiner, D.E.; Brunt, E.M.; Van Natta, M.; Behling, C.; Contos, M.J.; Cummings, O.W.; Ferrell, L.D.; Liu, Y.-C.; Torbenson, M.S.; Unalp-Arida, A.; et al. Design and Validation of a Histological Scoring System for Nonalcoholic Fatty Liver Disease. Hepatology 2005, 41, 1313–1321. [Google Scholar] [CrossRef]
- Zhang, Y.N.; Fowler, K.J.; Hamilton, G.; Cui, J.Y.; Sy, E.Z.; Balanay, M.; Hooker, J.C.; Szeverenyi, N.; Sirlin, C.B. Liver Fat Imaging—A Clinical Overview of Ultrasound, CT, and MR Imaging. Br. J. Radiol. 2018, 91, 20170959. [Google Scholar] [CrossRef]
- Permutt, Z.; Le, T.-A.; Peterson, M.R.; Seki, E.; Brenner, D.A.; Sirlin, C.; Loomba, R. Correlation between Liver Histology and Novel Magnetic Resonance Imaging in Adult Patients with Non-Alcoholic Fatty Liver Disease-MRI Accurately Quantifies Hepatic Steatosis in NAFLD. Aliment. Pharmacol. Ther. 2012, 36, 22–29. [Google Scholar] [CrossRef]
- Choi, S.J.; Kim, S.M.; Kim, Y.S.; Kwon, O.S.; Shin, S.K.; Kim, K.K.; Lee, K.; Park, I.B.; Choi, C.S.; Chung, D.H.; et al. Magnetic Resonance-Based Assessments Better Capture Pathophysiologic Profiles and Progression in Nonalcoholic Fatty Liver Disease. Diabetes Metab. J. 2021, 45, 739–752. [Google Scholar] [CrossRef]
- Stern, C.; Castera, L. Non-Invasive Diagnosis of Hepatic Steatosis. Hepatol. Int. 2016, 11, 70–78. [Google Scholar] [CrossRef]
- McHenry, S.; Park, Y.; Browning, J.D.; Sayuk, G.; Davidson, N.O. Dallas Steatosis Index Identifies Patients with Nonalcoholic Fatty Liver Disease. Clin. Gastroenterol. Hepatol. 2020, 18, 2073–2080.e7. [Google Scholar] [CrossRef]
- Dyson, J.K.; Anstee, Q.M.; McPherson, S. Non-Alcoholic Fatty Liver Disease: A Practical Approach to Treatment. Frontline Gastroenterol. 2014, 5, 277–286. [Google Scholar] [CrossRef]
- Long, M.T.; Pedley, A.; Colantonio, L.D.; Massaro, J.M.; Hoffmann, U.; Muntner, P.; Fox, C.S. Development and Validation of the Framingham Steatosis Index to Identify Persons with Hepatic Steatosis. Clin. Gastroenterol. Hepatol. 2016, 14, 1172–1180.e2. [Google Scholar] [CrossRef]
- Fedchuk, L.; Nascimbeni, F.; Pais, R.; Charlotte, F.; Housset, C.; Ratziu, V. Performance and Limitations of Steatosis Biomarkers in Patients with Nonalcoholic Fatty Liver Disease. Aliment. Pharmacol. Ther. 2014, 40, 1209–1222. [Google Scholar] [CrossRef]
- Lee, J.; Vali, Y.; Boursier, J.; Spijker, R.; Anstee, Q.M.; Bossuyt, P.M.; Zafarmand, M.H. Prognostic Accuracy of FIB-4, NAFLD Fibrosis Score and APRI for NAFLD-Related Events: A Systematic Review. Liver Int. 2021, 41, 261–270. [Google Scholar] [CrossRef]
- Vilar-Gomez, E.; Chalasani, N. Non-Invasive Assessment of Non-Alcoholic Fatty Liver Disease: Clinical Prediction Rules and Blood-Based Biomarkers. J. Hepatol. 2018, 68, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.H.; Al Juboori, A.; Petroski, G.F.; Diaz-Arias, A.A.; Syed-Abdul, M.M.; Wheeler, A.A.; Ganga, R.R.; Pitt, J.B.; Spencer, N.M.; Hammoud, G.M.; et al. The Utility and Diagnostic Accuracy of Transient Elastography in Adults with Morbid Obesity: A Prospective Study. J. Clin. Med. 2022, 11, 1201. [Google Scholar] [CrossRef]
- Newsome, P.N.; Sasso, M.; Deeks, J.J.; Paredes, A.; Boursier, J.; Chan, W.-K.; Yilmaz, Y.; Czernichow, S.; Zheng, M.-H.; Wong, V.W.-S.; et al. FibroScan-AST (FAST) Score for the Non-Invasive Identification of Patients with Non-Alcoholic Steatohepatitis with Significant Activity and Fibrosis: A Prospective Derivation and Global Validation Study. Lancet Gastroenterol. Hepatol. 2020, 5, 362–373. [Google Scholar] [CrossRef]
- Bae, J.S.; Dong Ho, L.; Suh, K.-S.; Lee, K.B.; Kim, H.; Lee, J.Y.; Han, J.K. Noninvasive Assessment of Hepatic Steatosis Using a Pathologic Reference Standard: Comparison of CT, MRI, and Ultrasound-Based Techniques. Ultrasonography 2021, 41, 344. [Google Scholar] [CrossRef]
- Ferraioli, G.; Maiocchi, L.; Savietto, G.; Tinelli, C.; Nichetti, M.; Rondanelli, M.; Calliada, F.; Preda, L.; Filice, C. Performance of the Attenuation Imaging Technology in the Detection of Liver Steatosis. J. Ultrasound Med. 2020, 40, 1325–1332. [Google Scholar] [CrossRef]
