First Case of COVID-19 Treated with Monoclonal Anti-Spike Antibodies in a Patient with Cystic Fibrosis in Romania
Abstract
:1. Introduction
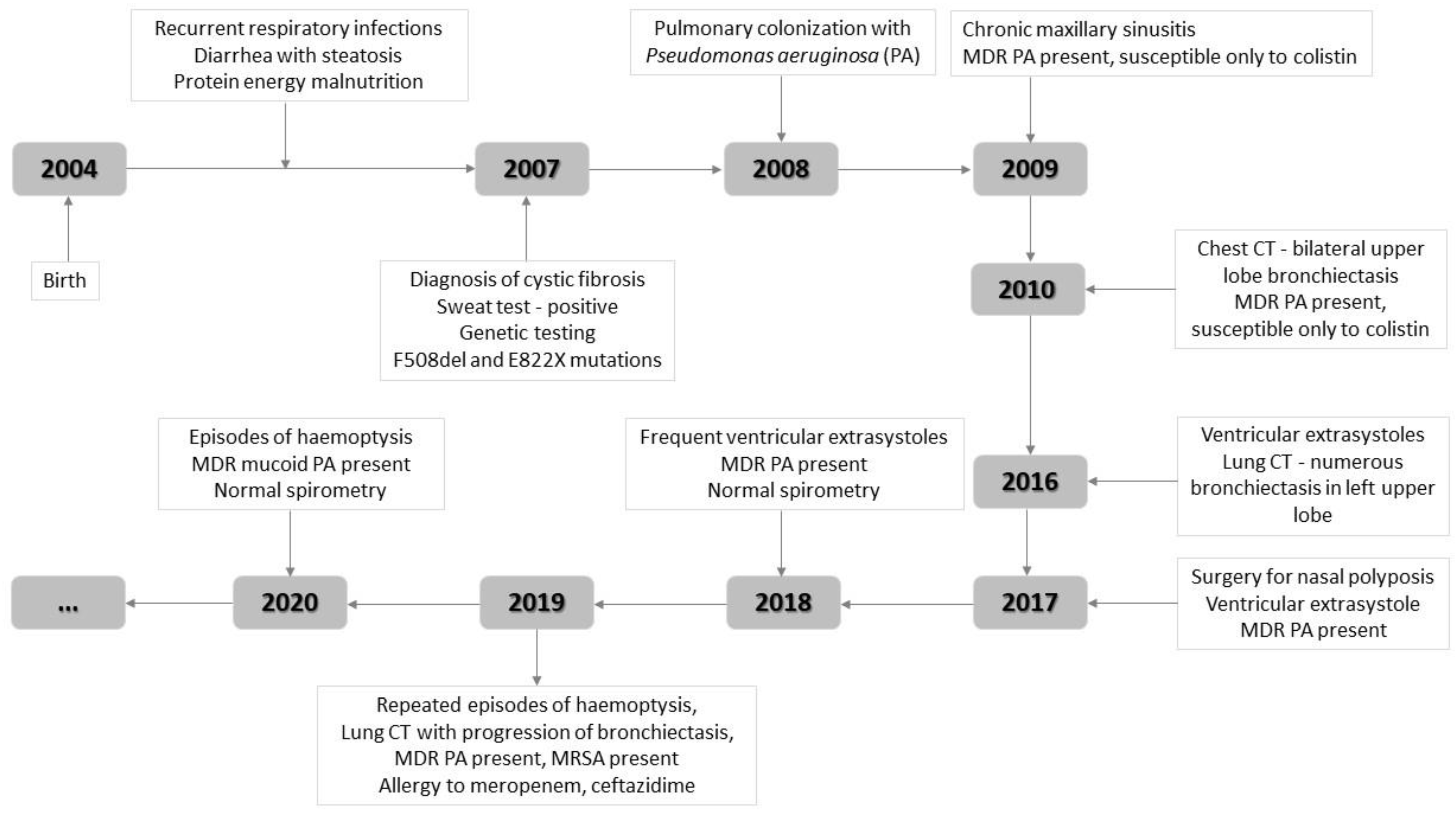
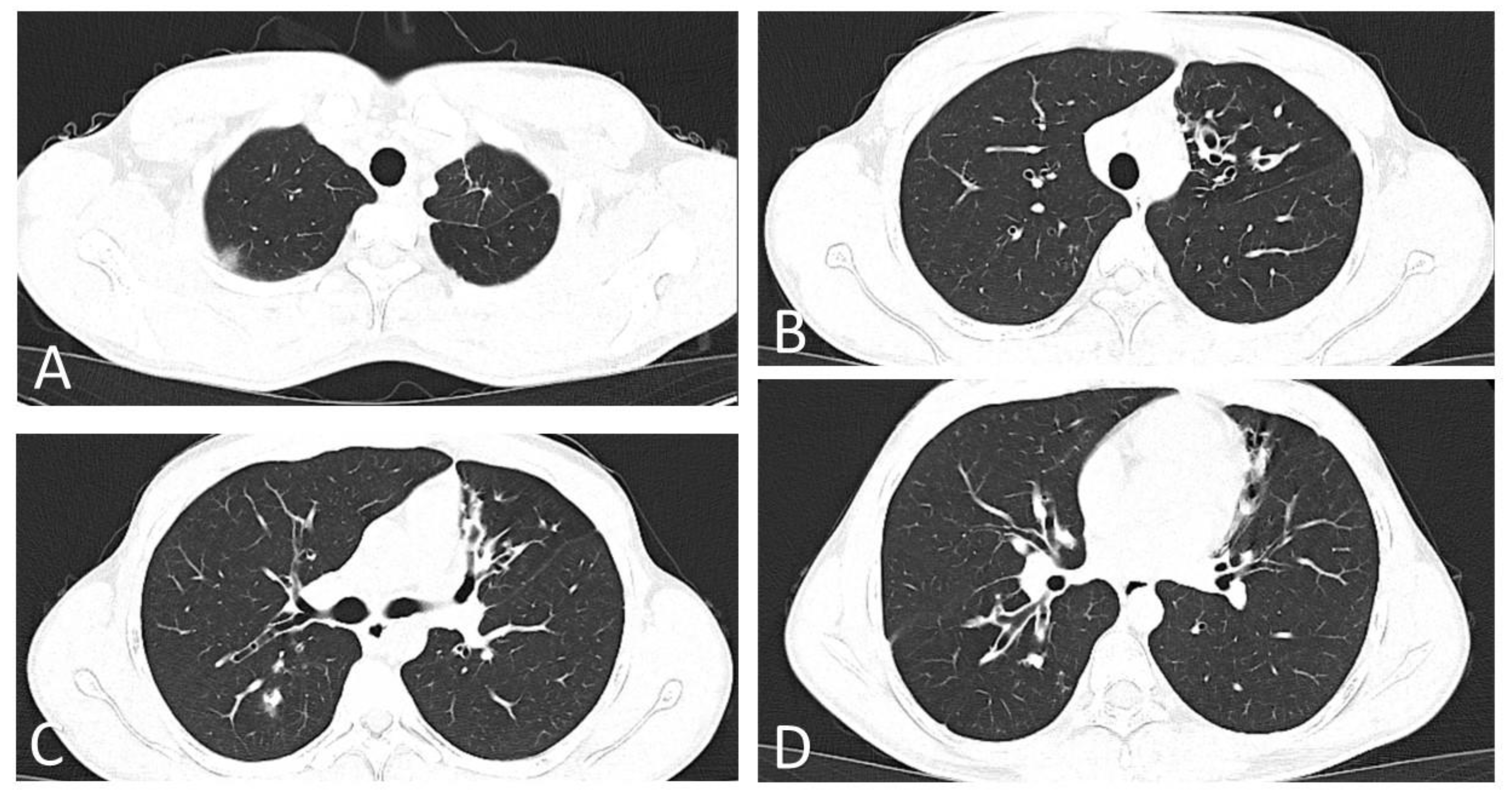
2. Case Report
3. Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mondejar-Lopez, P.; Quintana-Gallego, E.; Giron-Moreno, R.M.; Cortell-Aznar, I.; de Valbuena-Maiz, M.R.; Diab-Caceres, L.; Palma-Milla, S.; Perez-Ruiz, E.; Sole-Jover, A.; Velasco-Gonzalez, V.; et al. Impact of SARS-CoV-2 infection in patients with cystic fibrosis in Spain: Incidence and results of the national CF-COVID19-Spain survey. Respir. Med. 2020, 170, 106062. [Google Scholar] [CrossRef] [PubMed]
- Mathew, H.R.; Choi, M.Y.; Parkins, M.D.; Fritzler, M.J. Systematic review: Cystic fibrosis in the SARS-CoV-2/COVID-19 pandemic. BMC Pulm. Med. 2021, 21, 173. [Google Scholar] [CrossRef] [PubMed]
- Grehn, C.; Dittrich, A.-M.; Wosniok, J.; Holz, F.; Hafkemeyer, S.; Naehrlich, L.; Schwarz, C. Risk factors for cystic fibrosis arthropathy: Data from the German cystic fibrosis registry. J. Cyst. Fibros. 2021, 20, e87–e92. [Google Scholar] [CrossRef] [PubMed]
- Colombo, C.; Cipolli, M.; Daccò, V.; Medino, P.; Alghisi, F.; Ambroni, M.; Badolato, R.; Battistini, F.; Bignamini, E.; Casciaro, R.; et al. Clinical course and risk factors for severe COVID-19 among Italian patients with cystic fibrosis: A study within the Italian Cystic Fibrosis Society. Infection 2021, 49, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Burgel, P.