Clues to Disease Activity in Juvenile Dermatomyositis: Neopterin and Other Biomarkers
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Disease Activity Assessment
2.3. Methods
2.4. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Roberson, E.D.O.; Mesa, R.A.; Morgan, G.A.; Cao, L.; Marin, W.; Pachman, L.M. Transcriptomes of peripheral blood mono-nuclear cells from juvenile dermatomyositis patients show elevated inflammation even when clinically inactive. bioRxiv 2021. [Google Scholar] [CrossRef]
- Michalak, B.; Bulska, M.; Strząbała, K.; Szcześniak, P. Neopterin as a marker of cellular immunological response. Postepy Hig. Med. Dosw. 2017, 71, 727–736. [Google Scholar] [CrossRef] [PubMed]
- Molero-Luis, M.; Casas-Alba, D.; Orellana, G.; Ormazabal, A.; Sierra, C.; Oliva, C.; Valls, A.; Velasco, J.; Launes, C.; Cuadras, D.; et al. Cerebrospinal fluid neopterin as a biomarker of neuroinflammatory diseases. Sci. Rep. 2020, 10, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Thomas, B.; Bipath, P.; Viljoen, M. Comparison between plasma neopterin and the urine neopterin:creatinine ratio as inflammatory biomarkers. Afr Health Sci. 2019, 19, 2407–2413. [Google Scholar] [CrossRef]
- Chauvin, M.; Larsen, M.; Quirant, B.; Quentric, P.; Dorgham, K.; Royer, L.; Vallet, H.; Guihot, A.; Combadiere, B.; Combadiere, C.; et al. Elevated Neopterin Levels Predict Fatal Outcome in SARS-CoV-2-Infected Patients. Front. Cell Infect. Microbiol. 2021, 11, 709893. [Google Scholar] [CrossRef]
- Ibarra, M.F.; Klein-Gitelman, M.; Morgan, E.; Proytcheva, M.; Sullivan, C.; Morgan, G.; Pachman, L.M.; O’Gorman, M.R.G. Serum Neopterin Levels as a Diagnostic Marker of Hemophagocytic Lymphohistiocytosis Syndrome. Clin. Vaccine Immunol. 2011, 18, 609–614. [Google Scholar] [CrossRef]