- Ferraioli, G.; Maiocchi, L.; Raciti, M.V.; Tinelli, C.; De Silvestri, A.; Nichetti, M.; De Cata, P.; Rondanelli, M.; Chiovato, L.; Calliada, F.; et al. Detection of Liver Steatosis with a Novel Ultrasound-Based Technique. Clin. Transl. Gastroenterol. 2019, 10, e00081. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL–EASD–EASO Clinical Practice Guidelines for the Management of Non-Alcoholic Fatty Liver Disease. J. Hepatol. 2016, 64, 1388–1402. [Google Scholar] [CrossRef]
- Hernaez, R.; Lazo, M.; Bonekamp, S.; Kamel, I.; Brancati, F.L.; Guallar, E.; Clark, J.M. Diagnostic Accuracy and Reliability of Ultrasonography for the Detection of Fatty Liver: A Meta-Analysis. Hepatology 2011, 54, 1082–1090. [Google Scholar] [CrossRef] [PubMed]
- Décarie, P.-O.; Lepanto, L.; Billiard, J.-S.; Olivié, D.; Murphy-Lavallée, J.; Kauffmann, C.; Tang, A. Fatty Liver Deposition and Sparing: A Pictorial Review. Insights Imaging 2011, 2, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Barr, R.G. Ultrasound of Diffuse Liver Disease Including Elastography. Radiol. Clin. N. Am. 2019, 57, 549–562. [Google Scholar] [CrossRef] [PubMed]
- Ferraioli, G.; Soares Monteiro, L.B. Ultrasound-Based Techniques for the Diagnosis of Liver Steatosis. World J. Gastroenterol. 2019, 25, 6053–6062. [Google Scholar] [CrossRef]
- Webb, M.; Yeshua, H.; Zelber-Sagi, S.; Santo, E.; Brazowski, E.; Halpern, Z.; Oren, R. Diagnostic Value of a Computerized Hepatorenal Index for Sonographic Quantification of Liver Steatosis. Am. J. Roentgenol. 2009, 192, 909–914. [Google Scholar] [CrossRef]
- Moret, A.; Boursier, J.; Houssel Debry, P.; Riou, J.; Crouan, A.; Dubois, M.; Michalak Provost, S.; Aubé, C.; Paisant, A. Evaluation of the Hepatorenal B-Mode Ratio and the “Controlled Attenuation Parameter” for the Detection and Grading of Steatosis. Ultraschall Der Med. Eur. J. Ultrasound 2020. [Google Scholar] [CrossRef]
- Marshall, R.H.; Eissa, M.; Bluth, E.I.; Gulotta, P.M.; Davis, N.K. Hepatorenal Index as an Accurate, Simple, and Effective Tool in Screening for Steatosis. Am. J. Roentgenol. 2012, 199, 997–1002. [Google Scholar] [CrossRef]
- Shiralkar, K.; Johnson, S.; Bluth, E.I.; Marshall, R.H.; Dornelles, A.; Gulotta, P.M. Improved Method for Calculating Hepatic Steatosis Using the Hepatorenal Index. J. Ultrasound Med. 2015, 34, 1051–1059. [Google Scholar] [CrossRef]
- Petzold, G.; Lasser, J.; Rühl, J.; Bremer, S.C.B.; Knoop, R.F.; Ellenrieder, V.; Kunsch, S.; Neesse, A. Diagnostic Accuracy of B-Mode Ultrasound and Hepatorenal Index for Graduation of Hepatic Steatosis in Patients with Chronic Liver Disease. PLoS ONE 2020, 15, e0231044. [Google Scholar] [CrossRef]
- Johnson, S.I.; Fort, D.; Shortt, K.J.; Therapondos, G.; Galliano, G.E.; Nguyen, T.; Bluth, E.I. Ultrasound Stratification of Hepatic Steatosis Using Hepatorenal Index. Diagnostics 2021, 11, 1443. [Google Scholar] [CrossRef]
- Stahlschmidt, F.L.; Tafarel, J.R.; Menini-Stahlschmidt, C.M.; Baena, C.P. Hepatorenal Index for Grading Liver Steatosis with Concomitant Fibrosis. PLoS ONE 2021, 16, e0246837. [Google Scholar] [CrossRef]
- Hamaguchi, M.; Kojima, T.; Itoh, Y.; Harano, Y.; Fujii, K.; Nakajima, T.; Kato, T.; Takeda, N.; Okuda, J.; Ida, K.; et al. The Severity of Ultrasonographic Findings in Nonalcoholic Fatty Liver Disease Reflects the Metabolic Syndrome and Visceral Fat Accumulation. Am. J. Gastroenterol. 2007, 102, 2708–2715. [Google Scholar] [CrossRef]
- Kozłowska-Petriczko, K.; Wunsch, E.; Petriczko, J.; Syn, W.-K.; Milkiewicz, P. Diagnostic Accuracy of Non-Imaging and Ultrasound-Based Assessment of Hepatic Steatosis Using Controlled Attenuation Parameter (CAP) as Reference. J. Clin. Med. 2021, 10, 1507. [Google Scholar] [CrossRef]
- Berzigotti, A.; Ferraioli, G.; Bota, S.; Gilja, O.H.; Dietrich, C.F. Novel Ultrasound-Based Methods to Assess Liver Disease: The Game Has Just Begun. Dig. Liver Dis. 2018, 50, 107–112. [Google Scholar] [CrossRef]
- Ballestri, S.; Lonardo, A.; Romagnoli, D.; Carulli, L.; Losi, L.; Day, C.P.; Loria, P. Ultrasonographic Fatty Liver Indicator, a Novel Score Which Rules out NASH and Is Correlated with Metabolic Parameters in NAFLD. Liver Int. 2012, 32, 1242–1252. [Google Scholar] [CrossRef]
- Nelson, S.M.; Hoskins, J.D.; Lisanti, C.; Chaudhuri, J. Ultrasound Fatty Liver Indicator: A Simple Tool for Differentiating Steatosis from Nonalcoholic Steatohepatitis: Validity in the Average Obese Population. J. Ultrasound Med. 2019, 39, 749–759. [Google Scholar] [CrossRef]
