The Potential Roles of Cervical Plexus Abnormalities in Occipital Neuralgia: An Anatomic Variant Explored
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Data Collection and Analysis
3. Results
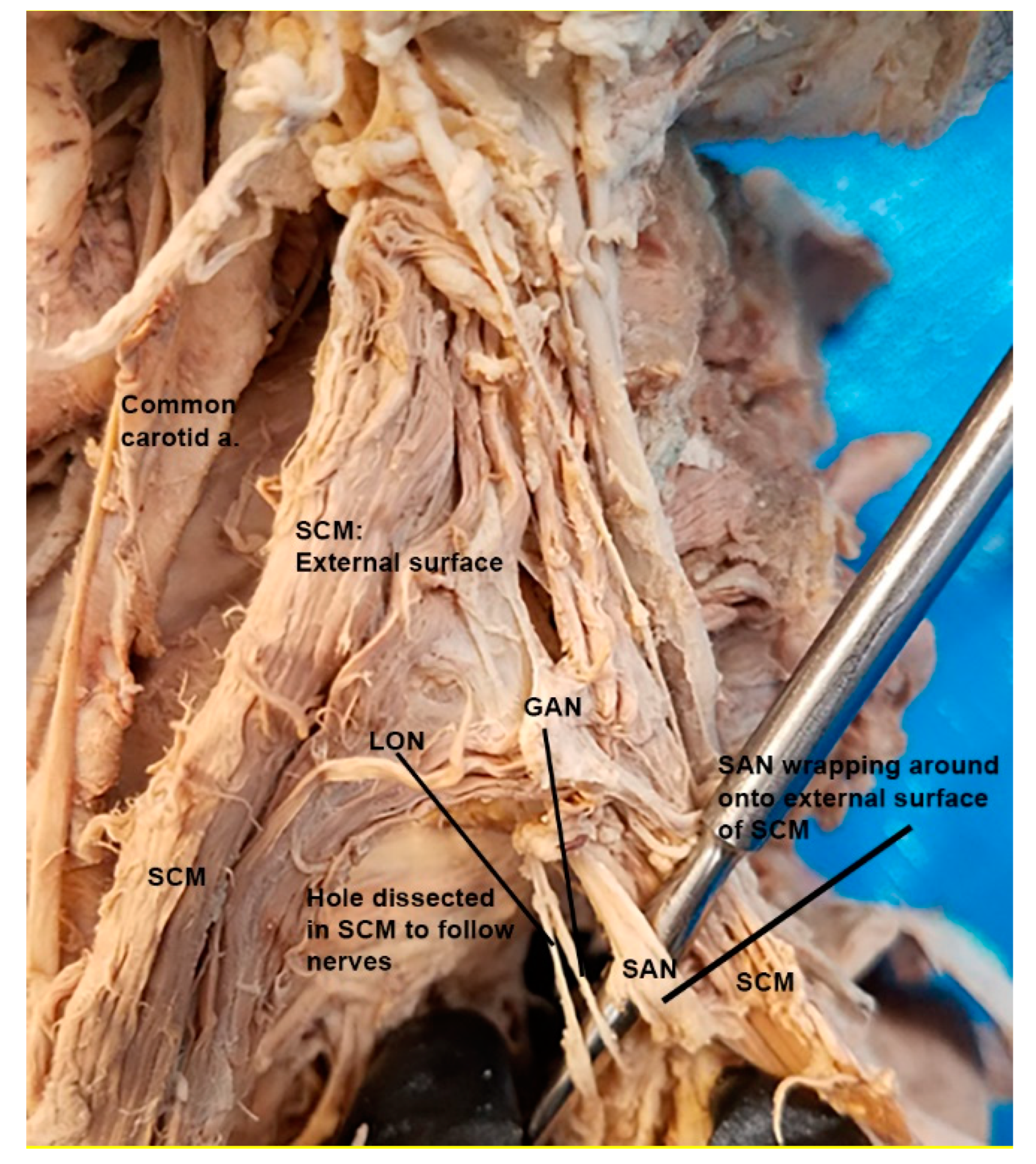
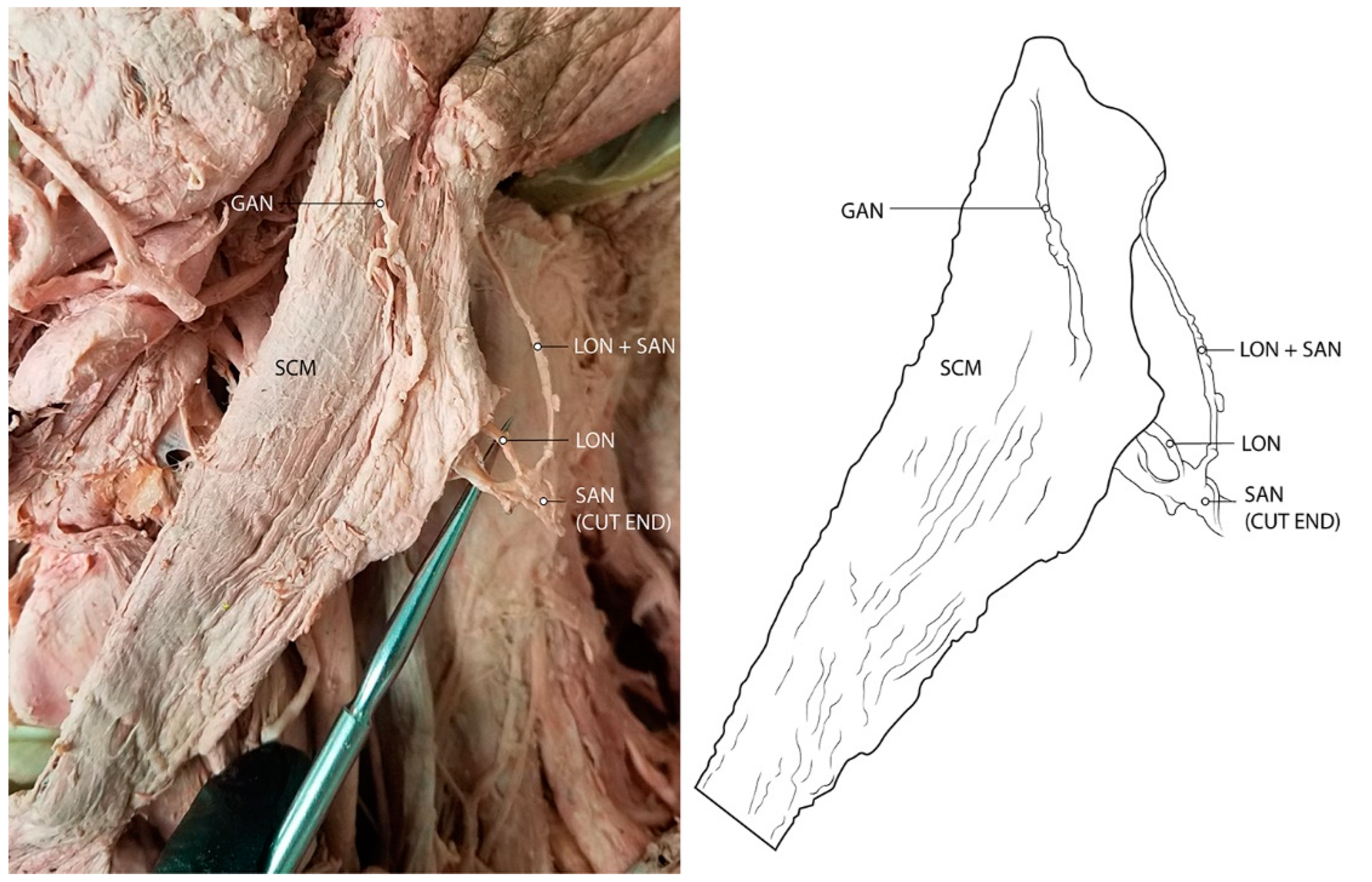
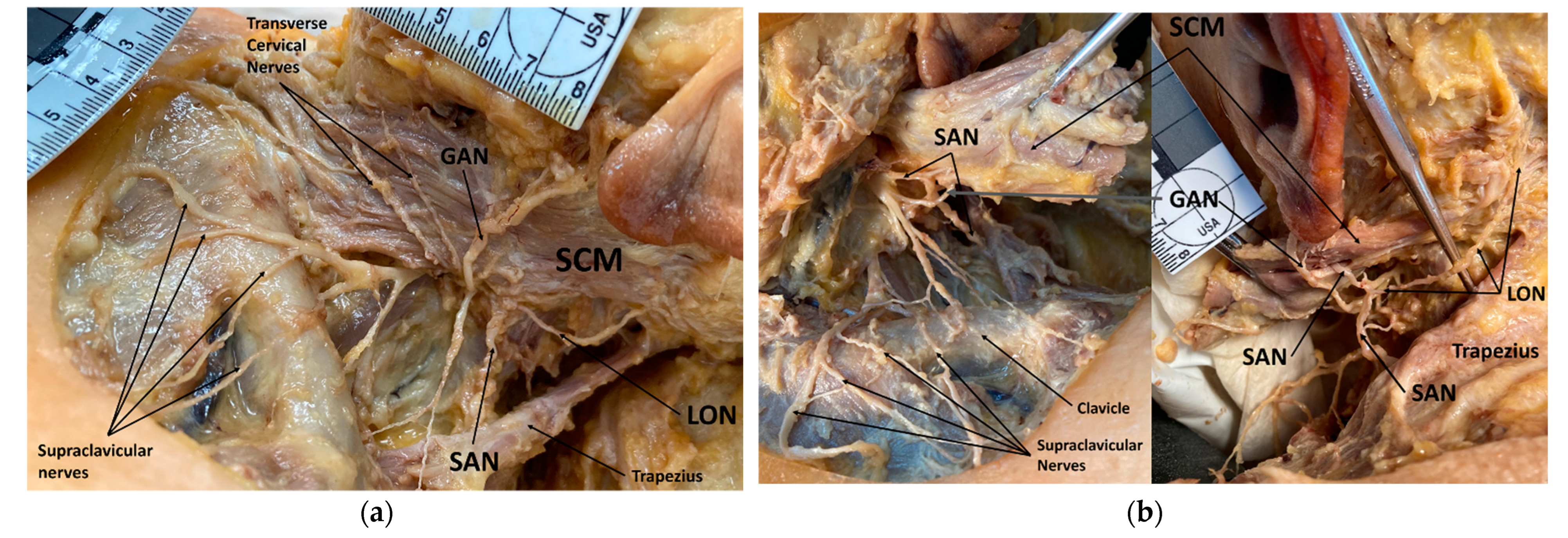
3.1. Spinal Accessory Nerve and Lesser Occipital Nerve Piercing Fusion
3.2. Lesser Occipital Nerve Piercing Sternocleidomastoid Alone
3.3. Greater Auricular Nerve Piercing Sternocleidomastoid Alone
3.4. Lesser Occipital and Greater Auricular Nerve Piercing Sternocleidomastoid
3.5. Statistical Analyses
4. Discussion
4.1. Clinical Implications of Spinal Accessory Nerve Fusion with Lesser Occipital Nerve
4.2. Clinical Implications of Lesser Occipital Nerve Piercing and Compression
4.3. Clinical Implications of Greater Auricular Nerve Piercing and Compression
4.4. Diagnosing Occipital Neuralgia
4.5. Treatment Options for Occipital Neuralgia
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barmherzig, R.; Kingston, W. Occipital Neuralgia and Cervicogenic Headache: Diagnosis and Management. Curr. Neurol. Neurosci. Rep. 2019, 19, 20. [Google Scholar] [CrossRef] [PubMed]
- Cesmebasi, A.; Muhleman, M.A.; Hulsberg, P. Occipital neuralgia: Anatomic considerations. Clin. Anat. 2015, 28, 101–108. [Google Scholar] [CrossRef]
