Diagnostic Performance of the Acute Kidney Injury Baseline Creatinine Equations in Children and Adolescents with Type 1 Diabetes Mellitus Onset
Abstract
:1. Introduction
2. Methods
2.1. AKI Definition
2.2. ebSCr Calculation
2.3. Statistical Analysis
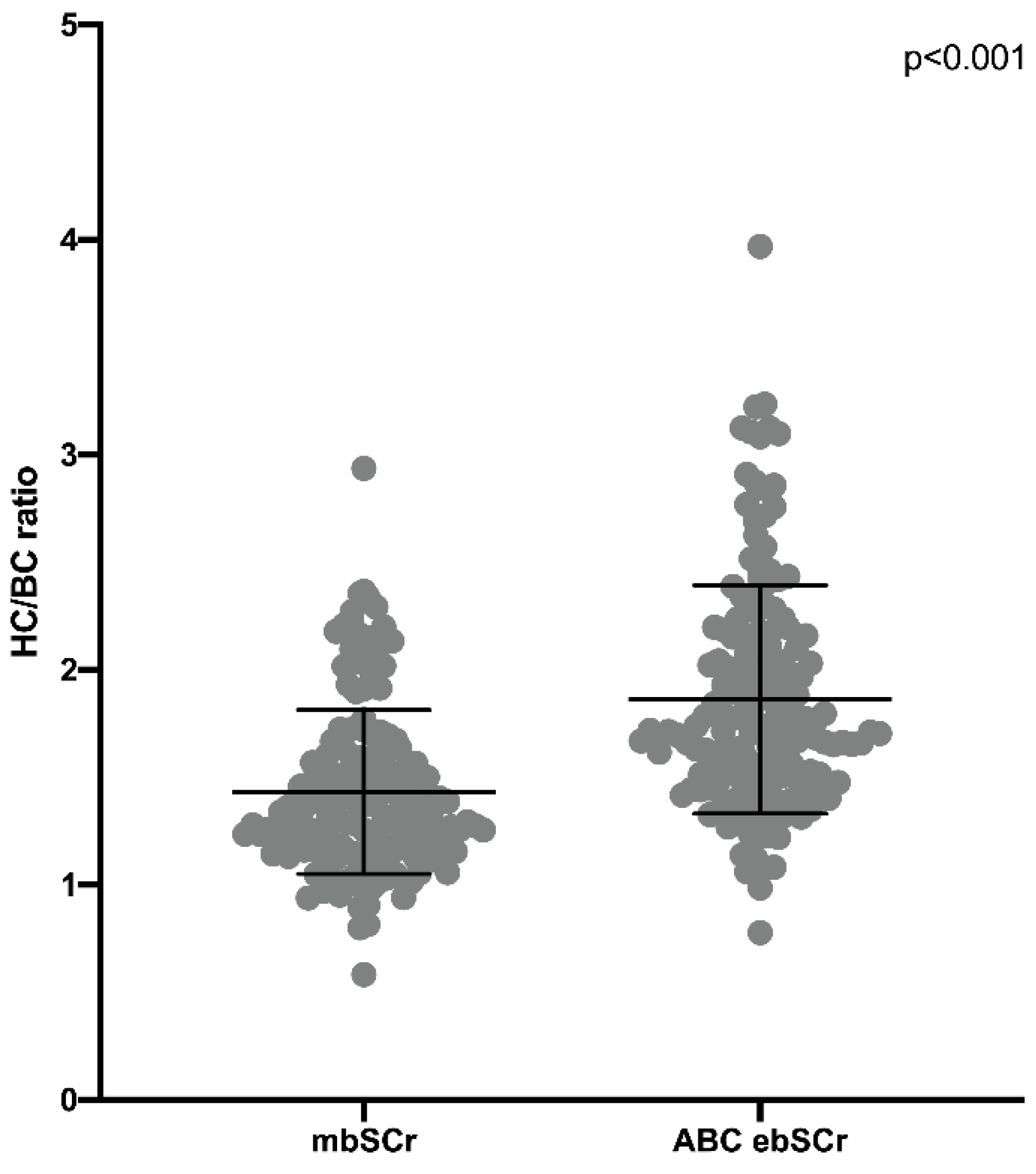
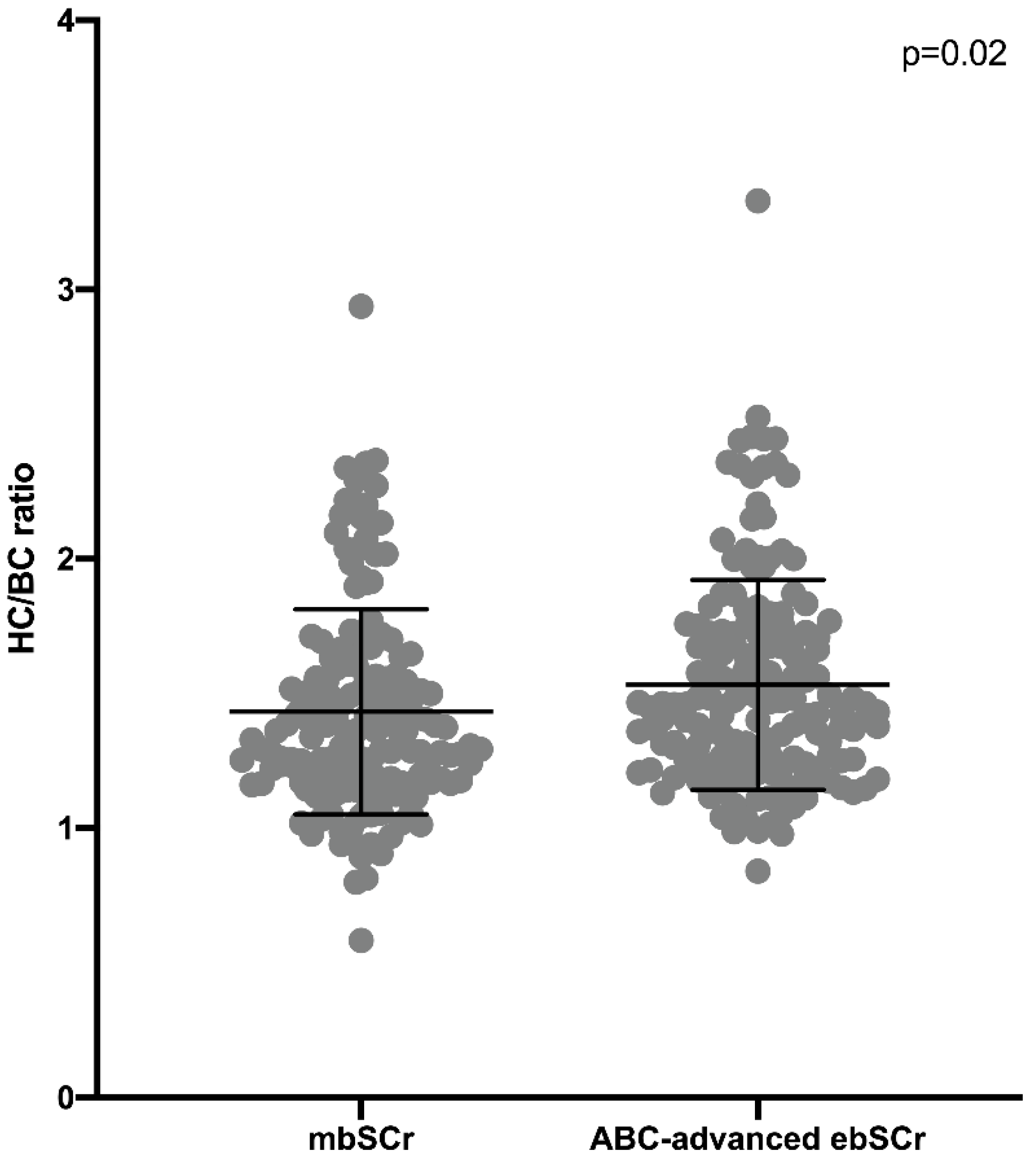
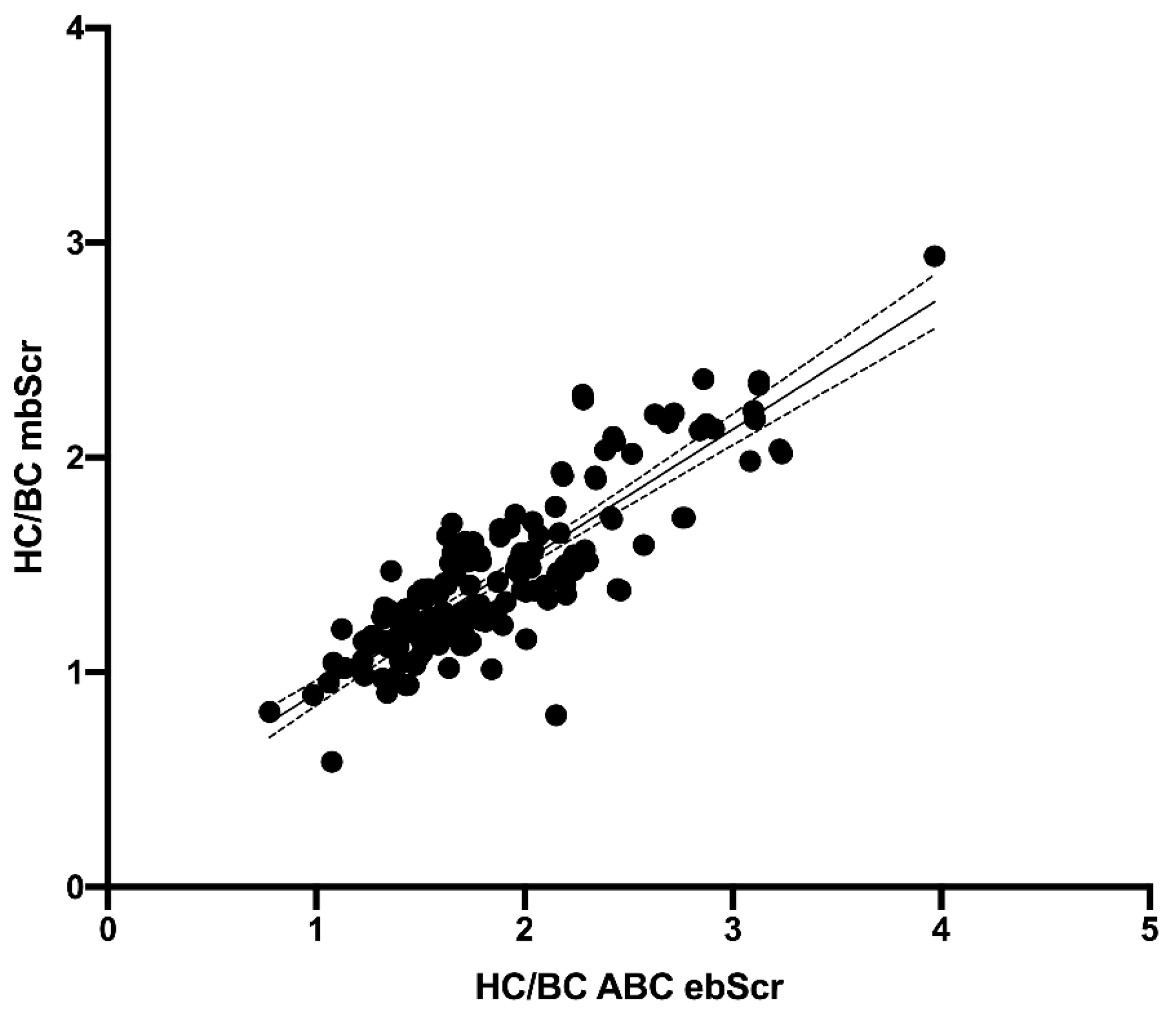
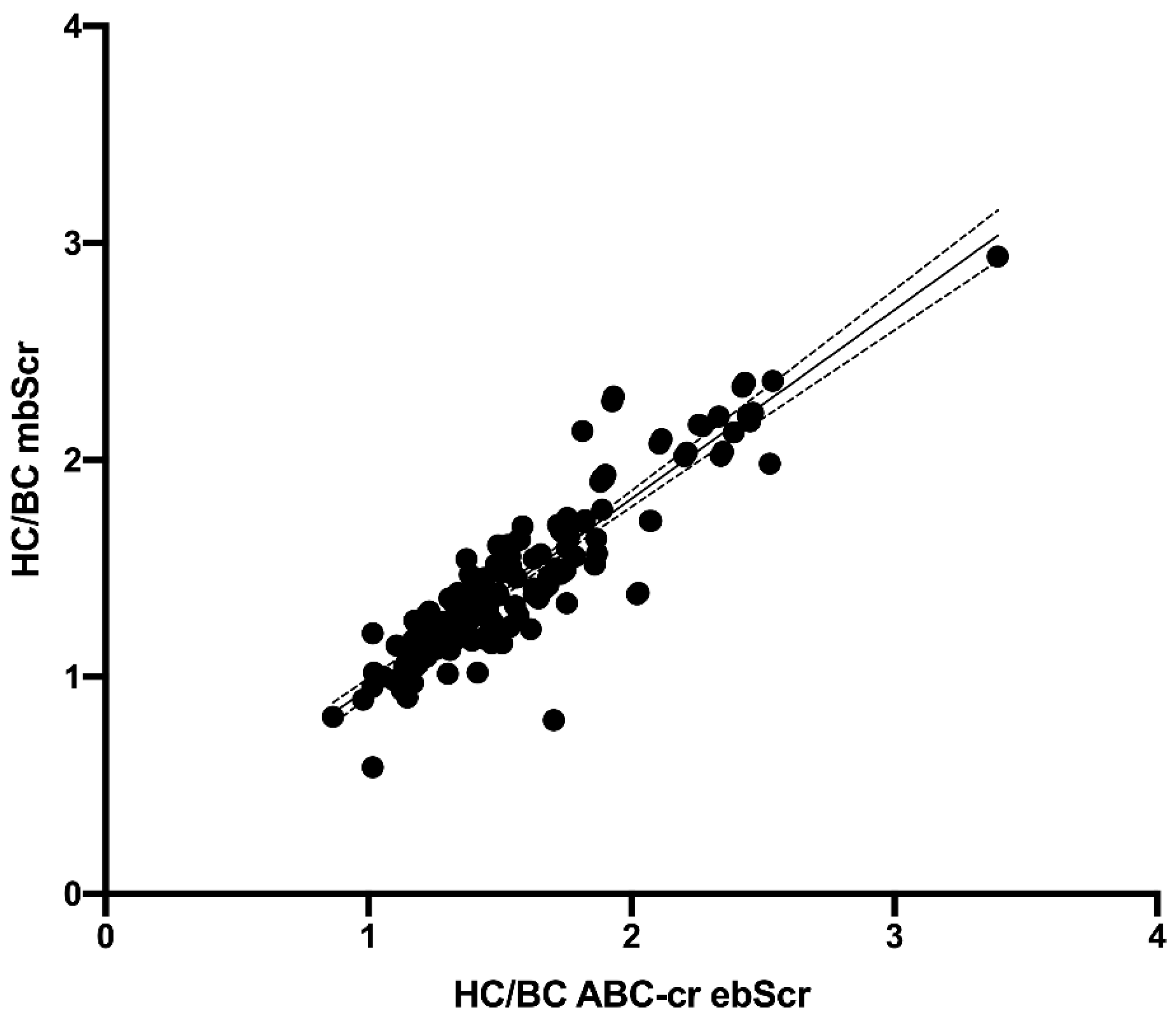
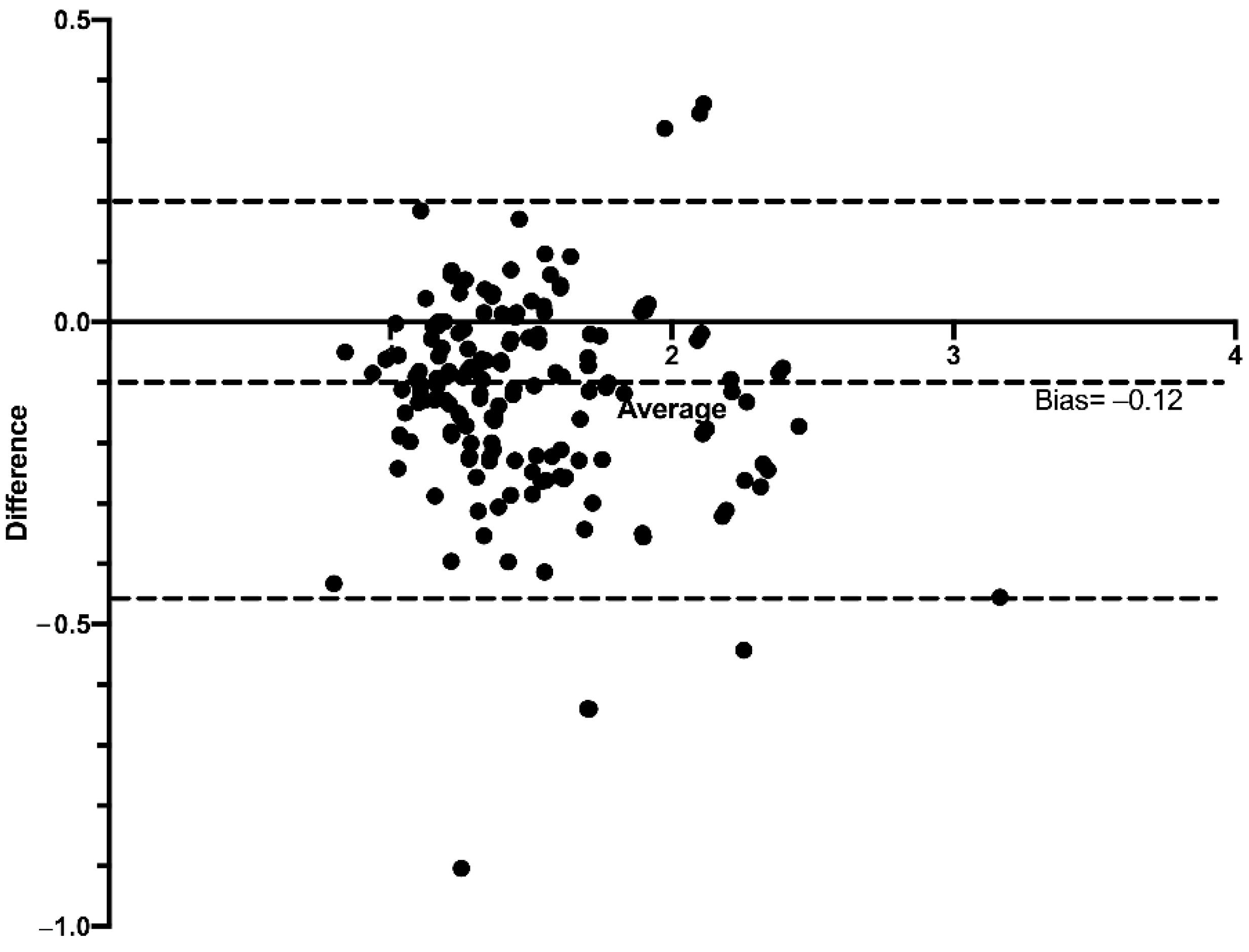
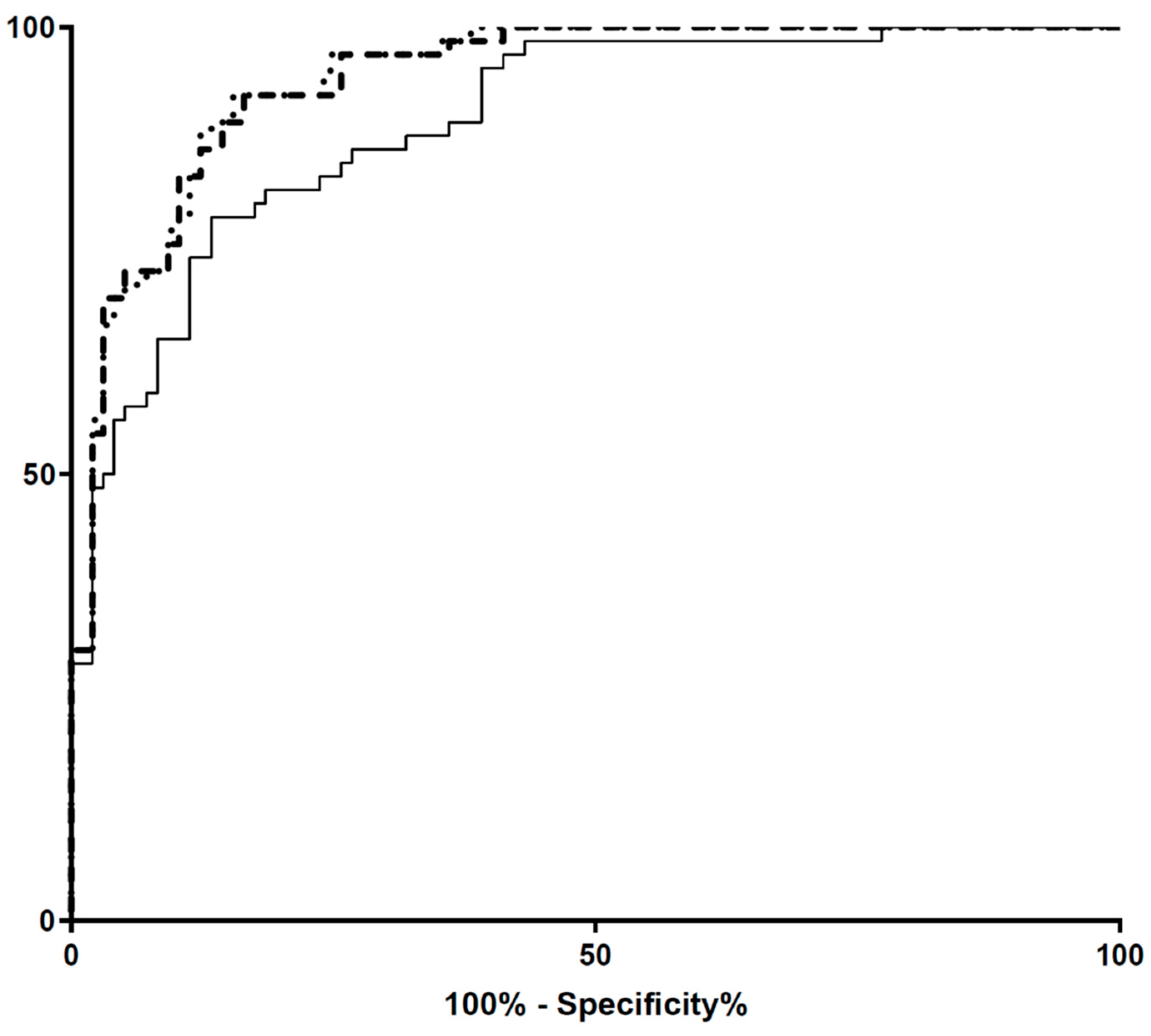
3. Results
Performance of the ebSCr Calculated on the Basis of the Examined Formulas Compared with mbSCr in Diagnosing AKI
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Marzuillo, P.; Pezzella, V.; Guarino, S.; Di Sessa, A.; Baldascino, M.; Polito, C.; Miraglia Del Giudice, E.; Nunziata, F. Acute Kidney Injury in children hospitalized for community acquired pneumonia. Pediatr. Nephrol. 2021, 36, 2883–2890. [Google Scholar] [CrossRef] [PubMed]