- Ballestri, S.; Nascimbeni, F.; Baldelli, E.; Marrazzo, A.; Romagnoli, D.; Targher, G.; Lonardo, A. Ultrasonographic Fatty Liver Indicator Detects Mild Steatosis and Correlates with Metabolic/Histological Parameters in Various Liver Diseases. Metabolism 2017, 72, 57–65. [Google Scholar] [CrossRef]
- Xavier, S.A.; Monteiro, S.O.; Arieira, C.M.; Castro, F.D.; Magalhães, J.T.; Leite, S.M.; Marinho, C.M.; Cotter, J.B. US-FLI Score–Is It Possible to Predict the Steatosis Grade with an Ultrasonographic Score? Mol. Genet. Metab. 2021, 132, 204–209. [Google Scholar] [CrossRef]
- Mohammadinia, A.R.; Bakhtavar, K.; Ebrahimi-Daryani, N.; Habibollahi, P.; Keramati, M.R.; Fereshtehnejad, S.M.; Abdollahzade, S. Correlation of Hepatic Vein Doppler Waveform and Hepatic Artery Resistance Index with the Severity of Nonalcoholic Fatty Liver Disease. J. Clin. Ultrasound 2010, 38, 346–352. [Google Scholar] [CrossRef]
- Alizadeh, A.; Mansour-Ghanaei, F.; Roozdar, A.; Joukar, F.; Sepehrimanesh, M.; Hojati, S.A.; Mansour-Ghanaei, A. Laboratory Tests, Liver Vessels Color Doppler Sonography, and FibroScan Findings in Patients with Nonalcoholic Fatty Liver Disease: An Observation Study. J. Clin. Imaging Sci. 2018, 8, 12. [Google Scholar] [CrossRef]
- Liu, L.-P.; Dong, B.-W.; Yu, X.-L.; Zhang, D.-K.; Kang, C.-S.; Zhao, X.-H. Evaluation of Focal Fatty Infiltration of the Liver Using Color Doppler and Contrast-Enhanced Sonography. J. Clin. Ultrasound 2008, 36, 560–566. [Google Scholar] [CrossRef]
- Liu, L.-P.; Dong, B.-W.; Yu, X.-L.; Zhang, D.-K.; Li, X.; Li, H. Analysis of Focal Spared Areas in Fatty Liver Using Color Doppler Imaging and Contrast-Enhanced Microvessel Display Sonography. J. Ultrasound Med. 2008, 27, 387–394. [Google Scholar] [CrossRef]
- Labyed, Y.; Milkowski, A. Novel Method for Ultrasound-Derived Fat Fraction Using an Integrated Phantom. J. Ultrasound Med. 2020, 39, 2427–2438. [Google Scholar] [CrossRef]
- Oelze, M.L.; Mamou, J. Review of Quantitative Ultrasound: Envelope Statistics and Backscatter Coefficient Imaging and Contributions to Diagnostic Ultrasound. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 2016, 63, 336–351. [Google Scholar] [CrossRef] [PubMed]
- Cook, R.D. Envelope Methods. WIREs Comput. Stat. 2019, 12, e1484. [Google Scholar] [CrossRef]
- Sirli, R.; Sporea, I. Controlled Attenuation Parameter for Quantification of Steatosis: Which Cut-Offs to Use? Can. J. Gastroenterol. Hepatol. 2021, 2021, 6662760. [Google Scholar] [CrossRef] [PubMed]
- Beyer, C.; Hutton, C.; Andersson, A.; Imajo, K.; Nakajima, A.; Kiker, D.; Banerjee, R.; Dennis, A. Comparison between Magnetic Resonance and Ultrasound-Derived Indicators of Hepatic Steatosis in a Pooled NAFLD Cohort. PLoS ONE 2021, 16, e0249491. [Google Scholar] [CrossRef]
- Ferraioli, G. Quantitative Assessment of Liver Steatosis Using Ultrasound Controlled Attenuation Parameter (Echosens). J. Med. Ultrason. 2021, 48, 489–495. [Google Scholar] [CrossRef]
- Caussy, C.; Alquiraish, M.H.; Nguyen, P.; Hernandez, C.; Cepin, S.; Fortney, L.E.; Ajmera, V.; Bettencourt, R.; Collier, S.; Hooker, J.; et al. Optimal Threshold of Controlled Attenuation Parameter with MRI-PDFF as the Gold Standard for the Detection of Hepatic Steatosis. Hepatology 2018, 67, 1348–1359. [Google Scholar] [CrossRef]
- Eddowes, P.J.; Sasso, M.; Allison, M.; Tsochatzis, E.; Anstee, Q.M.; Sheridan, D.; Guha, I.N.; Cobbold, J.F.; Deeks, J.J.; Paradis, V.; et al. Accuracy of FibroScan Controlled Attenuation Parameter and Liver Stiffness Measurement in Assessing Steatosis and Fibrosis in Patients with Nonalcoholic Fatty Liver Disease. Gastroenterology 2019, 156, 1717–1730. [Google Scholar] [CrossRef]
- Ferraioli, G.; Kumar, V.; Ozturk, A.; Nam, K.; de Korte, C.L.; Barr, R.G. US Attenuation for Liver Fat Quantification: An AIUM-RSNA QIBA Pulse-Echo Quantitative Ultrasound Initiative. Radiology 2022, 302, 495–506. [Google Scholar] [CrossRef]
- Karlas, T.; Petroff, D.; Sasso, M.; Fan, J.-G.; Mi, Y.-Q.; de Lédinghen, V.; Kumar, M.; Lupsor-Platon, M.; Han, K.-H.; Cardoso, A.C.; et al. Individual Patient Data Meta-Analysis of Controlled Attenuation Parameter (CAP) Technology for Assessing Steatosis. J. Hepatol. 2017, 66, 1022–1030. [Google Scholar] [CrossRef]