-R.; Bellis, G.; Olesen, H.V.; Viviani, L.; Zolin, A.; Blasi, F.; Elborn, J.S. Future trends in cystic fibrosis demography in 34 European countries. Eur. Respir. J. 2015, 46, 133–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.Y.; Jang, Y.R.; Hong, J.H.; Jung, J.G.; Park, J.H.; Streinu-Cercel, A.; Lee, G.S.; Lee, Y.J.; Lee, Y.M.; Kim, Y.S.; et al. Safety, Virologic Efficacy, and Pharmacokinetics of CT-P59, a Neutralizing Monoclonal Antibody Against SARS-CoV-2 Spike Receptor-Binding Protein: Two Randomized, Placebo-Controlled, Phase I Studies in Healthy Individuals and Patients with Mild SARS-CoV-2 Infection. Clin. Ther. 2021, 43, 1706–1727. [Google Scholar] [PubMed]
- Weinreich, D.M.; Sivapalasingam, S.; Norton, T.; Ali, S.; Gao, H.; Bhore, R.; Musser, B.J.; Soo, Y.; Rofail, D.; Im, J.; et al. REGN-COV2, a Neutralizing Antibody Cocktail, in Outpatients with COVID-19. N. Engl. J. Med. 2021, 384, 238–251. [Google Scholar] [CrossRef] [PubMed]
- Deeks, E.D. Casirivimab/Imdevimab: First Approval. Drugs 2021, 81, 2047–2055. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Parker, B.M.; Priyadarshi, V.; Parker, J. Cardiac Adverse Events with Remdesivir in COVID-19 Infection. Cureus 2020, 12, e11132. [Google Scholar] [CrossRef] [PubMed]
- Somers, E.C.; Eschenauer, G.A.; Troost, J.P.; Golob, J.L.; Gandhi, T.N.; Wang, L.; Zhou, N.; Petty, L.A.; Baang, J.H.; Dillman, N.O.; et al. Tocilizumab for Treatment of Mechanically Ventilated Patients With COVID-19. Clin. Infect. Dis. 2020, 73, e445–e454. [Google Scholar] [CrossRef] [PubMed]
- Wiltshire, D.A.; Vahora, I.S.; Tsouklidis, N.; Kumar, R.; Khan, S. H1N1 Influenza Virus in Patients with Cystic Fibrosis: A Literature Review Examining Both Disease Entities and Their Association in Light of the 2009 Pandemic. Cureus 2020, 12, e9218. [Google Scholar] [CrossRef] [PubMed]