- Marzuillo, P.; Baldascino, M.; Guarino, S.; Perrotta, S.; Miraglia del Giudice, E.; Nunziata, F. Acute Kidney Injury in children hospitalized for acute gastroenteritis: Prevalence and risk factors. Pediatr. Nephrol. 2021, 36, 1627–1635. [Google Scholar] [CrossRef] [PubMed]
- Marzuillo, P.; Coppola, C.; Caiazzo, R.; Macchini, G.; Di Sessa, A.; Guarino, S.; Esposito, F.; del Giudice, E.M.; Tipo, V. Acute Kidney Injury in Children with Acute Appendicitis. Children 2022, 9, 620. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.; Neu, A.; Fadrowski, J. AKI in Hospitalized Children: Poorly Documented (and Underrecognized). Front. Pediatr. 2022, 9, 790509. [Google Scholar] [CrossRef] [PubMed]
- Marzuillo, P.; Iafusco, D.; Zanfardino, A.; Guarino, S.; Piscopo, A.; Casaburo, F.; Capalbo, D.; Ventre, M.; Arienzo, M.R.; Cirillo, G.; et al. Acute kidney injury and renal tubular damage in children with type 1 diabetes mellitus onset. J. Clin. Endocrinol. Metab. 2021, 106, e2720–e2737. [Google Scholar] [CrossRef] [PubMed]