- Mildvan, D.; Spritzler, J.; Grossberg, S.E.; Fahey, J.L.; Johnston, D.M.; Schock, B.R.; Kagan, J. Serum Neopterin, an Immune Activation Marker, Independently Predicts Disease Progression in Advanced HIV-1 Infection. Clin. Infect. Dis. 2005, 40, 853–858. [Google Scholar] [CrossRef]
- Pachman, L.M.; Khojah, A.M. Advances in Juvenile Dermatomyositis: Myositis Specific Antibodies Aid in Understand-ing Disease Heterogeneity. J. Pediatr. 2018, 195, 16–27. [Google Scholar] [CrossRef]
- Mendez, E.P.; Lipton, R.; Ramsey-Goldman, R.; Roettcher, P.; Bowyer, S.; Dyer, A.; Pachman, L.M. For the NIAMS Juvenile DM Registry Physician Referral Group US incidence of juvenile dermatomyositis, 1995-1998: Results from the National Institute of Arthritis and Musculoskeletal and Skin Diseases Registry. Arthritis Rheum. 2003, 49, 300–305. [Google Scholar] [CrossRef]
- Bohan, A.; Peter, J.B. Polymyositis and dermatomyositis (second of two parts). N. Engl J. Med. 1975, 292, 403–407. [Google Scholar] [CrossRef]
- Bohan, A.; Peter, J.B. Polymyositis and dermatomyositis (first of two parts). N. Engl J. Med. 1975, 292, 344–347. [Google Scholar] [CrossRef]
- LeClair, V.; Lundberg, I.E. New Myositis Classification Criteria—What We Have Learned Since Bohan and Peter. Curr. Rheumatol. Rep. 2018, 20, 1–8. [Google Scholar] [CrossRef]
- Pachman, L.M.; Nolan, B.E.; DeRanieri, D.; Khojah, A.M. Juvenile Dermatomyositis: New Clues to Diagnosis and Therapy. Curr. Treat. Options Rheumatol. 2021, 7, 39–62. [Google Scholar] [CrossRef]
- Lee, C.K.; Rao, D.T.; Gertner, R.; Gimeno, R.; Frey, A.B.; Levy, D.E. Distinct requirements for IFNs and STAT1 in NK cell func-tion. J. Immunol. 2000, 165, 3571–3577. [Google Scholar] [CrossRef]
- Nguyen, K.B.; Salazar-Mather, T.P.; Dalod, M.; Van Deusen, J.B.; Wei, X.-Q.; Liew, F.Y.; Caligiuri, M.A.; Durbin, J.E.; Biron, C.A. Coordinated and Distinct Roles for IFN-αβ, IL-12, and IL-15 Regulation of NK Cell Responses to Viral Infection. J. Immunol. 2002, 169, 4279–4287. [Google Scholar] [CrossRef]