- Burgel, P.-R.; Goss, C. COVID-19 outcomes in people with cystic fibrosis. Curr. Opin. Pulm. Med. 2021, 27, 538–543. [Google Scholar] [CrossRef] [PubMed]
| Type of Laboratory Analysis | Date | 2 Nov. | 5 Nov. | 11 Nov. | 19 Nov. | 23 Nov. |
|---|---|---|---|---|---|---|
| Day of Illness | 6 | 9 | 15 | 23 | 27 | |
| Normal Range | ||||||
| WBCs | 5–12 × 103/μL | 10.32 | 8.04 | 9.64 | - | - |
| Lymphocytes # | 1.5–5.2 × 103/μL | 2.43 | 3.29 | 2.86 | - | - |
| Lymphocytes % | 32–48% | 23.5 | 40.9 | 29.7 | - | - |
| Neutrophils # | 1.5–8.0 × 103/μL | 6.85 | 3.78 | 5.67 | - | - |
| Neutrophils % | 35–55% | 66.4 | 47.1 | 58.8 | - | - |
| Hemoglobin | 13–15 g/dL | 17.7 | 16.5 | 16.1 | - | - |
| Platelets | 150–450 × 103/μL | 231 | 248 | 292 | - | - |
| C-reactive protein | <0.5 mg/dL | 1.23 | 0.48 | 0.41 | - | - |
| Fibrinogen | 160–390 mg/dL | 432 | 392 | 341 | - | - |
| ESR | <15 mm/h | 3 | 10 | 7 | - | - |
| IL-6 | 0–7 pg/mL | 14.55 | 2.51 | 2.42 | - | - |
| AST | 10–37 U/L | 28 | 15 | 23 | - | - |
| ALT | 10–60 U/L | 37 | 28 | 40 | - | - |
| Urea | 15–35 mg/dL | 35 | 33 | 35 | - | - |
| Creatinine | 0.4–1.4 mg/dL | 0.7 | 0.6 | 0.8 | - | - |
| Ferritin | 20–200 μg/L | 120 | 149 | - | - | - |
| D-dimer | 0–0.5 mg/dL | 0.2 | 0.2 | 0.3 | - | - |
| IgM a (SARS-CoV-2) | - | negative | positive | - | positive | - |
| IgG a (SARS-CoV-2) | - | negative | negative | - | positive | - |
| IgM b (SARS-CoV-2) | positive > 1 | 0 | 1.44 | - | 18.53 | - |
| IgG b (SARS-CoV-2) | positive > 1 | 0 | 0 | - | 1.81 | - |
| RT-PCR SARS-CoV-2 | - | positive | positive | positive | positive | positive |
| Type of Drug | Name | Administration |
|---|---|---|
| Inhalation antibiotic therapy | Colistin | 1,000,000 IU b.i.d.—wet nebulization |