- Coca, S.G.; Singanamala, S.; Parikh, C.R. Chronic kidney disease after acute kidney injury: A systematic review and meta-analysis. Kidney Int. 2012, 81, 442–448. [Google Scholar] [CrossRef] [PubMed]
- Harel, Z.; Wald, R.; Bargman, J.M.; Mamdani, M.; Etchells, E.; Garg, A.X.; Ray, J.G.; Luo, J.; Li, P.; Quinn, R.R.; et al. Nephrologist follow-up improves all-cause mortality of severe acute kidney injury survivors. Kidney Int. 2013, 83, 901–908. [Google Scholar] [CrossRef] [PubMed]
- Rivetti, G.; Hursh, B.E.; Miraglia del Giudice, E.; Marzuillo, P. Acute and chronic kidney complications in children with type 1 diabetes mellitus. Pediatr. Nephrol. 2022, in press. [Google Scholar] [CrossRef] [PubMed]
- Kellum, J.A.; Lameire, N.; Aspelin, P.; Barsoum, R.S.; Burdmann, E.A.; Goldstein, S.L.; Herzog, C.A.; Joannidis, M.; Kribben, A.; Levey, A.S.; et al. Kidney disease: Improving global outcomes (KDIGO) acute kidney injury work group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int. Suppl. 