- Juškys, R.; Šustickas, G. Effectiveness of treatment of occipital neuralgia using the nerve block technique: A prospective analysis of 44 patients. Acta Med. Litu. 2018, 25, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Narouze, S. Occipital Neuralgia Diagnosis and Treatment: The Role of Ultrasound. Headache 2016, 56, 801–807. [Google Scholar] [CrossRef]
- Sweet, J.A.; Mitchell, L.S.; Narouze, S. Occipital Nerve Stimulation for the Treatment of Patients with Medically Refractory Occipital Neuralgia: Congress of Neurological Surgeons Systematic Review and Evidence-Based Guideline. Neurosurgery 2015, 77, 332–341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bogduk, N.; Govind, J. Cervicogenic headache: An assessment of the evidence on clinical diagnosis, invasive tests, and treatment. Lancet Neurol. 2009, 8, 959–968. [Google Scholar] [CrossRef]
- Koopman, J.S.; Dieleman, J.P.; Huygen, F.J.; de Mos, M.; Martin, C.G.; Sturkenboom, M.C. Incidence of facial pain in the general population. Pain 2009, 147, 122–127. [Google Scholar] [CrossRef]
- Yu, M.; Wang, S.M. Anatomy, Head and Neck, Occipital Nerves; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Bordoni, B.; Reed, R.R.; Tadi, P. Neuroanatomy, Cranial Nerve 11 (Accessory); StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Amirlak, B.; Lu, K.B.; Erickson, C.R. In-Depth Look at the Anatomical Relationship between the Lesser Occipital Nerve, Greater Auricular Nerve, and Spinal Accessory Nerve and Their Implication in Safety of Operations in the Posterior Triangle of the Neck. Plast. Reconstr. Surg. 2020, 146, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Johnston, M.M.; Jordan, S.E. Pain referral patterns of the C1 to C3 nerves: Implications for headache disorders. Ann. Neurol. 2013, 74, 145–148. [Google Scholar] [CrossRef]