- Briones, M.R.; Morgan, G.A.; Amoruso, M.C.; Rahmani, B.; Ryan, M.E.; Pachman, L.M. Decreased CD3-CD16+CD56+ natural killer cell counts in children with orbital myositis: A clue to disease activity. RMD Open 2017, 3, e000385. [Google Scholar] [CrossRef][Green Version]
- Brinkmann, V.; Geiger, T.; Alkan, S.; Heusser, C.H. Interferon alpha increases the frequency of interferon gam-ma-producing human CD4+ T cells. J. Exp. Med. 1993, 178, 1655–1663. [Google Scholar] [CrossRef]
- Rider, L.G.; Schiffenbauer, A.; Zito, M.; Lim, K.L.; Ahmed, A.; Zemel, L.S.; Rennebohm, R.M.; Passo, M.H.; Summers, R.M.; Hicks, J.E.; et al. Neopterin and quinolinic acid are surrogate measures of disease activity in the juvenile idiopathic inflammatory myopathies. Clin. Chem. 2002, 48, 1681–1688. [Google Scholar]
- De Benedetti, F.; De Amici, M.; Aramini, L.; Ruperto, N.; Martini, A. Correlation of serum neopterin concentrations with disease activity in juvenile dermatomyositis. Arch. Dis. Child. 1993, 69, 232–235. [Google Scholar] [CrossRef]
- Bode, R.K.; Klein-Gitelman, M.S.; Miller, M.L.; Lechman, T.S.; Pachman, L.M. Disease activity score for children with juve-nile dermatomyositis: Reliability and validity evidence. Arthritis Rheum. 2003, 49, 7–15. [Google Scholar] [CrossRef]
- Takken, T.; Elst, E.; Spermon, N.; Helders, P.J.M.; Prakken, A.B.J.; Van Der Net, J. The physiological and physical determinants of functional ability measures in children with juvenile dermatomyositis. Rheumatology 2003, 42, 591–595. [Google Scholar] [CrossRef][Green Version]
- Khojah, A.; Liu, V.; Savani, S.I.; Morgan, G.; Shore, R.; Bellm, J.; Pachman, L.M. Studies of 96 children with Juvenile Dermatomyositis: P155/140, is associated with loss of nailfold capillaries, but not generalized lipodystrophy. Arthritis Care Res. 2020. [Google Scholar] [CrossRef]
