Longitudinal Changes in Brain Diffusion MRI Indices during and after Proton Beam Therapy in a Child with Pilocytic Astrocytoma: A Case Report
Abstract
:1. Introduction
2. Materials and Methods
3. Results
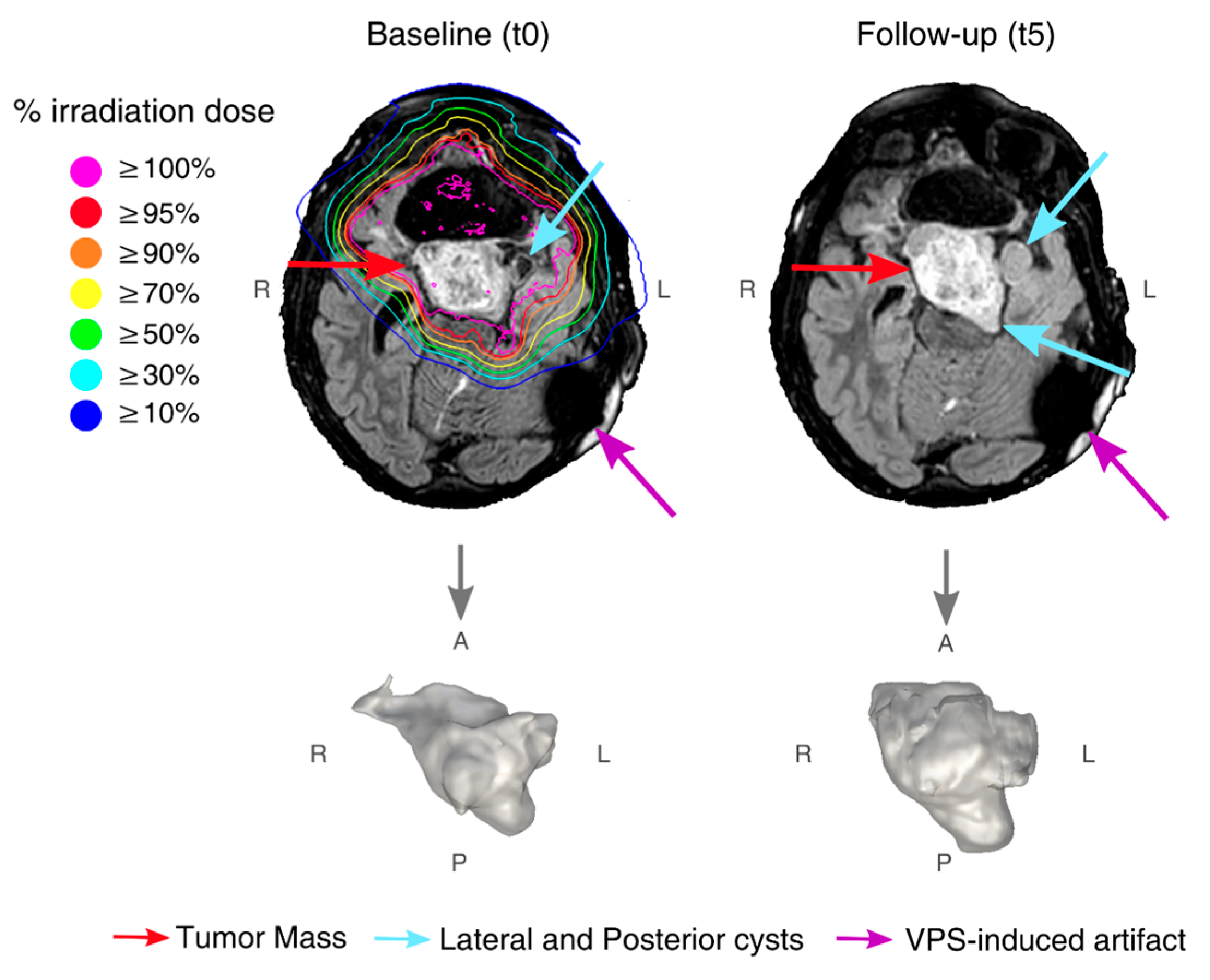
3.1. Findings at Conventional MRI
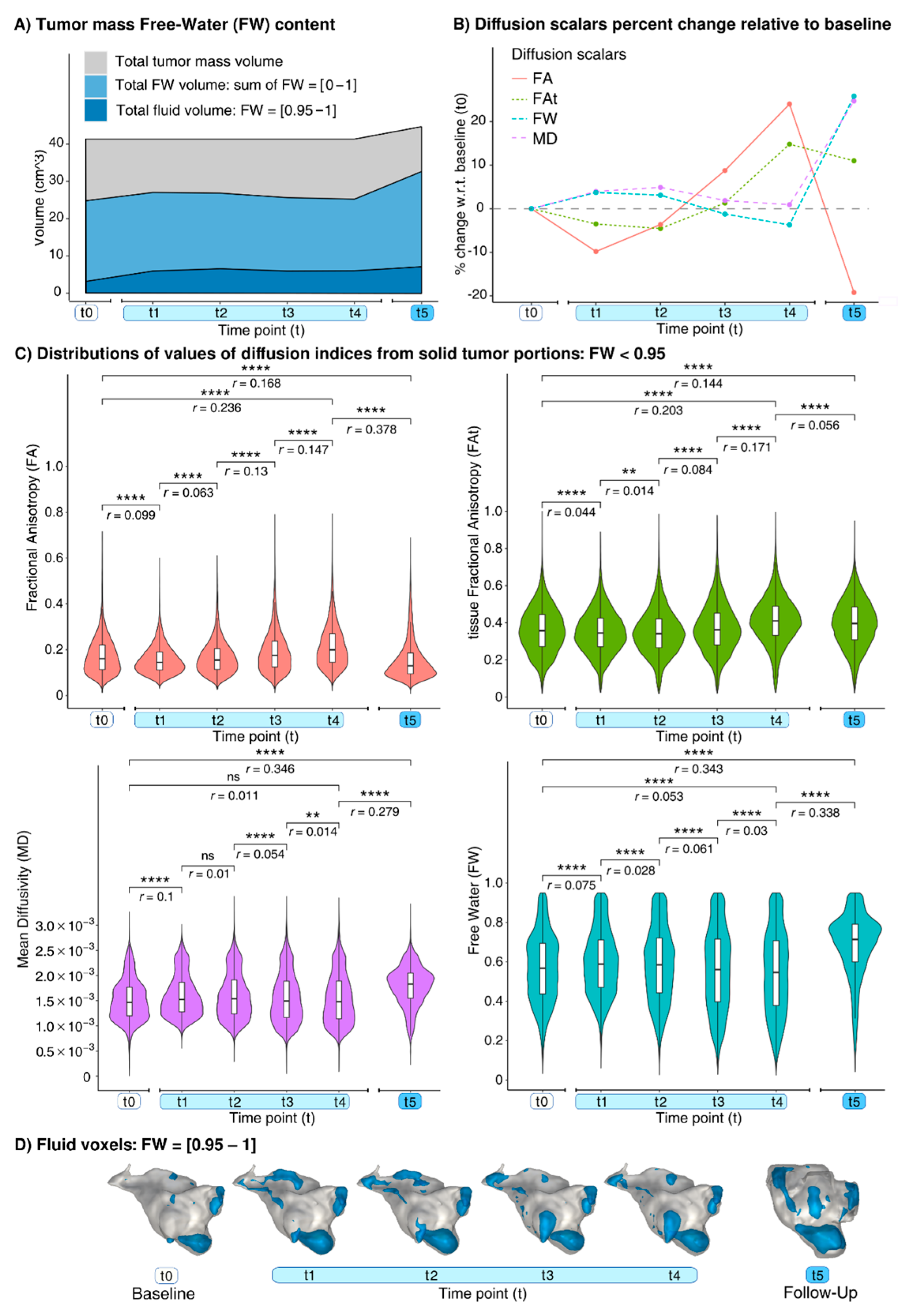
3.2. Findings at dMRI
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Levin, W.P.; Kooy, H.; Loeffler, J.S.; DeLaney, T.F. Proton beam therapy. Br. J. Cancer 2005, 93, 849–854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Han, S.; Zhu, J.; Wang, X.; Li, Y.; Wang, Z.; Lin, E.; Wang, X.; Molkentine, D.P.; Blanchard, P.; et al. Proton versus photon radiation–induced cell death in head and neck cancer cells. Head Neck 2019, 41, 46–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kralik, S.F.; Ho, C.Y.; Finke, W.; Buchsbaum, J.C.; Haskins, C.P.; Shih, C.-S. Radiation necrosis in pediatric patients with brain tumors treated with proton radiotherapy. AJNR Am. J. Neuroradiol. 2015, 36, 1572–1578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chenevert, T.L.; Stegman, L.D.; Taylor, J.M.; Robertson, P.L.; Greenberg, H.S.; Rehemtulla, A.; Ross, B.D. Diffusion magnetic resonance imaging: An early surrogate marker of therapeutic efficacy in brain tumors. JNCI J. Natl. Cancer Inst. 2000, 92, 2029–2036. [Google Scholar] [CrossRef] [PubMed]
- Connor, M.; Karunamuni, R.; McDonald, C.; Seibert, T.; White, N.; Moiseenko, V.; Bartsch, H.; Farid, N.; Kuperman, J.; Krishnan, A.; et al. Regional susceptibility to dose-dependent white matter damage after brain radiotherapy. Radiother. Oncol. 2017, 123, 209–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basser, P.J.; Mattiello, J.; LeBihan, D. MR diffusion tensor spectroscopy and imaging. Biophys. J. 1994, 66, 259–267. [Google Scholar] [CrossRef] [Green Version]