- Safran, M.R. Nerve injury about the shoulder in athletes, part 2: Long thoracic nerve, spinal accessory nerve, burners/stingers, thoracic outlet syndrome. Am. J. Sports Med. 2004, 32, 1063–1076. [Google Scholar] [CrossRef]
- Dash, K.S.; Janis, J.E.; Guyuron, B. The lesser and third occipital nerves and migraine headaches. Plast. Reconstr. Surg. 2005, 115, 1752–1760. [Google Scholar] [CrossRef]
- Peled, Z.M.; Pietramaggiori, G.; Scherer, S. Anatomic and Compression Topography of the Lesser Occipital Nerve. Plast. Reconstr. Surg. Glob. Open. 2016, 4, e639. [Google Scholar] [CrossRef] [PubMed]
- Eghtesadi, M.; Leroux, E.; Vargas-Schaffer, G. A case report of complex auricular neuralgia treated with the great auricular nerve and facet blocks. J. Pain Res. 2017, 10, 435–438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loukas, M.; El-Sedfy, A.; Tubbs, R.S. Identification of greater occipital nerve landmarks for the treatment of occipital neuralgia. Folia Morphol. 2006, 65, 337–342. [Google Scholar]
- Shin, K.J.; Kim, H.S.; O, J.; Kwon, H.J.; Yang, H.M. Anatomical consideration of the occipital cutaneous nerves and artery for the safe treatment of occipital neuralgia. Clin. Anat. 2018, 31, 1058–1064. [Google Scholar] [CrossRef]
