Feasibility of Recombinant Human TSH as a Preparation for Radioiodine Therapy in Patients with Distant Metastases from Papillary Thyroid Cancer: Comparison of Long-Term Survival Outcomes with Thyroid Hormone Withdrawal
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Treatment Protocol
2.3. Preparation for RAI Treatment
2.4. Statistical Analysis
3. Results
3.1. Patients
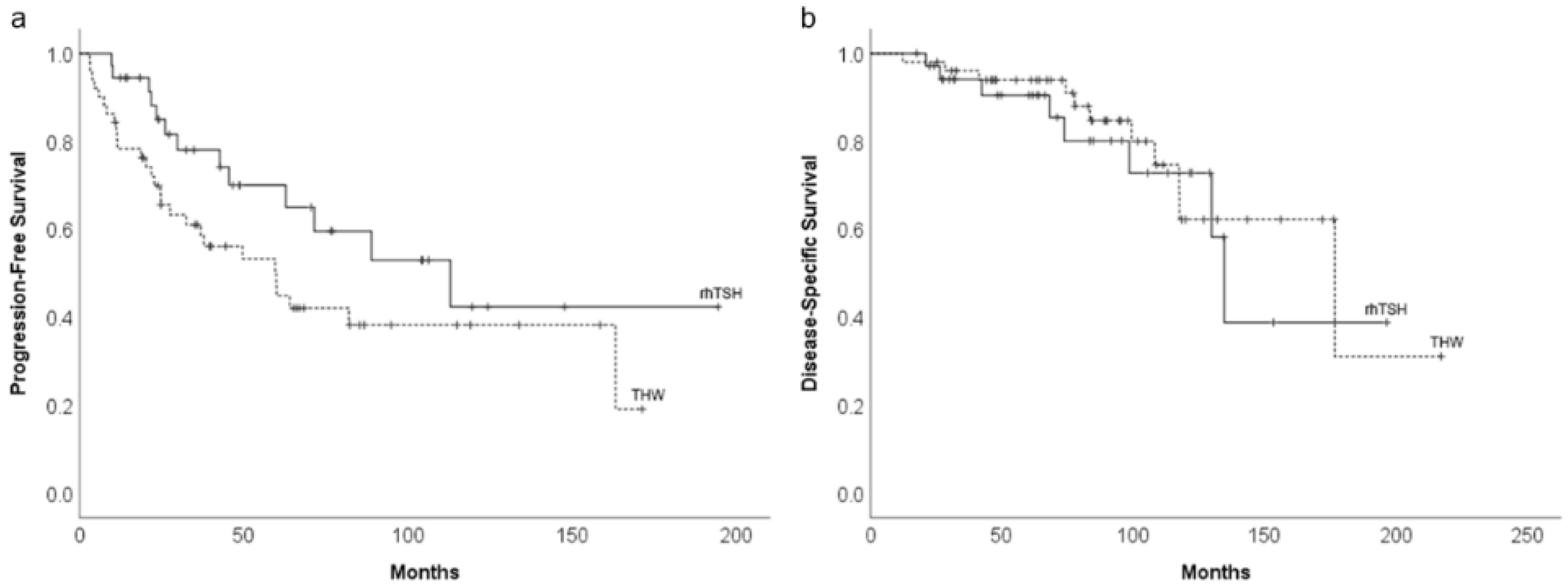
3.2. Long-Term Survival Impact of THW or rhTSH Preparation
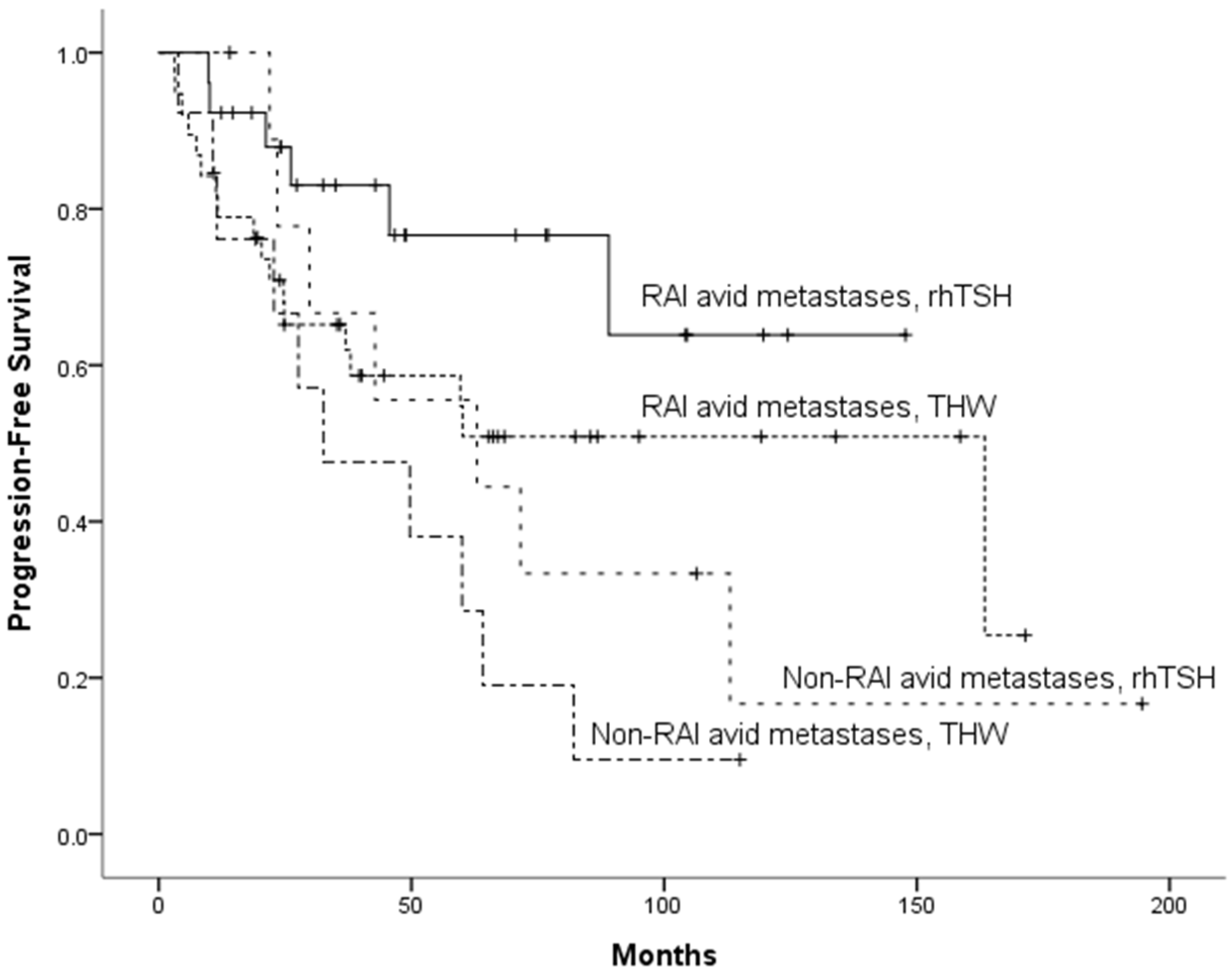
3.3. Risk Factors for PFS
3.4. Risk Factors for DSS
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Luster, M.; Sherman, S.I.; Skarulis, M.C.; Reynolds, J.R.; Lassmann, M.; Hanscheid, H.; Reiners, C. Comparison of radioiodine biokinetics following the administration of recombinant human thyroid stimulating hormone and after thyroid hormone withdrawal in thyroid carcinoma. Eur. J. Nucl. Med. Mol. Imaging 2003, 30, 1371–1377. [Google Scholar] [CrossRef]
- Hanscheid, H.; Lassmann, M.; Luster, M.; Thomas, S.R.; Pacini, F.; Ceccarelli, C.; Ladenson, P.W.; Wahl, R.L.; Schlumberger, M.; Ricard, M.; et al. Iodine biokinetics and dosimetry in radioiodine therapy of thyroid cancer: Procedures and results of a prospective international controlled study of ablation after rhTSH or hormone withdrawal. J. Nucl. Med. 2006, 47, 648–654. [Google Scholar] [PubMed]
- Luster, M. Acta Oncologica Lecture. Present status of the use of recombinant human TSH in thyroid cancer management. Acta Oncol. 2006, 45, 1018–1030. [Google Scholar] [CrossRef] [PubMed]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tu, J.; Wang, S.; Huo, Z.; Lin, Y.; Li, X.; Wang, S. Recombinant human thyrotropin-aided versus thyroid hormone withdrawal-aided radioiodine treatment for differentiated thyroid cancer after total thyroidectomy: A meta-analysis. Radiother. Oncol. 2014, 110, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Rani, D.; Kaisar, S.; Awasare, S.; Kamaldeep; Abhyankar, A.; Basu, S. Examining recombinant human TSH primed 131I therapy protocol in patients with metastatic differentiated thyroid carcinoma: Comparison with the traditional thyroid hormone withdrawal protocol. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 1767–1780. [Google Scholar] [CrossRef] [PubMed]