| Mucolytic | Alfa-dornase | 2500 IU q.d.—wet nebulization |
| Hypertonic saline 3% | 3 mL b.i.d.—wet nebulization | |
| Bronchodilator | Ipratropium bromide 20 μg * | 1 puff b.i.d.—inhaler |
| Enzyme replacement therapy | Pancreatin | 10,000 UI/kg/day—orally |
| Fat-soluble vitamins | Vitamin A | 3000 μg/day—orally |
| Vitamin D | 2000 IU/day—orally | |
| Vitamin E | 200 mg/day—orally | |
| Vitamin K | 200 μg/day—orally |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stan, I.V.; Miron, V.D.; Vangheli, I.A.; Gheorghiu, R.M.; Streinu-Cercel, A.; Săndulescu, O.; Craiu, M. First Case of COVID-19 Treated with Monoclonal Anti-Spike Antibodies in a Patient with Cystic Fibrosis in Romania. Diagnostics 2022, 12, 137. https://doi.org/10.3390/diagnostics12010137
Stan IV, Miron VD, Vangheli IA, Gheorghiu RM, Streinu-Cercel A, Săndulescu O, Craiu M. First Case of COVID-19 Treated with Monoclonal Anti-Spike Antibodies in a Patient with Cystic Fibrosis in Romania. Diagnostics. 2022; 12(1):137. https://doi.org/10.3390/diagnostics12010137
Chicago/Turabian StyleStan, Iustina Violeta, Victor Daniel Miron, Ioana Alexandra Vangheli, Radu Marian Gheorghiu, Anca Streinu-Cercel, Oana Săndulescu, and Mihai Craiu. 2022. "First Case of COVID-19 Treated with Monoclonal Anti-Spike Antibodies in a Patient with Cystic Fibrosis in Romania" Diagnostics 12, no. 1: 137. https://doi.org/10.3390/diagnostics12010137
APA StyleStan, I. V., Miron, V. D., Vangheli, I. A., Gheorghiu, R. M., Streinu-Cercel, A., Săndulescu, O., & Craiu, M. (2022). First Case of COVID-19 Treated with Monoclonal Anti-Spike Antibodies in a Patient with Cystic Fibrosis in Romania. Diagnostics, 12(1), 137. https://doi.org/10.3390/diagnostics12010137