- Pasternak, O.; Sochen, N.; Gur, Y.; Intrator, N.; Assaf, Y. Free water elimination and mapping from diffusion MRI. Magn. Reson. Med. Off. J. Int. Soc. Magn. Reson. Med. 2009, 62, 717–730. [Google Scholar] [CrossRef]
- Albi, A.; Pasternak, O.; Minati, L.; Marizzoni, M.; Bartrés-Faz, D.; Bargallo, N.; Bosch, B.; Rossini, P.M.; Marra, C.; Müller, B.; et al. Free water elimination improves test–retest reproducibility of diffusion tensor imaging indices in the brain: A longitudinal multisite study of healthy elderly subjects. Hum. Brain Mapp. 2017, 38, 12–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collins, V.P.; Jones, D.T.; Giannini, C. Pilocytic astrocytoma: Pathology, molecular mechanisms and markers. Acta Neuropathol. 2015, 129, 775–788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srinivas, U.S.; Tan, B.W.; Vellayappan, B.A.; Jeyasekharan, A.D. ROS and the DNA damage response in cancer. Redox Biol. 2019, 25, 101084. [Google Scholar] [CrossRef]
- Krisnawan, V.E.; Stanley, J.A.; Schwarz, J.K.; DeNardo, D.G. Tumor Microenvironment as a Regulator of Radiation Therapy: New Insights into Stromal-Mediated Radioresistance. Cancers 2020, 12, 2916. [Google Scholar] [CrossRef] [PubMed]