- Pingree, M.J.; Sole, J.S.; O’Brien, T.G.; Eldrige, J.S.; Moeschler, S.M. Clinical Efficacy of an Ultrasound-Guided Greater Occipital Nerve Block at the Level of C2. Reg. Anesth. Pain Med. 2017, 42, 99–104. [Google Scholar] [CrossRef]
- Kapural, L.; Stillman, M.; Kapural, M.; McIntyre, P.; Guirgius, M.; Mekhail, N. Botulinum toxin occipital nerve block for the treatment of severe occipital neuralgia: A case series. Pain Pract. 2007, 7, 337–340. [Google Scholar] [CrossRef]
- Taylor, M.; Silva, S.; Cottrell, C. Botulinum toxin type-A (BOTOX) in the treatment of occipital neuralgia: A pilot study. Headache 2008, 48, 1476–1481. [Google Scholar] [CrossRef]
- Palamar, D.; Uluduz, D. Ultrasound-guided greater occipital nerve block: An efficient technique in chronic refractory migraine without aura? Pain Physician 2015, 18, 153–162. [Google Scholar] [CrossRef]
- Shim, J.H.; Ko, S.Y. Ultrasound-guided greater occipital nerve block for patients with occipital headache and short term follow up. Korean J. Anesthesiol. 2011, 61, 50–54. [Google Scholar] [CrossRef]
- Vanderhoek, M.D.; Hoang, H.T. Ultrasound-guided greater occipital nerve blocks and pulsed radiofrequency ablation for diagnosis and treatment of occipital neuralgia. Anesthesiol. Pain Med. 2013, 3, 256–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodrigo, D.; Acin, P.; Bermejo, P. Occipital Nerve Stimulation for Refractory Chronic Migraine: Results of a Long-Term Prospective Study. Pain Physician 2017, 20, E151–E159. [Google Scholar] [CrossRef] [PubMed]