- Petroff, D.; Blank, V.; Newsome, P.N.; Shalimar; Voican, C.S.; Thiele, M.; de Lédinghen, V.; Baumeler, S.; Chan, W.K.; Perlemuter, G.; et al. Assessment of Hepatic Steatosis by Controlled Attenuation Parameter Using the M and XL Probes: An Individual Patient Data Meta-Analysis. Lancet Gastroenterol. Hepatol. 2021, 6, 185–198. [Google Scholar] [CrossRef]
- Grgurevic, I.; Salkic, N.; Madir, A.; Aralica, G. Steatosis Assessment by Controlled Attenuation Parameter in Patients with Compensated Advanced Chronic Liver Disease. Liver Int. 2020, 40, 1784–1785. [Google Scholar] [CrossRef]
- Shao, C.X.; Ye, J.; Dong, Z.; Li, F.; Lin, Y.; Liao, B.; Feng, S.; Zhong, B. Steatosis Grading Consistency between Controlled Attenuation Parameter and MRI-PDFF in Monitoring Metabolic Associated Fatty Liver Disease. Ther. Adv. Chronic Dis. 2021, 12, 204062232110331. [Google Scholar] [CrossRef]
- Chan, W.-K.; Nik Mustapha, N.R.; Mahadeva, S.; Wong, V.W.-S.; Cheng, J.Y.-K.; Wong, G.L.-H. Can the Same Controlled Attenuation Parameter Cut-Offs Be Used for M and XL Probes for Diagnosing Hepatic Steatosis? J. Gastroenterol. Hepatol. 2018, 33, 1787–1794. [Google Scholar] [CrossRef]
- Ng, Y.Z.; Lai, L.L.; Wong, S.W.; Mohamad, S.Y.; Chuah, K.H.; Chan, W.K. Attenuation Parameter and Liver Stiffness Measurement Using FibroTouch vs. Fibroscan in Patients with Chronic Liver Disease. PLoS ONE 2021, 16, e0250300. [Google Scholar] [CrossRef]
- Qu, Y.; Song, Y.-Y.; Chen, C.-W.; Fu, Q.-C.; Shi, J.-P.; Xu, Y.; Xie, Q.; Yang, Y.-F.; Zhou, Y.-J.; Li, L.-P.; et al. Diagnostic Performance of FibroTouch Ultrasound Attenuation Parameter and Liver Stiffness Measurement in Assessing Hepatic Steatosis and Fibrosis in Patients with Nonalcoholic Fatty Liver Disease. Clin. Transl. Gastroenterol. 2021, 12, e00323. [Google Scholar] [CrossRef]
- Zhu, S.-H.; Zheng, K.I.; Hu, D.-S.; Gao, F.; Rios, R.S.; Li, G.; Li, Y.-Y.; Byrne, C.D.; Targher, G.; Chen, Y.-P.; et al. Optimal Thresholds for Ultrasound Attenuation Parameter in the Evaluation of Hepatic Steatosis Severity: Evidence from a Cohort of Patients with Biopsy-Proven Fatty Liver Disease. Eur. J. Gastroenterol. Hepatol. 2020, 33, 430–435. [Google Scholar] [CrossRef]
- Bae, J.S.; Lee, D.H.; Lee, J.Y.; Kim, H.; Yu, S.J.; Lee, J.-H.; Cho, E.J.; Lee, Y.B.; Han, J.K.; Choi, B.I. Assessment of Hepatic Steatosis by Using Attenuation Imaging: A Quantitative, Easy-To-Perform Ultrasound Technique. Eur. Radiol. 2019, 29, 6499–6507. [Google Scholar] [CrossRef]
- Lee, D.H. Quantitative Assessment of Fatty Liver Using Ultrasound Attenuation Imaging. J. Med. Ultrason. 2021, 48, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, K.; Abe, M.; Oshiro, H.; Takahashi, H.; Kakegawa, T.; Tomita, Y.; Yoshimasu, Y.; Takeuchi, H.; Itoi, T. The Most Appropriate Region-of-Interest Position for Attenuation Coefficient Measurement in the Evaluation of Liver Steatosis. J. Med. Ultrason. 2021, 48, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Tada, T.; Iijima, H.; Kobayashi, N.; Yoshida, M.; Nishimura, T.; Kumada, T.; Kondo, R.; Yano, H.; Kage, M.; Nakano, C.; et al. Usefulness of Attenuation Imaging with an Ultrasound Scanner for the Evaluation of Hepatic Steatosis. Ultrasound Med. Biol. 2019, 45, 2679–2687. [Google Scholar] [CrossRef]
- Jeon, S.K.; Lee, J.M.; Joo, I.; Yoon, J.H.; Lee, D.H.; Lee, J.Y.; Han, J.K. Prospective Evaluation of Hepatic Steatosis Using Ultrasound Attenuation Imaging in Patients with Chronic Liver Disease with Magnetic Resonance Imaging Proton Density Fat Fraction as the Reference Standard. Ultrasound Med. Biol. 2019, 45, 1407–1416. [Google Scholar] [CrossRef] [PubMed]
- Dioguardi Burgio, M.; Ronot, M.; Reizine, E.; Rautou, P.-E.; Castera, L.; Paradis, V.; Garteiser, P.; Van Beers, B.; Vilgrain, V. Quantification of Hepatic Steatosis with Ultrasound: Promising Role of Attenuation Imaging Coefficient in a Biopsy-Proven Cohort. Eur. Radiol. 2019, 30, 2293–2301. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, K.; Moriyasu, F.; Oshiro, H.; Takeuchi, H.; Abe, M.; Yoshimasu, Y.; Kasai, Y.; Sakamaki, K.; Hara, T.; Itoi, T. The Role of Multiparametric US of the Liver for the Evaluation of Nonalcoholic Steatohepatitis. Radiology 2020, 296, 532–540. [Google Scholar] [CrossRef]