- Klubo-Gwiezdzinska, J.; Burman, K.D.; Van Nostrand, D.; Mete, M.; Jonklaas, J.; Wartofsky, L. Potential use of recombinant human thyrotropin in the treatment of distant metastases in patients with differentiated thyroid cancer. Endocr. Pract. 2013, 19, 139–148. [Google Scholar] [CrossRef] [Green Version]
- Klubo-Gwiezdzinska, J.; Burman, K.D.; Van Nostrand, D.; Mete, M.; Jonklaas, J.; Wartofsky, L. Radioiodine treatment of metastatic thyroid cancer: Relative efficacy and side effect profile of preparation by thyroid hormone withdrawal versus recombinant human thyrotropin. Thyroid 2012, 22, 310–317. [Google Scholar] [CrossRef] [Green Version]
- Tala, H.; Robbins, R.; Fagin, J.A.; Larson, S.M.; Tuttle, R.M. Five-year survival is similar in thyroid cancer patients with distant metastases prepared for radioactive iodine therapy with either thyroid hormone withdrawal or recombinant human TSH. J. Clin. Endocrinol. Metab. 2011, 96, 2105–2111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taieb, D.; Jacob, T.; Zotian, E.; Mundler, O. Lack of efficacy of recombinant human thyrotropin versus thyroid hormone withdrawal for radioiodine therapy imaging in a patient with differentiated thyroid carcinoma lung metastases. Thyroid 2004, 14, 465–467. [Google Scholar] [CrossRef]
- Driedger, A.A.; Kotowycz, N. Two cases of thyroid carcinoma that were not stimulated by recombinant human thyrotropin. J. Clin. Endocrinol. Metab. 2004, 89, 585–590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Nostrand, D.; Khorjekar, G.R.; O’Neil, J.; Moreau, S.; Atkins, F.B.; Kharazi, P.; Mete, M.; Chennupati, S.P.; Burman, K.D.; Wartofsky, L. Recombinant human thyroid-stimulating hormone versus thyroid hormone withdrawal in the identification of metastasis in differentiated thyroid cancer with 131I planar whole-body imaging and 124I PET. J. Nucl. Med. 2012, 53, 359–362. [Google Scholar] [CrossRef] [Green Version]
- Plyku, D.; Hobbs, R.F.; Huang, K.; Atkins, F.; Garcia, C.; Sgouros, G.; Van Nostrand, D. Recombinant Human Thyroid-Stimulating Hormone Versus Thyroid Hormone Withdrawal in 124I PET/CT-Based Dosimetry for 131I Therapy of Metastatic Differentiated Thyroid Cancer. J. Nucl. Med. 2017, 58, 1146–1154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, D.H.; Chang, S.H.; Chang, T.C. The impact of locoregional recurrences and distant metastases on the survival of patients with papillary thyroid carcinoma. Clin. Endocrinol. 