- Indelicato, D.J.; Rotondo, R.L.; Uezono, H.; Sandler, E.S.; Aldana, P.R.; Ranalli, N.J.; Beier, A.D.; Morris, C.G.; Bradley, J.A. Outcomes following proton therapy for pediatric low-grade glioma. Int. J. Radiat. Oncol. Biol. Phys. 2019, 104, 149–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hou, P.; Zhu, K.H.; Park, P.C.; Li, H.; Mahajan, A.; Grosshans, D.R. Proton Therapy for Juvenile Pilocytic Astrocytoma: Quantifying Treatment Responses by Magnetic Resonance Diffusion Tensor Imaging. Int. J. Part. Ther. 2016, 3, 414–420. [Google Scholar] [CrossRef] [Green Version]
- Golub, M.; Neto Henriques, R.; Gouveia Nunes, R. Free-water DTI estimates from single b-value data might seem plausible but must be interpreted with care. Magn. Reson. Med. 2021, 85, 2537–2551. [Google Scholar] [CrossRef] [PubMed]
| Time Points (t) | |||||||
|---|---|---|---|---|---|---|---|
| Baseline | Proton Therapy Treatment | Follow-Up | |||||
| t0 | t1 | t2 | t3 | t4 | t5 | ||
| dMRI acquisition from treatment start (days) | − | 9 | 17 | 24 | 30 | 269 | |
| Cumulative dose (Gy) (percentage of total dose) | none | 16.2 (30%) | 30.6 (56%) | 43.2 (80%) | 54 (100%) | none | |
| FA | median | 0.153 | 0.136 | 0.141 | 0.159 | 0.182 | 0.118 |
| ∆ | − | −11.1% | −7.8% | +3.9% | +19% | −22.9% | |
| Q1 Q3 | 0.104 0.214 | 0.1 0.181 | 0.099 0.193 | 0.108 0.225 | 0.126 0.255 | 0.084 0.172 | |
| FAt | median | 0.344 | 0.321 | 0.315 | 0.337 | 0.386 | 0.367 |
| ∆ | − | −6.7% | −8.4% | −2% | +12.2% | +6.7% | |
| Q1 Q3 | 0.247 0.436 | 0.221 0.41 | 0.208 0.403 | 0.223 0.434 | 0.269 0.474 | 0.238 0.465 | |
| MD (×10−3) | median | 1.506 | 1.618 | 1.656 | 1.615 | 1.607 | 1.913 |
| ∆ | − | +7.4% | +10% | +7.2% | +6.7% | +27% | |
| Q1 Q3 | 1.215 1.855 | 1.321 2.191 | 1.294 2.285 | 1.217 2.211 | 1.193 2.215 | 1.615 2.249 | |