- Tada, T.; Kumada, T.; Toyoda, H.; Nakamura, S.; Shibata, Y.; Yasuda, S.; Watanuki, Y.; Tsujii, K.; Fukuda, N.; Fujioka, M.; et al. Attenuation Imaging Based on Ultrasound Technology for Assessment of Hepatic Steatosis: A Comparison with Magnetic Resonance Imaging-Determined Proton Density Fat Fraction. Hepatol. Res. 2020, 50, 1319–1327. [Google Scholar] [CrossRef]
- Lee, D.H.; Cho, E.J.; Bae, J.S.; Lee, J.Y.; Yu, S.J.; Kim, H.; Lee, K.B.; Han, J.K.; Choi, B.I. Accuracy of Two-Dimensional Shear Wave Elastography and Attenuation Imaging for Evaluation of Patients with Nonalcoholic Steatohepatitis. Clin. Gastroenterol. Hepatol. 2021, 19, 797–805.e7. [Google Scholar] [CrossRef]
- Hsu, P.-K.; Wu, L.-S.; Yen, H.-H.; Huang, H.P.; Chen, Y.-Y.; Su, P.-Y.; Su, W.-W. Attenuation Imaging with Ultrasound as a Novel Evaluation Method for Liver Steatosis. J. Clin. Med. 2021, 10, 965. [Google Scholar] [CrossRef]
- Kwon, E.Y.; Kim, Y.R.; Kang, D.M.; Yoon, K.H.; Lee, Y.H. Usefulness of US Attenuation Imaging for the Detection and Severity Grading of Hepatic Steatosis in Routine Abdominal Ultrasonography. Clin. Imaging 2021, 76, 53–59. [Google Scholar] [CrossRef]
- Jang, J.K.; Kim, S.Y.; Yoo, I.W.; Cho, Y.B.; Kang, H.J.; Lee, D.H. Diagnostic Performance of Ultrasound Attenuation Imaging for Assessing Low-Grade Hepatic Steatosis. Eur. Radiol. 2021, 32, 2070–2077. [Google Scholar] [CrossRef]
- Kuroda, H.; Abe, T.; Fujiwara, Y.; Nagasawa, T.; Takikawa, Y. Diagnostic Accuracy of Ultrasound-Guided Attenuation Parameter as a Noninvasive Test for Steatosis in Non-Alcoholic Fatty Liver Disease. J. Med. Ultrason. 2021, 48, 471–480. [Google Scholar] [CrossRef]
- Yoo, J.; Lee, J.M.; Joo, I.; Lee, D.H.; Yoon, J.H.; Kang, H.-J.; Ahn, S.J. Reproducibility of Ultrasound Attenuation Imaging for the Noninvasive Evaluation of Hepatic Steatosis. Ultrasonography 2020, 39, 121–129. [Google Scholar] [CrossRef]
- Gao, J.; Lee, R.; Trujillo, M. Reliability of Performing Multiparametric Ultrasound in Adult Livers. J. Ultrasound Med. 2021, 41, 699–711. [Google Scholar] [CrossRef]
- Cerit, M.; Şendur, H.N.; Cindil, E.; Erbaş, G.; Yalçın, M.M.; Cerit, E.T.; Allahverdiyeva, S.; Oktar, S.Ö.; Yücel, C. Quantification of Liver Fat Content with Ultrasonographic Attenuation Measurement Function: Correlation with Unenhanced Multidimensional Computerized Tomography. Clin. Imaging 2020, 65, 85–93. [Google Scholar] [CrossRef]
- Tamaki, N.; Koizumi, Y.; Hirooka, M.; Yada, N.; Takada, H.; Nakashima, O.; Kudo, M.; Hiasa, Y.; Izumi, N. Novel Quantitative Assessment System of Liver Steatosis Using a Newly Developed Attenuation Measurement Method. Hepatol. Res. 2018, 48, 821–828. [Google Scholar] [CrossRef]
- Tamaki, N.; Kurosaki, M.; Yasui, Y.; Tsuchiya, K.; Izumi, N. Attenuation Coefficient (ATT) Measurement for Liver Fat Quantification in Chronic Liver Disease. J. Med. Ultrason. 2021, 48, 481–487. [Google Scholar] [CrossRef]
- Koizumi, Y.; Hirooka, M.; Tamaki, N.; Yada, N.; Nakashima, O.; Izumi, N.; Kudo, M.; Hiasa, Y. New Diagnostic Technique to Evaluate Hepatic Steatosis Using the Attenuation Coefficient on Ultrasound B Mode. PLoS ONE 2019, 14, e0221548. [Google Scholar] [CrossRef]
- Popa, A.; Bende, F.; Șirli, R.; Popescu, A.; Bâldea, V.; Lupușoru, R.; Cotrău, R.; Fofiu, R.; Foncea, C.; Sporea, I. Quantification of Liver Fibrosis, Steatosis, and Viscosity Using Multiparametric Ultrasound in Patients with Non-Alcoholic Liver Disease: A “Real-Life” Cohort Study. Diagnostics 2021, 11, 783. [Google Scholar] [CrossRef]
- Tada, T.; Kumada, T.; Toyoda, H.; Kobayashi, N.; Sone, Y.; Oguri, T.; Kamiyama, N. Utility of Attenuation Coefficient Measurement Using an Ultrasound-Guided Attenuation Parameter for Evaluation of Hepatic Steatosis: Comparison with MRI-Determined Proton Density Fat Fraction. Am. J. Roentgenol. 2019, 212, 332–341. [Google Scholar] [CrossRef]