2015, 82, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.; Devesa, S.S.; Sosa, J.A.; Check, D.; Kitahara, C.M. Trends in Thyroid Cancer Incidence and Mortality in the United States, 1974–2013. JAMA 2017, 317, 1338–1348. [Google Scholar] [CrossRef] [PubMed]
- Edge, S.B.; Byrd, D.R.; Carducci, M.A.; Compton, C.C.; Fritz, A.; Greene, F. AJCC Cancer Staging Manual; Springer: New York, NY, USA, 2010; Volume 7. [Google Scholar]
- Lin, J.D.; Kuo, S.F.; Huang, B.Y.; Lin, S.F.; Chen, S.T. The efficacy of radioactive iodine for the treatment of well-differentiated thyroid cancer with distant metastasis. Nucl. Med. Commun. 2018, 39, 1091–1096. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, H.I.; Kim, S.W.; Jung, J.; Jeon, M.J.; Kim, W.G.; Kim, T.Y.; Kim, H.K.; Kang, H.C.; Han, J.M.; et al. Prognosis of Differentiated Thyroid Carcinoma with Initial Distant Metastasis: A Multicenter Study in Korea. Endocrinol. Metab. 2018, 33, 287–295. [Google Scholar] [CrossRef]
- Lang, B.H.; Wong, K.P.; Cheung, C.Y.; Wan, K.Y.; Lo, C.Y. Evaluating the prognostic factors associated with cancer-specific survival of differentiated thyroid carcinoma presenting with distant metastasis. Ann. Surg. Oncol. 2013, 20, 1329–1335. [Google Scholar] [CrossRef] [Green Version]
- Shoup, M.; Stojadinovic, A.; Nissan, A.; Ghossein, R.A.; Freedman, S.; Brennan, M.F.; Shah, J.P.; Shaha, A.R. Prognostic indicators of outcomes in patients with distant metastases from differentiated thyroid carcinoma. J. Am. Coll. Surg. 2003, 197, 191–197. [Google Scholar] [CrossRef]
- Kakudo, K.; Bychkov, A.; Bai, Y.; Li, Y.; Liu, Z.; Jung, C.K. The new 4th edition World Health Organization classification for thyroid tumors, Asian perspectives. Pathol. Int. 2018, 68, 641–664. [Google Scholar] [CrossRef] [Green Version]
- Schlumberger, M.; Tahara, M.; Wirth, L.J.; Robinson, B.; Brose, M.S.; Elisei, R.; Habra, M.A.; Newbold, K.; Shah, M.H.; Hoff, A.O.; et al. Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N. Engl. J. Med. 2015, 372, 621–630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brose, M.S.; Nutting, C.M.; Jarzab, B.; Elisei, R.; Siena, S.; Bastholt, L.; de la Fouchardiere, C.; Pacini, F.; Paschke, R.; Shong, Y.K.; et al. Sorafenib in radioactive iodine-refractory, locally advanced or metastatic differentiated thyroid cancer: A randomised, double-blind, phase 3 trial. Lancet 2014, 384, 319–328. [Google Scholar] [CrossRef] [Green Version]