2012, 2, 1–138. [Google Scholar] [CrossRef]
- Hessey, E.; Ali, R.; Dorais, M.; Morissette, G.; Pizzi, M.; Rink, N.; Jouvet, P.; Lacroix, J.; Phan, V.; Zappitelli, M. Evaluation of height-dependent and height-independent methods of estimating baseline serum creatinine in critically ill children. Pediatr. Nephrol. 2017, 32, 1953–1962. [Google Scholar] [CrossRef] [PubMed]
- Guarino, S.; Rivetti, G.; Di Sessa, A.; De Lucia, M.; Palma, P.L.; Miraglia del Giudice, E.; Polito, C.; Marzuillo, P. Diagnostic Performance of Height-Estimated Baseline Creatinine in Diagnosing Acute Kidney Injury in Children with Type 1 Diabetes Mellitus Onset. Children 2022, 9, 899. [Google Scholar] [CrossRef] [PubMed]
- Braun, C.; Rahman, A.K.M.F.; Macomb, E.; Askenazi, D.; Bjornstad, E.C. Derivation and evaluation of baseline creatinine equations for hospitalized children and adolescents: The AKI baseline creatinine equation. Pediatr. Nephrol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, G.J.; Furth, S.L. Glomerular filtration rate measurement and estimation in chronic kidney disease. Pediatr. Nephrol. 2007, 22, 1839–1848. [Google Scholar] [CrossRef] [PubMed]
- Hursh, B.E.; Ronsley, R.; Islam, N.; Mammen, C.; Panagiotopoulos, C. Acute Kidney Injury in Children With Type 1 Diabetes Hospitalized for Diabetic Ketoacidosis. JAMA Pediatr. 2017, 171, e170020. [Google Scholar] [CrossRef] [PubMed]
- Marzuillo, P.; Grandone, A.; Di Sessa, A.; Guarino, S.; Diplomatico, M.; Umano, G.R.; Polito, C.; La Manna, A.; Perrone, L.; Miraglia del Giudice, E. Anthropometric and Biochemical Determinants of Estimated Glomerular Filtration Rate in a Large Cohort of Obese Children. J. Ren. Nutr. 2018, 28, 359–362. [Google Scholar] [CrossRef] [PubMed]