- Yao, L.X.; Zagzebski, J.A.; Madsen, E.L. Backscatter Coefficient Measurements Using a Reference Phantom to Extract Depth-Dependent Instrumentation Factors. Ultrason. Imaging 1990, 12, 58–70. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, Y.; Kuroda, H.; Abe, T.; Ishida, K.; Oguri, T.; Noguchi, S.; Sugai, T.; Kamiyama, N.; Takikawa, Y. The B-Mode Image-Guided Ultrasound Attenuation Parameter Accurately Detects Hepatic Steatosis in Chronic Liver Disease. Ultrasound Med. Biol. 2018, 44, 2223–2232. [Google Scholar] [CrossRef] [PubMed]
- Ogino, Y.; Wakui, N.; Nagai, H.; Igarashi, Y. The Ultrasound-Guided Attenuation Parameter Is Useful in Quantification of Hepatic Steatosis in Non-Alcoholic Fatty Liver Disease. JGH Open 2021, 5, 947–952. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, H.; Fujiwara, Y.; Abe, T.; Nagasawa, T.; Oguri, T.; Noguchi, S.; Kamiyama, N.; Takikawa, Y. Two-Dimensional Shear Wave Elastography and Ultrasound-Guided Attenuation Parameter for Progressive Non-Alcoholic Steatohepatitis. PLoS ONE 2021, 16, e0249493. [Google Scholar] [CrossRef]
- Tada, T.; Kumada, T.; Toyoda, H.; Yasuda, S.; Sone, Y.; Hashinokuchi, S.; Ogawa, S.; Oguri, T.; Kamiyama, N.; Chuma, M.; et al. Liver Stiffness Does Not Affect Ultrasound-Guided Attenuation Coefficient Measurement in the Evaluation of Hepatic Steatosis. Hepatol. Res. 2019, 50, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Imajo, K.; Toyoda, H.; Yasuda, S.; Suzuki, Y.; Sugimoto, K.; Kuroda, H.; Akita, T.; Tanaka, J.; Yasui, Y.; Tamaki, N.; et al. Utility of Ultrasound-Guided Attenuation Parameter for Grading Steatosis with Reference to MRI-PDFF in a Large Cohort. Clin. Gastroenterol. Hepatol. 2021, in press. [Google Scholar] [CrossRef]
- Bende, F.; Sporea, I.; Șirli, R.; Bâldea, V.; Lazăr, A.; Lupușoru, R.; Fofiu, R.; Popescu, A. Ultrasound-Guided Attenuation Parameter (UGAP) for the Quantification of Liver Steatosis Using the Controlled Attenuation Parameter (CAP) as the Reference Method. Med. Ultrason. 2021, 23, 7. [Google Scholar] [CrossRef]
- Jeon, S.K.; Lee, J.M.; Joo, I.; Park, S.-J. Quantitative Ultrasound Radiofrequency Data Analysis for the Assessment of Hepatic Steatosis in Nonalcoholic Fatty Liver Disease Using Magnetic Resonance Imaging Proton Density Fat Fraction as the Reference Standard. Korean J. Radiol. 2021, 22, 1077. [Google Scholar] [CrossRef]
- Jeon, S.K.; Joo, I.; Kim, S.Y.; Jang, J.K.; Park, J.; Park, H.S.; Lee, E.S.; Lee, J.M. Quantitative Ultrasound Radiofrequency Data Analysis for the Assessment of Hepatic Steatosis Using the Controlled Attenuation Parameter as a Reference Standard. Ultrasonography 2021, 40, 136–146. [Google Scholar] [CrossRef]
- Han, A.; Zhang, Y.N.; Boehringer, A.S.; Montes, V.; Andre, M.P.; Erdman, J.W.; Loomba, R.; Valasek, M.A.; Sirlin, C.B.; O’Brien, W.D. Assessment of Hepatic Steatosis in Nonalcoholic Fatty Liver Disease by Using Quantitative US. Radiology 2020, 295, 106–113. [Google Scholar] [CrossRef]
- Lin, S.C.; Heba, E.; Wolfson, T.; Ang, B.; Gamst, A.; Han, A.; Erdman, J.W.; O’Brien, W.D.; Andre, M.P.; Sirlin, C.B.; et al. Noninvasive Diagnosis of Nonalcoholic Fatty Liver Disease and Quantification of Liver Fat Using a New Quantitative Ultrasound Technique. Clin. Gastroenterol. Hepatol. 2015, 13, 1337–1345.e6. [Google Scholar] [CrossRef]
- Gao, J.; Wong, C.; Maar, M.; Park, D. Reliability of Performing Ultrasound Derived SWE and Fat Fraction in Adult Livers. Clin. Imaging 2021, 80, 424–429. [Google Scholar] [CrossRef]
- Han, A.; Labyed, Y.; Sy, E.Z.; Boehringer, A.S.; Andre, M.P.; Erdman, J.W.; Loomba, R.; Sirlin, C.B.; O’Brien, W.D. Inter-Sonographer Reproducibility of Quantitative Ultrasound Outcomes and Shear Wave Speed Measured in the Right Lobe of the Liver in Adults with Known or Suspected Non-Alcoholic Fatty Liver Disease. Eur. Radiol. 2018, 28, 4992–5000. [Google Scholar] [CrossRef]
- Barr, R.G.; Cestone, A.; De Silvestri, A. A Pre-Release Algorithm with a Confidence Map for Estimating the Attenuation Coefficient for Liver Fat Quantification. J. Ultrasound Med. 2021, 41, 1939–1948. [Google Scholar] [CrossRef]
- Bai, Y.; Wai-Liang, J. Marine Structural Design; Butterworth-Heinemann: Oxford, UK, 2016. [Google Scholar]
- Kuroda, H.; Kakisaka, K.; Kamiyama, N.; Oikawa, T.; Onodera, M.; Sawara, K.; Oikawa, K.; Endo, R.; Takikawa, Y.; Suzuki, K. Non-Invasive Determination of Hepatic Steatosis by Acoustic Structure Quantification from Ultrasound Echo Amplitude. World J. Gastroenterol. 2012, 18, 3889–3895. [Google Scholar] [CrossRef]