| FW | median | 0.589 | 0.627 | 0.635 | 0.614 | 0.603 | 0.745 |
| ∆ | − | +6.5% | +7.8% | +4.2% | +2.3% | +26.5% | |
| Q1 Q3 | 0.448 0.726 | 0.492 0.809 | 0.471 0.841 | 0.426 0.815 | 0.407 0.811 | 0.628 0.857 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Novello, L.; Agarwal, N.; Vennarini, S.; Lorentini, S.; Zacà, D.; Mussano, A.; Pasternak, O.; Jovicich, J. Longitudinal Changes in Brain Diffusion MRI Indices during and after Proton Beam Therapy in a Child with Pilocytic Astrocytoma: A Case Report. Diagnostics 2022, 12, 26. https://doi.org/10.3390/diagnostics12010026
Novello L, Agarwal N, Vennarini S, Lorentini S, Zacà D, Mussano A, Pasternak O, Jovicich J. Longitudinal Changes in Brain Diffusion MRI Indices during and after Proton Beam Therapy in a Child with Pilocytic Astrocytoma: A Case Report. Diagnostics. 2022; 12(1):26. https://doi.org/10.3390/diagnostics12010026
Chicago/Turabian StyleNovello, Lisa, Nivedita Agarwal, Sabina Vennarini, Stefano Lorentini, Domenico Zacà, Anna Mussano, Ofer Pasternak, and Jorge Jovicich. 2022. "Longitudinal Changes in Brain Diffusion MRI Indices during and after Proton Beam Therapy in a Child with Pilocytic Astrocytoma: A Case Report" Diagnostics 12, no. 1: 26. https://doi.org/10.3390/diagnostics12010026
APA StyleNovello, L., Agarwal, N., Vennarini, S., Lorentini, S., Zacà, D., Mussano, A., Pasternak, O., & Jovicich, J. (2022). Longitudinal Changes in Brain Diffusion MRI Indices during and after Proton Beam Therapy in a Child with Pilocytic Astrocytoma: A Case Report. Diagnostics, 12(1), 26. https://doi.org/10.3390/diagnostics12010026