- Tansley, S.L.; Simou, S.; Shaddick, G.; Betteridge, Z.E.; Almeida, B.; Gunawardena, H.; Thomson, W.; Beresford, M.W.; Midgley, A.; Muntoni, F.; et al. Autoantibodies in juvenile-onset myositis: Their diagnostic value and associated clinical phenotype in a large UK cohort. J. Autoimmun. 2017, 84, 55–64. [Google Scholar] [CrossRef]
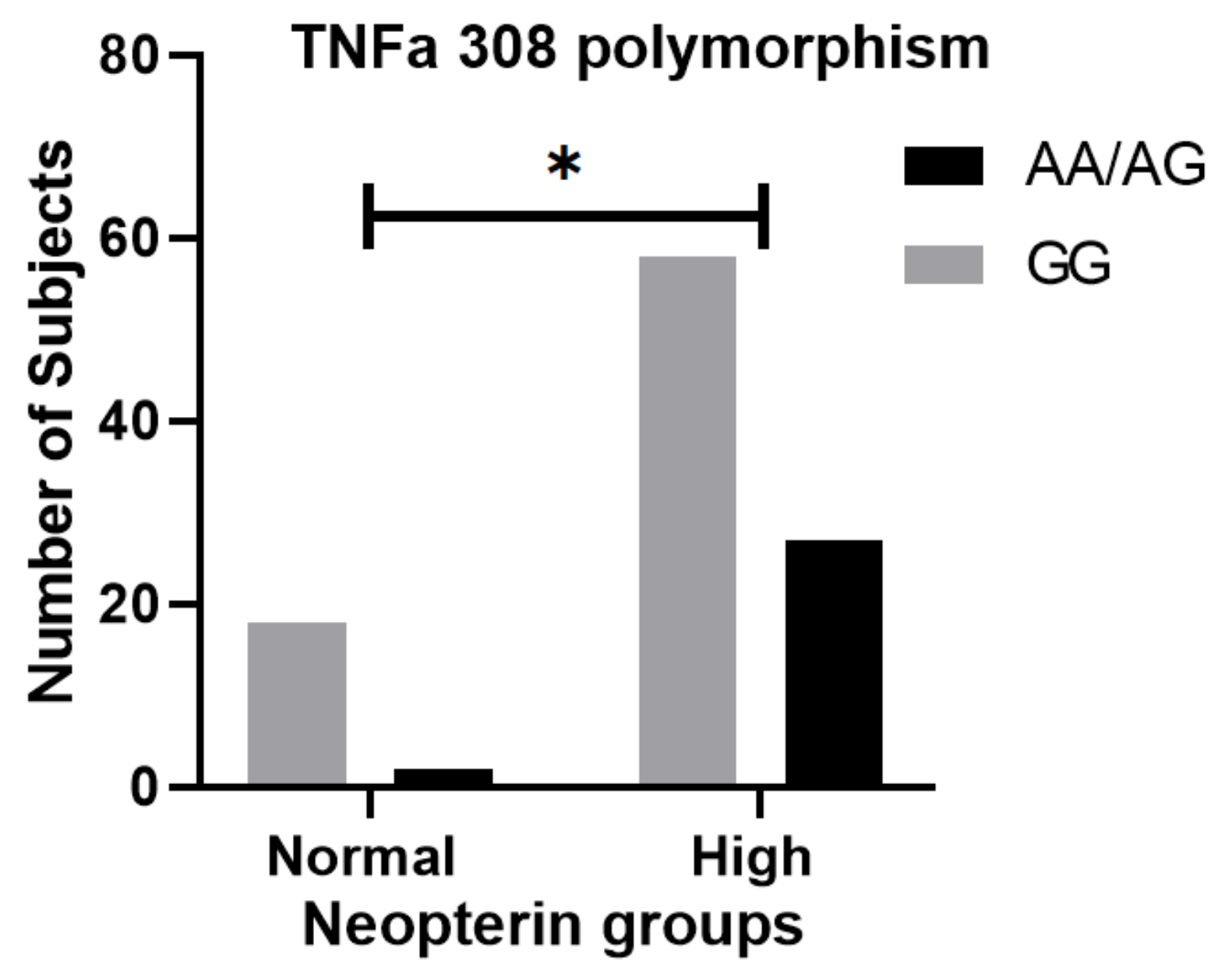
- Pachman, L.M.; Liotta-Davis, M.R.; Hong, D.K.; Kinsella, T.R.; Mendez, E.P.; Kinder, J.M.; Chen, E.H. TNFalpha-308A allele in juve-nile dermatomyositis: Association with increased production of tumor necrosis factor alpha, disease duration, and pathologic calcifications. Arthritis Rheum. 2000, 43, 2368–2377. [Google Scholar] [CrossRef]
- Tawalbeh, S.M.; Marin, W.; Morgan, G.A.; Dang, U.J.; Hathout, Y.; Pachman, L.M. Serum protein biomarkers for juvenile dermatomyositis: A pilot study. BMC Rheumatol. 2020, 4, 1–15. [Google Scholar] [CrossRef]
- Peng, Q.; Zhang, Y.; Liang, L.; Liu, X.; Ye, L.; Yang, H.; Zhang, L.; Shu, X.M.; Wang, G. A high level of serum neopterin is associated with rapidly progressive interstitial lung disease and reduced survival in dermatomyositis. Clin. Exp. Immunol. 2020, 199, 314–325. [Google Scholar] [CrossRef]
- Nishioka, A.; Tsunoda, S.; Abe, T.; Yoshikawa, T.; Takata, M.; Kitano, M.; Matsui, K.; Nakashima, R.; Hosono, Y.; Ohmura, K.; et al. Serum neopterin as well as ferritin, soluble interleukin-2 receptor, KL-6 and anti-MDA5 antibody titer provide markers of the response to therapy in patients with interstitial lung disease complicating anti-MDA5 antibody-positive dermatomyositis. Mod. Rheumatol. 2019, 29, 814–820. [Google Scholar] [CrossRef] [PubMed]