- Karlas, T.; Berger, J.; Garnov, N.; Lindner, F.; Busse, H.; Linder, N.; Schaudinn, A.; Relke, B.; Chakaroun, R.; Tröltzsch, M.; et al. Estimating Steatosis and Fibrosis: Comparison of Acoustic Structure Quantification with Established Techniques. World J. Gastroenterol. 2015, 21, 4894. [Google Scholar] [CrossRef]
- Bae, J.S.; Lee, D.H.; Lee, J.Y.; Kim, H.; Yu, S.J.; Lee, J.-H.; Cho, E.J.; Lee, Y.B.; Han, J.K.; Choi, B.I. Quantitative Assessment of Fatty Liver Using Ultrasound with Normalized Local Variance Technique. Ultraschall Der Med. Eur. J. Ultrasound 2020, 42, 599–606. [Google Scholar] [CrossRef]
- Lin, Y.-H.; Wan, Y.-L.; Tai, D.-I.; Tseng, J.-H.; Wang, C.-Y.; Tsai, Y.-W.; Lin, Y.-R.; Chang, T.-Y.; Tsui, P.-H. Considerations of Ultrasound Scanning Approaches in Non-Alcoholic Fatty Liver Disease Assessment through Acoustic Structure Quantification. Ultrasound Med. Biol. 2019, 45, 1955–1969. [Google Scholar] [CrossRef]
- Son, J.-Y.; Lee, J.Y.; Yi, N.-J.; Lee, K.-W.; Suh, K.-S.; Kim, K.G.; Lee, J.M.; Han, J.K.; Choi, B.I. Hepatic Steatosis: Assessment with Acoustic Structure Quantification of US Imaging. Radiology 2016, 278, 257–264. [Google Scholar] [CrossRef]
- Lee, D.H.; Lee, J.Y.; Park, M.S.; Han, J.K. Non-Invasive Monitoring of Hepatic Steatosis via Acoustic Structure Quantification of Ultrasonography with MR Spectroscopy as the Reference Standard. Ultrasonography 2020, 39, 70–78. [Google Scholar] [CrossRef]
- Lee, D.H.; Lee, J.Y.; Lee, K.B.; Han, J.K. Evaluation of Hepatic Steatosis by Using Acoustic Structure Quantification US in a Rat Model: Comparison with Pathologic Examination and MR Spectroscopy. Radiology 2017, 285, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Keller, J.; Kaltenbach, T.E.-M.; Haenle, M.M.; Oeztuerk, S.; Graeter, T.; Mason, R.A.; Seufferlein, T.; Kratzer, W. Comparison of Acoustic Structure Quantification (ASQ), Shearwave Elastography and Histology in Patients with Diffuse Hepatopathies. BMC Med. Imaging 2015, 15, 58. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, C.; Xu, S.; Zhang, H.; Wei, S.; Huang, P.; Zhang, L.; Wong, Y.N.; Xu, W.; Huang, P. Quantitative Evaluation of Hepatic Steatosis Using Novel Ultrasound Technology Normalized Local Variance (NLV) and Its Standard Deviation with Different ROIs in Patients with Metabolic-Associated Fatty Liver Disease: A Pilot Study. Abdom. Radiol. 2021, 47, 693–703. [Google Scholar] [CrossRef] [PubMed]
- Duck, F.A. Physical Properties of Tissues; Cademic Press: London, UK, 1990; pp. 73–135. [Google Scholar]
- Dioguardi Burgio, M.; Imbault, M.; Ronot, M.; Faccinetto, A.; Van Beers, B.E.; Rautou, P.-E.; Castera, L.; Gennisson, J.-L.; Tanter, M.; Vilgrain, V. Ultrasonic Adaptive Sound Speed Estimation for the Diagnosis and Quantification of Hepatic Steatosis: A Pilot Study. Ultraschall Der Med. Eur. J. Ultrasound 2018, 40, 722–733. [Google Scholar] [CrossRef]
- Imbault, M.; Faccinetto, A.; Osmanski, B.-F.; Tissier, A.; Deffieux, T.; Gennisson, J.-L.; Vilgrain, V.; Tanter, M. Robust Sound Speed Estimation for Ultrasound-Based Hepatic Steatosis Assessment. Phys. Med. Biol. 2017, 62, 3582–3598. [Google Scholar] [CrossRef]
- Popa, A.; Șirli, R.; Popescu, A.; Bâldea, V.; Lupușoru, R.; Bende, F.; Cotrău, R.; Sporea, I. Ultrasound-Based Quantification of Fibrosis and Steatosis with a New Software Considering Transient Elastography as Reference in Patients with Chronic Liver Diseases. Ultrasound Med. Biol. 2021, 47, 1692–1703. [Google Scholar] [CrossRef]
- Mikolašević, I.; Filipec Kanižaj, T.; Targher, G. Nonalcoholic Fatty Liver Disease-A Growing Public Health Problem. Croat. Med. J. 2021, 62, 1–3. [Google Scholar] [CrossRef]
- Grgurevic, I.; Podrug, K.; Mikolasevic, I.; Kukla, M.; Madir, A.; Tsochatzis, E.A. Natural History of Nonalcoholic Fatty Liver Disease: Implications for Clinical Practice and an Individualized Approach. Can. J. Gastroenterol. Hepatol. 2020, 2020, 9181368. [Google Scholar] [CrossRef]
- Grgurevic, I.; Bozin, T.; Mikus, M.; Kukla, M.; O’Beirne, J. Hepatocellular Carcinoma in Non-Alcoholic Fatty Liver Disease: From Epidemiology to Diagnostic Approach. Cancers 2021, 13, 5844. [Google Scholar] [CrossRef]