- Marzuillo, P.; Guarino, S.; Grandone, A.; Di Somma, A.; Diplomatico, M.; Rambaldi, P.F.; Decimo, F.; Miraglia del Giudice, E.; La Manna, A.; Polito, C. Congenital solitary kidney size at birth could predict reduced eGFR levels later in life. J. Perinatol. 2019, 39, 129–134. [Google Scholar] [CrossRef]
- Hanley, J.A.; McNeil, B.J. A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology 1983, 148, 839–843. [Google Scholar] [CrossRef]
- Donaghue, K.; Marcovecchio, M.; Wadwa, R.; Chew, E.; Wong, T.; Calliari, L.; Zabeen, B.; Salem, M.; Craig, M. ISPAD Clinical Practice Consensus Guidelines 2018: Microvascular and macrovascular complications in children and adolescents. Pediatr. Diabetes 2018, 19 (Suppl. 2), 262–274. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.X.; Casper, T.C.; Pitts, C.; Myers, S.; Loomba, L.; Ramesh, J.; Kuppermann, N.; Glaser, N. Association of Acute Kidney Injury during Diabetic Ketoacidosis with Risk of Microalbuminuria in Children with Type 1 Diabetes. JAMA Pediatr. 2021, 176, 169–175. [Google Scholar] [CrossRef] [PubMed]