| THW (n = 51) | rhTSH (n = 37) | p-Value | |
|---|---|---|---|
| Age at diagnosis, n (%) | |||
| <55 years | 38 (75%) | 20 (54%) | 0.068 |
| ≥55 years | 13 (25%) | 17 (46%) | |
| Sex, n (%) | |||
| Man | 23 (45%) | 13 (35%) | 0.386 |
| Woman | 28 (55%) | 24 (65%) | |
| Site of distant metastasis, n (%) | |||
| Isolated pulmonary metastases | 37 (73%) | 25 (68%) | 0.642 |
| Other sites 1 | 14 (27%) | 12 (32%) | |
| Diagnostic time of distant metastasis, n (%) | |||
| Initial | 25 (49%) | 19 (51%) | 1.000 |
| Late | 26 (51%) | 18 (49%) | |
| Stage at diagnosis 2, n (%) | |||
| I–II | 35 (69%) | 21 (57%) | 0.271 |
| III–IV | 16 (31%) | 16 (43%) | |
| Cycles of RAI therapy, median (range) | 6 (1–16) | 5 (2–16) | 0.769 |
| RAI avidity, n (%) | |||
| RAI avid | 38 (75%) | 26 (70%) | 0.809 |
| RAI non-avid | 13 (25%) | 11 (30%) | |
| Targeted therapy, n (%) | |||
| No | 41 (80%) | 28 (76%) | 0.610 |
| Yes | 10 (20%) | 9 (24%) |
| Univariate Analysis | p-Value | Multivariate Analysis | p-Value | |
|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | |||
| Sex | ||||
| Woman vs. man | 0.566 (0.305–1.049) | 0.071 | ||
| Age at diagnosis | ||||
| <55 year vs. ≥55 year | 0.741 (0.386–1.422) | 0.367 | ||
| Preparation | ||||
| rhTSH vs. THW | 0.551 (0.285–1.065) | 0.076 | 0.499 (0.256―0.971) | 0.041 |
| Diagnostic time of distant metastasis | ||||
| Initial vs. late | 0.892 (0.482–1.651) | 0.716 | ||
| Site of distant metastasis | ||||
| Isolated pulmonary metastases vs. other sites 1 | 1.011 (0.515–1.985) | 0.974 | ||
| Stage at diagnosis 2 | ||||
| I + II vs. III + IV | 0.657 (0.339–1.273) | 0.214 | ||
| RAI avidity | ||||
| RAI-avid vs. Non-RAI-avid | 0.539 (0.290–1.004) | 0.052 | 0.488 (0.260―0.914) | 0.025 |
| Targeted therapy | ||||
| No vs. Yes | 0.701 (0.362–1.356) | 0.291 |
| Univariate Analysis | p-Value | Multivariate Analysis | p-Value | |
|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | |||
| Sex | ||||
| Woman vs. man | 0.301 (0.114–0.795) | 0.015 | 0.305 (0.115–0.808) | 0.017 |
| Age at diagnosis | ||||