- Bovim, G.; Fredriksen, T.A.; Stolt-Nielsen, A.; Sjaastad, O. Neurolysis of the greater occipital nerve in cervicogenic headache. A follow up study. Headache 1992, 32, 175–179. [Google Scholar] [CrossRef] [PubMed]
| Number of Presentations | SAN Fusing w/LON, Piercing SCM | LON Piercing SCM Alone | GAN Piercing SCM Alone | LON and GAN Piercing SCM |
|---|---|---|---|---|
| Number of Presentations | 4 (4F) | 68 (27F/41M) | 29 (12F/17M) | 25 (11F/14M) |
| Percentage (%) | 3.77% | 58.1% | 25.9% | 24.5% |
| Female | 55 | 49 | 51 | 42 |
| Male | 51 | 68 | 61 | 60 |
| Total | 106 | 117 | 112 | 102 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mirande, M.H.; Smith, H.F. The Potential Roles of Cervical Plexus Abnormalities in Occipital Neuralgia: An Anatomic Variant Explored. Diagnostics 2022, 12, 139. https://doi.org/10.3390/diagnostics12010139
Mirande MH, Smith HF. The Potential Roles of Cervical Plexus Abnormalities in Occipital Neuralgia: An Anatomic Variant Explored. Diagnostics. 2022; 12(1):139. https://doi.org/10.3390/diagnostics12010139
Chicago/Turabian StyleMirande, Mitchell H., and Heather F. Smith. 2022. "The Potential Roles of Cervical Plexus Abnormalities in Occipital Neuralgia: An Anatomic Variant Explored" Diagnostics 12, no. 1: 139. https://doi.org/10.3390/diagnostics12010139
APA StyleMirande, M. H., & Smith, H. F. (2022). The Potential Roles of Cervical Plexus Abnormalities in Occipital Neuralgia: An Anatomic Variant Explored. Diagnostics, 12(1), 139. https://doi.org/10.3390/diagnostics12010139