- CDC, Surveillance System: Laboratory Reporting Using IDMS-Traceable Creatinine Calibration. Available online: https://nccd.cdc.gov/ckd/detail.aspx?Qnum=Q223 (accessed on 4 April 2022).




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palma, P.L.; Guarino, S.; Di Sessa, A.; Rivetti, G.; Barlabà, A.; Scaglione, F.; Capalbo, D.; Papparella, A.; Miraglia del Giudice, E.; Marzuillo, P. Diagnostic Performance of the Acute Kidney Injury Baseline Creatinine Equations in Children and Adolescents with Type 1 Diabetes Mellitus Onset. Diagnostics 2022, 12, 2268. https://doi.org/10.3390/diagnostics12102268
Palma PL, Guarino S, Di Sessa A, Rivetti G, Barlabà A, Scaglione F, Capalbo D, Papparella A, Miraglia del Giudice E, Marzuillo P. Diagnostic Performance of the Acute Kidney Injury Baseline Creatinine Equations in Children and Adolescents with Type 1 Diabetes Mellitus Onset. Diagnostics. 2022; 12(10):2268. https://doi.org/10.3390/diagnostics12102268
Chicago/Turabian StylePalma, Pier Luigi, Stefano Guarino, Anna Di Sessa, Giulio Rivetti, Annalisa Barlabà, Federica Scaglione, Daniela Capalbo, Alfonso Papparella, Emanuele Miraglia del Giudice, and Pierluigi Marzuillo. 2022. "Diagnostic Performance of the Acute Kidney Injury Baseline Creatinine Equations in Children and Adolescents with Type 1 Diabetes Mellitus Onset" Diagnostics 12, no. 10: 2268. https://doi.org/10.3390/diagnostics12102268
APA StylePalma, P. L., Guarino, S., Di Sessa, A., Rivetti, G., Barlabà, A., Scaglione, F., Capalbo, D., Papparella, A., Miraglia del Giudice, E., & Marzuillo, P. (2022). Diagnostic Performance of the Acute Kidney Injury Baseline Creatinine Equations in Children and Adolescents with Type 1 Diabetes Mellitus Onset. Diagnostics, 12(10), 2268. https://doi.org/10.3390/diagnostics12102268