- Mikolasevic, I.; Orlic, L.; Franjic, N.; Hauser, G.; Stimac, D.; Milic, S. Transient Elastography (FibroScan®) with Controlled Attenuation Parameter in the Assessment of Liver Steatosis and Fibrosis in Patients with Nonalcoholic Fatty Liver Disease-Where Do We Stand? World J. Gastroenterol. 2016, 22, 7236. [Google Scholar] [CrossRef]
- Podrug, K.; Sporea, I.; Lupusoru, R.; Pastrovic, F.; Mustapic, S.; Bâldea, V.; Bozin, T.; Bokun, T.; Salkic, N.; Șirli, R.; et al. Diagnostic Performance of 2-D Shear-Wave Elastography with Propagation Maps and Attenuation Imaging in Patients with Non-Alcoholic Fatty Liver Disease. Ultrasound Med. Biol. 2021, 47, 2128–2137. [Google Scholar] [CrossRef]
- Simon, T.G.; Roelstraete, B.; Khalili, H.; Hagström, H.; Ludvigsson, J.F. Mortality in Biopsy-Confirmed Nonalcoholic Fatty Liver Disease: Results from a Nationwide Cohort. Gut 2020, 70, 1375–1382. [Google Scholar] [CrossRef]
- Angulo, P.; Kleiner, D.E.; Dam-Larsen, S.; Adams, L.A.; Bjornsson, E.S.; Charatcharoenwitthaya, P.; Mills, P.R.; Keach, J.C.; Lafferty, H.D.; Stahler, A.; et al. Liver Fibrosis, but No Other Histologic Features, Is Associated with Long-Term Outcomes of Patients with Nonalcoholic Fatty Liver Disease. Gastroenterology 2015, 149, 389–397.e10. [Google Scholar] [CrossRef] [PubMed]
- Allen, A.M.; Hicks, S.B.; Mara, K.C.; Larson, J.J.; Therneau, T.M. The Risk of Incident Extrahepatic Cancers Is Higher in Non-Alcoholic Fatty Liver Disease than Obesity–A Longitudinal Cohort Study. J. Hepatol. 2019, 71, 1229–1236. [Google Scholar] [CrossRef] [PubMed]
- Stine, J.G.; Munaganuru, N.; Barnard, A.; Wang, J.L.; Kaulback, K.; Argo, C.K.; Singh, S.; Fowler, K.J.; Sirlin, C.B.; Loomba, R. Change in MRI-PDFF and Histologic Response in Patients with Nonalcoholic Steatohepatitis: A Systematic Review and Meta-Analysis. Clin. Gastroenterol. Hepatol. 2021, 19, 2274–2283.e5. [Google Scholar] [CrossRef] [PubMed]

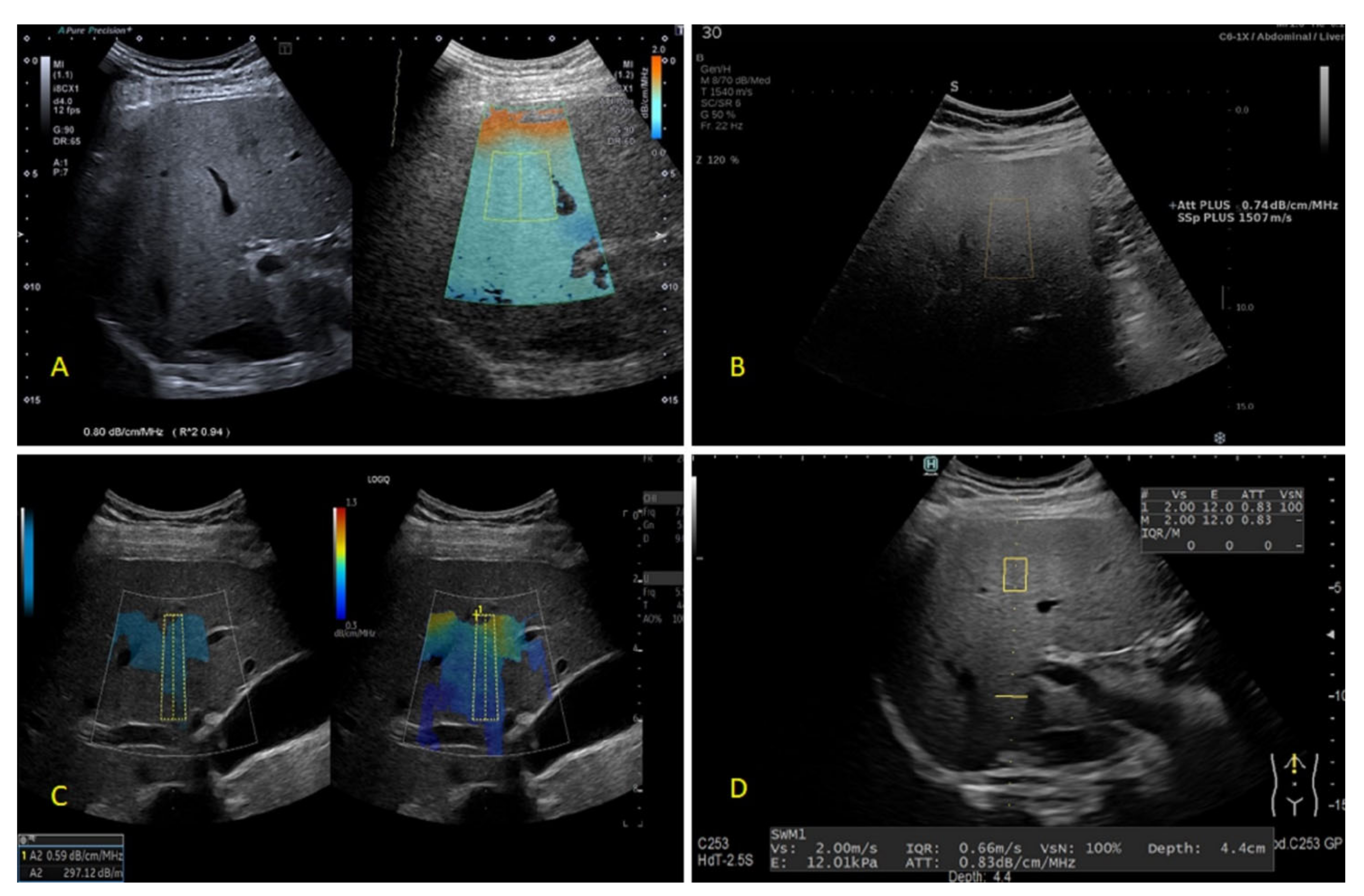
| Steatosis Grade | Fatty Transformed Hepatocytes (%) |
|---|---|
| 0 Normal liver | <5% |
| 1 Mild | 5–33% |
| 2 Moderate | 33–66% |
| 3 Severe | >66% |
| Steatosis Grade | Sonographic Features |
|---|---|
| 0 Normal liver | Normal liver echogenicity |
| 1 Mild | Mildly hyperechoic liver parencyhma, no vessel blurring, normal diaphragm visualization |
| 2 Moderate | Moderately hyperechoic liver parenchyma, blurred liver vessels, impaired visualization of diaphragm |
| 3 Severe | Remarkably hyperechoic liver parenchyma, inadequate visualization of posterior portion of the right lobe, liver vessels and diaphragm |
| Participant recommendations: |
|---|
| Fast at least 3 h prior to the exam |
| Take the supine lying position |
| Place the right arm in the maximum adduction |
| Suspended respiration during measurement—holding a breath for a few seconds at the end of expiration |
| Investigator recommendation: |
| When placing ROI avoid parts of liver parencyhma with blood vessels, biliary ducts and focal liver lesions Place ROI at least 2 cm under liver capsule to avoid reverberation artifact If color map is available, avoid areas that are markedly differently colored Maintain the acoustic radiation force impulse perpendicular to the liver capsule |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bozic, D.; Podrug, K.; Mikolasevic, I.; Grgurevic, I. Ultrasound Methods for the Assessment of Liver Steatosis: A Critical Appraisal. Diagnostics 2022, 12, 2287. https://doi.org/10.3390/diagnostics12102287
Bozic D, Podrug K, Mikolasevic I, Grgurevic I. Ultrasound Methods for the Assessment of Liver Steatosis: A Critical Appraisal. Diagnostics. 2022; 12(10):2287. https://doi.org/10.3390/diagnostics12102287
Chicago/Turabian StyleBozic, Dorotea, Kristian Podrug, Ivana Mikolasevic, and Ivica Grgurevic. 2022. "Ultrasound Methods for the Assessment of Liver Steatosis: A Critical Appraisal" Diagnostics 12, no. 10: 2287. https://doi.org/10.3390/diagnostics12102287
APA StyleBozic, D., Podrug, K., Mikolasevic, I., & Grgurevic, I. (2022). Ultrasound Methods for the Assessment of Liver Steatosis: A Critical Appraisal. Diagnostics, 12(10), 2287. https://doi.org/10.3390/diagnostics12102287