- Prestridge, A.; Morgan, G.; Ferguson, L.; Huang, C.-C.; Pachman, L.M. Pulmonary Function Tests in Idiopathic Inflammatory Myopathy: Association with Clinical Parameters in Children. Arthritis Rheum. 2013, 65, 1424–1431. [Google Scholar] [CrossRef] [PubMed]
- Fedczyna, T.O.; Lutz, J.; Pachman, L.M. Expression of TNFalpha by muscle fibers in biopsies from children with un-treated juvenile dermatomyositis: Association with the TNFalpha-308A allele. Clin. Immunol. 2001, 100, 236–239. [Google Scholar] [CrossRef] [PubMed]
- Lopez De Padilla, C.M.; Vallejo, A.N.; Lacomis, D.; McNallan, K.; Reed, A.M. Extranodal lymphoid microstructures in in-flamed muscle and disease severity of new-onset juvenile dermatomyositis. Arthritis Rheum. 2009, 60, 1160–1172. [Google Scholar] [CrossRef] [PubMed]
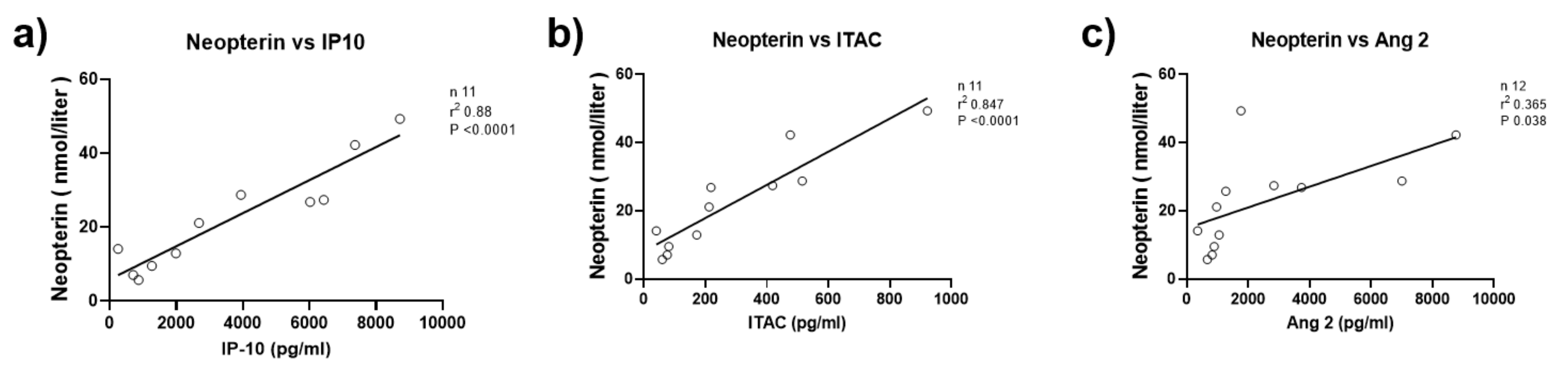
- Wienke, J.; Pachman, L.M.; Morgan, G.A.; Yeo, J.G.; Amoruso, M.C.; Hans, V.; Kamphuis, S.S.M.; Hoppenreijs, E.P.A.H.; Armbrust, W.; Berg, J.v.D.; et al. Endothelial and Inflammation Biomarker Profiles at Diagnosis Reflecting Clinical Heterogeneity and Serving as a Prognostic Tool for Treatment Response in Two Independent Cohorts of Patients with Juvenile Dermatomyositis. Arthritis Rheumatol. 2020, 72, 1214–1226. [Google Scholar] [CrossRef]
- Conklin, L.S.; Merkel, P.A.; Pachman, L.M.; Parikh, H.; Tawalbeh, S.; Damsker, J.M.; Cuthberthson, D.D.; Morgan, G.A.; Monach, P.; Hathout, Y.; et al. Serum biomarkers of glucocorti-coid response and safety in anti-neutrophil cytoplasmic antibody-associated vasculitis and juvenile dermatomyositis. Steroids 2018, 140, 159–166. [Google Scholar] [CrossRef]
- Smith, R.L.; Sundberg, J.; Shamiyah, E.; Dyer, A.; Pachman, L.M. Skin involvement in juvenile dermatomyositis is associated with loss of end row nailfold capillary loops. J. Rheumatol. 2004, 31, 1644–1649. [Google Scholar]