| <55 year vs. ≥55 year | 0.250 (0.099–0.627) | 0.003 | 0.246 (0.098–0.617) | 0.003 |
| Preparation | ||||
| rhTSH vs. THW | 0.814 (0.327–2.027) | 0.658 | ||
| Diagnostic time of distant metastasis | ||||
| Initial vs. late | 0.870 (0.349–2.172) | 0.766 | ||
| Site of distant metastasis | ||||
| Isolated pulmonary metastases vs. other sites 1 | 0.430 (0.174–1.063) | 0.068 | 0.383 (0.149–0.985) | 0.047 |
| Stage at diagnosis 2 | ||||
| I + II vs. III + IV | 0.335 (0.133–0.844) | 0.020 | ||
| RAI avidity | ||||
| RAI-avid vs. Non-RAI-avid | 0.410 (0.166–1.012) | 0.053 | ||
| Targeted therapy | ||||
| No vs. yes | 0.891 (0.337–2.358) | 0.816 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, H.-C.; Ho, K.-C.; Chen, S.-H.; Tseng, J.-R.; Yang, L.-Y.; Lin, K.-J.; Cheng, J.-C.; Liou, M.-J. Feasibility of Recombinant Human TSH as a Preparation for Radioiodine Therapy in Patients with Distant Metastases from Papillary Thyroid Cancer: Comparison of Long-Term Survival Outcomes with Thyroid Hormone Withdrawal. Diagnostics 2022, 12, 221. https://doi.org/10.3390/diagnostics12010221
Tsai H-C, Ho K-C, Chen S-H, Tseng J-R, Yang L-Y, Lin K-J, Cheng J-C, Liou M-J. Feasibility of Recombinant Human TSH as a Preparation for Radioiodine Therapy in Patients with Distant Metastases from Papillary Thyroid Cancer: Comparison of Long-Term Survival Outcomes with Thyroid Hormone Withdrawal. Diagnostics. 2022; 12(1):221. https://doi.org/10.3390/diagnostics12010221
Chicago/Turabian StyleTsai, Hsi-Chen, Kung-Chu Ho, Shih-Hsin Chen, Jing-Ren Tseng, Lan-Yan Yang, Kun-Ju Lin, Ju-Chin Cheng, and Miaw-Jene Liou. 2022. "Feasibility of Recombinant Human TSH as a Preparation for Radioiodine Therapy in Patients with Distant Metastases from Papillary Thyroid Cancer: Comparison of Long-Term Survival Outcomes with Thyroid Hormone Withdrawal" Diagnostics 12, no. 1: 221. https://doi.org/10.3390/diagnostics12010221
APA StyleTsai, H.-C., Ho, K.-C., Chen, S.-H., Tseng, J.-R., Yang, L.-Y., Lin, K.-J., Cheng, J.-C., & Liou, M.-J. (2022). Feasibility of Recombinant Human TSH as a Preparation for Radioiodine Therapy in Patients with Distant Metastases from Papillary Thyroid Cancer: Comparison of Long-Term Survival Outcomes with Thyroid Hormone Withdrawal. Diagnostics, 12(1), 221. https://doi.org/10.3390/diagnostics12010221