- Ostrowski, R.A.; Sullivan, C.L.; Seshadri, R.; Morgan, G.A.; Pachman, L.M. Association of normal nailfold end row loop numbers with a shorter duration of untreated disease in children with juvenile dermatomyositis. Arthritis Rheum. 2010, 62, 1533–1538. [Google Scholar] [CrossRef]

| Elevated Serum Neopterin Group | Normal Serum Neopterin Group | p-Value | |
|---|---|---|---|
| Number of subjects | 110 | 29 | |
| Age at onset of symptoms in years (mean/SD) | 6.97 | 6.81 | 0.832 |
| Duration of untreated disease in months (mean/SD) | 8.19 | 20.63 | 0.030 |
| Gender | |||
| Female | 84 (76%) | 24 (83%) | 0.462 |
| Male | 26 (24%) | 5 (17%) | |
| Race/ethnicity | |||
| White, Non-Hispanic | 79 (72%) | 23 (79%) | 0.840 |
| White, Hispanic | 18 (16%) | 4 (14%) | |
| African American | 5 (5%) | 1 (3%) | |
| Asian | 4 (4%) | 0 (0%) | |
| Others | 3 (3%) | 1 (3%) | |
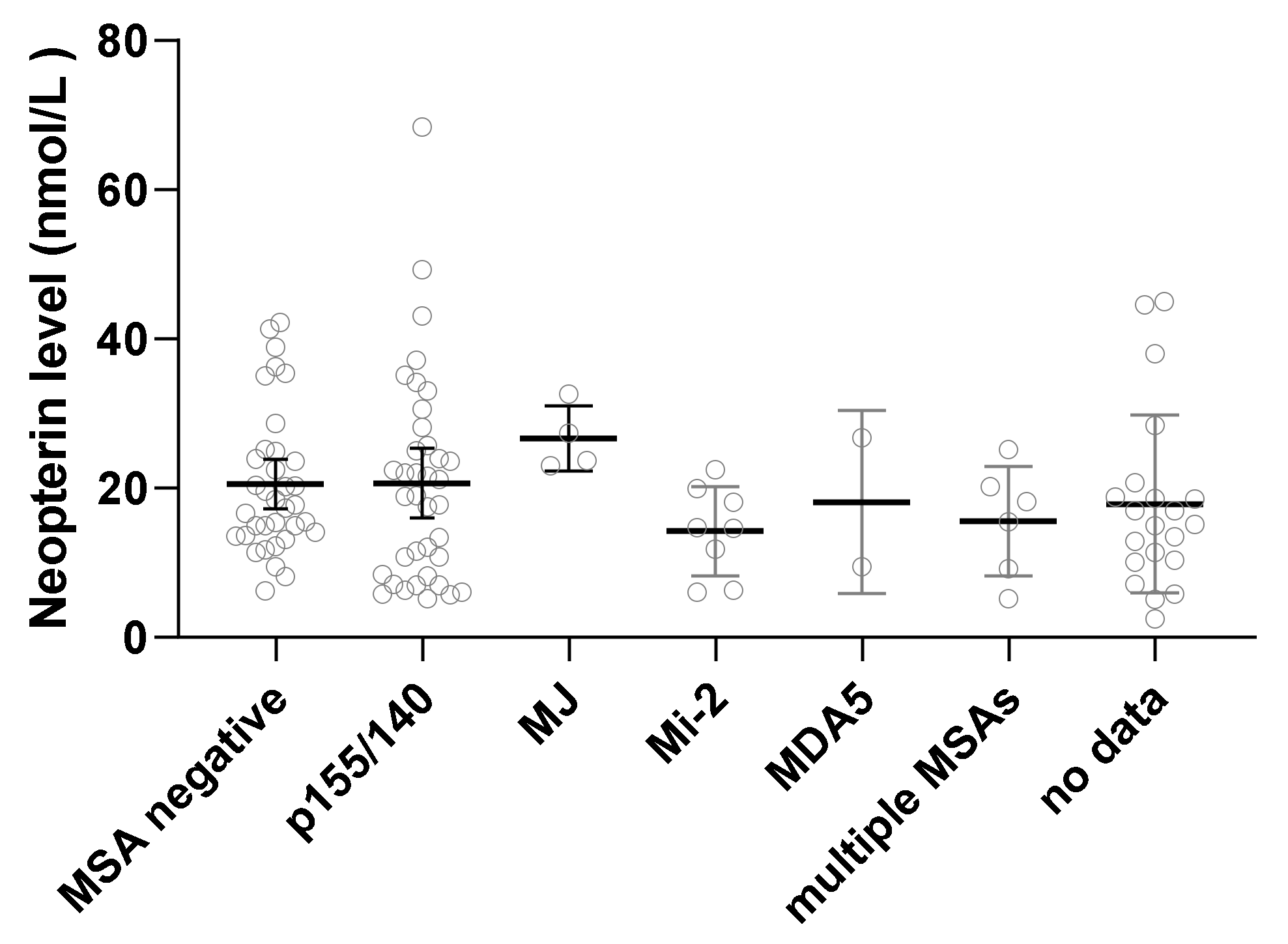
| Myositis-specific antibodies | |||
| P155/140 | 30 (27%) | 11 (38%) | 0.167 |
| MJ | 7 (6%) | 0 (0%) | |
| Mi2 | 8 (7%) | 2 (7%) | |
| MDA5 | 1 (1%) | 1 (3%) | |
| Multiple MSAs | 4 (4%) | 4 (14%) | |
| Negative | 33 (30%) | 4 (14%) | |
| Not done | 26 (24%) | 7 (24%) | |
| Disease course | |||
| Monophasic | 48 (44%) | 13 (45%) | 0.674 |
| Polyphasic | 15 (14%) | 6 (21%) | |
| Chronic | 21 (19%) | 3 (10%) | |
| Unknown | 26 (24%) | 7 (24%) |
| Clinical Findings | Elevated Serum Neopterin Group | Normal Serum Neopterin Group | p-Value |
|---|---|---|---|
| Clinical disease activity indicator | |||
| Disease activity score—total | 11.92 ± 3.19 | 8.13 ± 3.57 | <0.0001 |
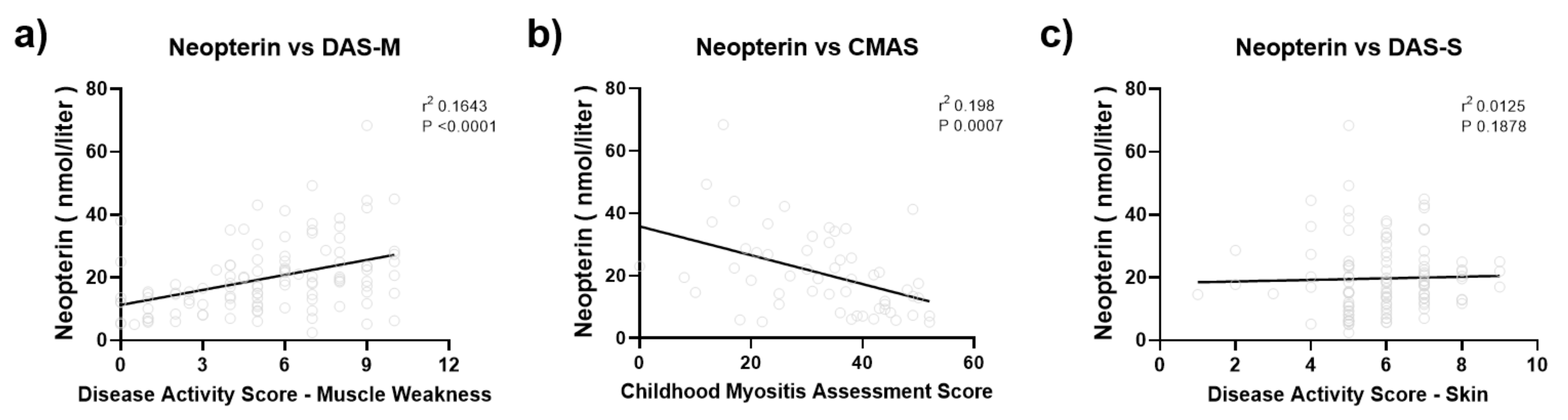
| Disease activity score—skin | 6.06 ± 1.47 | 4.86 ± 1.58 | 0.0002 |
| Disease activity score—muscle weakness | 5.84 ± 2.69 | 3.13 ± 2.89 | <0.0001 |
| Childhood Myositis Assessment Scale (CMAS) | 30.05 ± 12.60 | 40.43 ± 9.94 | 0.007 |
| Nailfold capillary end row loops (ERL) | 4.86 ± 1.70 | 4.99 ± 1.25 | 0.749 |
| Laboratory disease activity indicator | |||
| Erythrocyte sedimentation rate (ESR) | 19.8 ± 14.63 | 11.55 ± 9.03 | 0.01 |
| von Willebrand factor antigen | 171.1 ± 79.68 | 109.15 ± 57.23 | <0.0001 |
| Muscle enzymes | |||
| Creatine phosphokinase (CK) | 2486.51 ± 7494.67 | 724.81 ± 3116.15 | 0.244 |
| Aspartate aminotransferase (AST) | 136.45 ± 224.89 | 53.96 ± 103.02 | 0.008 |
| Lactate dehydrogenase (LDH) | 520.23 ± 407.79 | 273.19 ± 153.15 | <0.0001 |
| Aldolase | 24 ± 36.99 | 7.59 ± 3.47 | <0.0001 |
| Flow cytometry | |||
| Total T cells (CD3+) | 1494.36 ± 673.79 | 2278.43 ± 1264.74 | 0.008 |
| T helper cells (CD3+ CD4+) | 1004.06 ± 463.22 | 1533.22 ± 869.07 | 0.009 |
| T cytotoxic cells (CD3+ CD8+) | 462.97 ± 251.984 | 671.61 ± 379.72 | 0.019 |
| B cells (CD19+) | 747.81 ± 424.01 | 924.39 ± 550.99 | 0.097 |
| NK cells (CD16+/CD56+) | 144.94 ± 124.67 | 240.13 ± 159.41 | 0.003 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khojah, A.; Morgan, G.; Pachman, L.M. Clues to Disease Activity in Juvenile Dermatomyositis: Neopterin and Other Biomarkers. Diagnostics 2022, 12, 8. https://doi.org/10.3390/diagnostics12010008
Khojah A, Morgan G, Pachman LM. Clues to Disease Activity in Juvenile Dermatomyositis: Neopterin and Other Biomarkers. Diagnostics. 2022; 12(1):8. https://doi.org/10.3390/diagnostics12010008
Chicago/Turabian StyleKhojah, Amer, Gabrielle Morgan, and Lauren M. Pachman. 2022. "Clues to Disease Activity in Juvenile Dermatomyositis: Neopterin and Other Biomarkers" Diagnostics 12, no. 1: 8. https://doi.org/10.3390/diagnostics12010008
APA StyleKhojah, A., Morgan, G., & Pachman, L. M. (2022). Clues to Disease Activity in Juvenile Dermatomyositis: Neopterin and Other Biomarkers. Diagnostics, 12(1), 8. https://doi.org/10.3390/diagnostics12010008







