Beat-to-Beat P-Wave Analysis Outperforms Conventional P-Wave Indices in Identifying Patients with a History of Paroxysmal Atrial Fibrillation during Sinus Rhythm
Abstract
:1. Introduction
2. Materials and Methods
2.1. Standard P-Wave Indices
2.1.1. The 12-Lead ECG Indices
2.1.2. Orthogonal Morphology
2.2. P-Wave Beat-to-Beat Analysis
2.2.1. Beat-to-Beat Classification into Main and Secondary Morphology
2.2.2. Time-Domain Analysis
2.2.3. Time-Frequency Domain Analysis
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
Appendix A.1. Standard P-Wave Indices
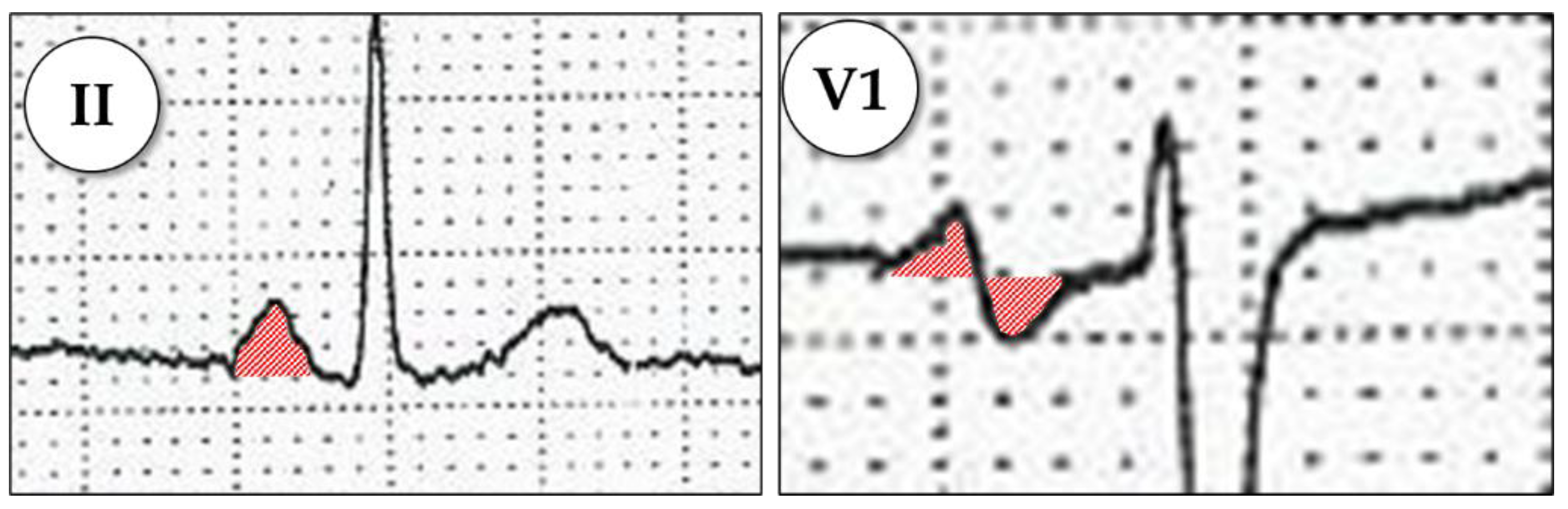
Appendix A.1.1. Advanced Interatrial Block

Appendix A.1.2. P-Wave Dispersion
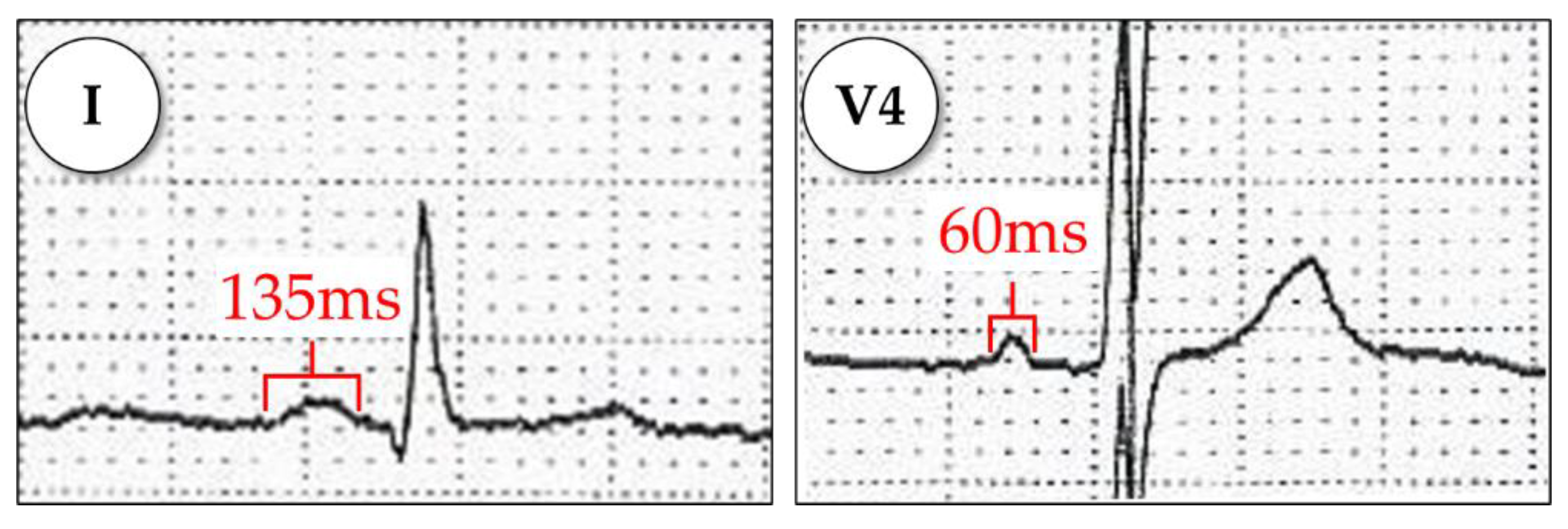
Appendix A.1.3. P-Wave Area
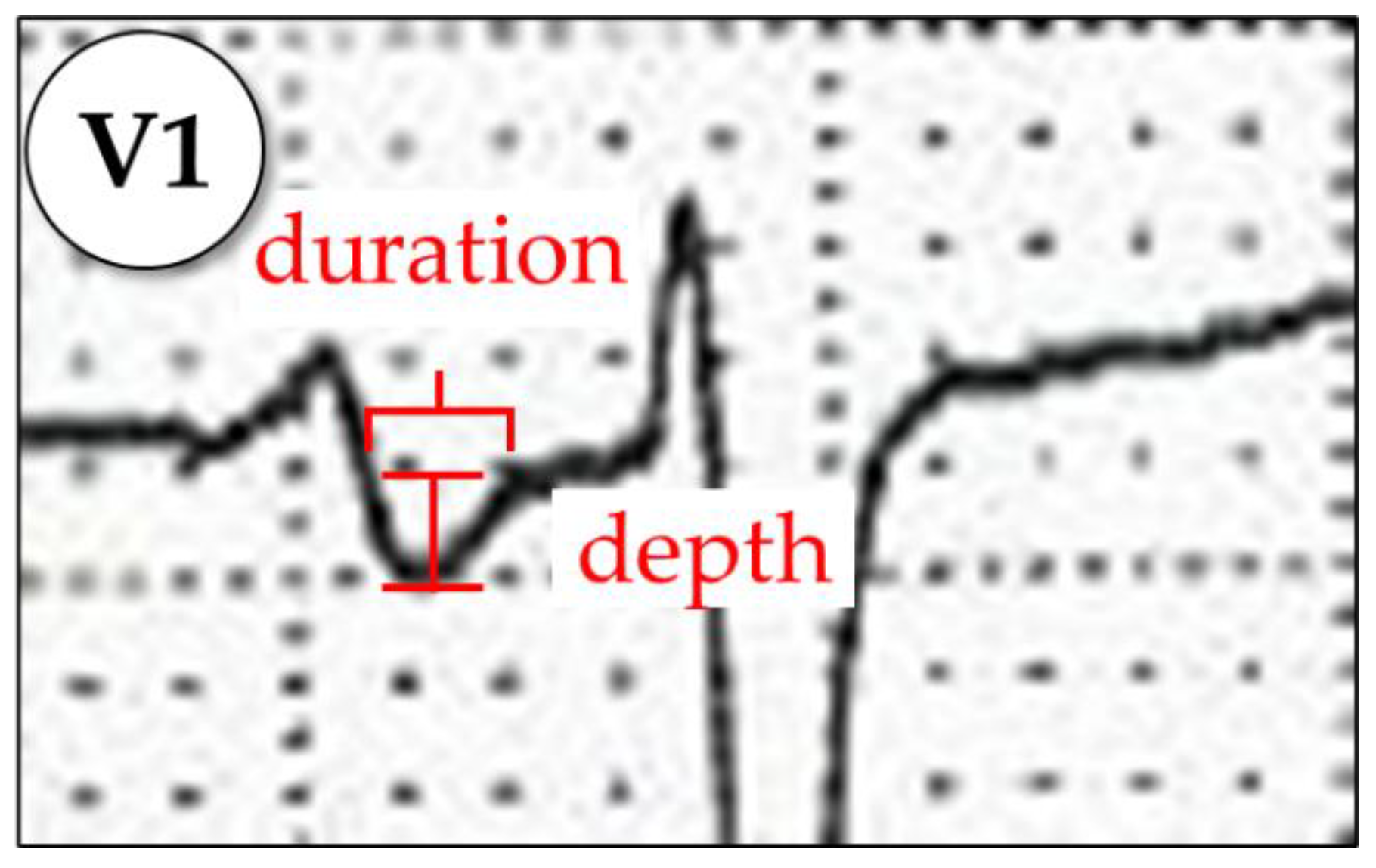
Appendix A.1.4. P-Wave Terminal Force in V1 (PTFV1)
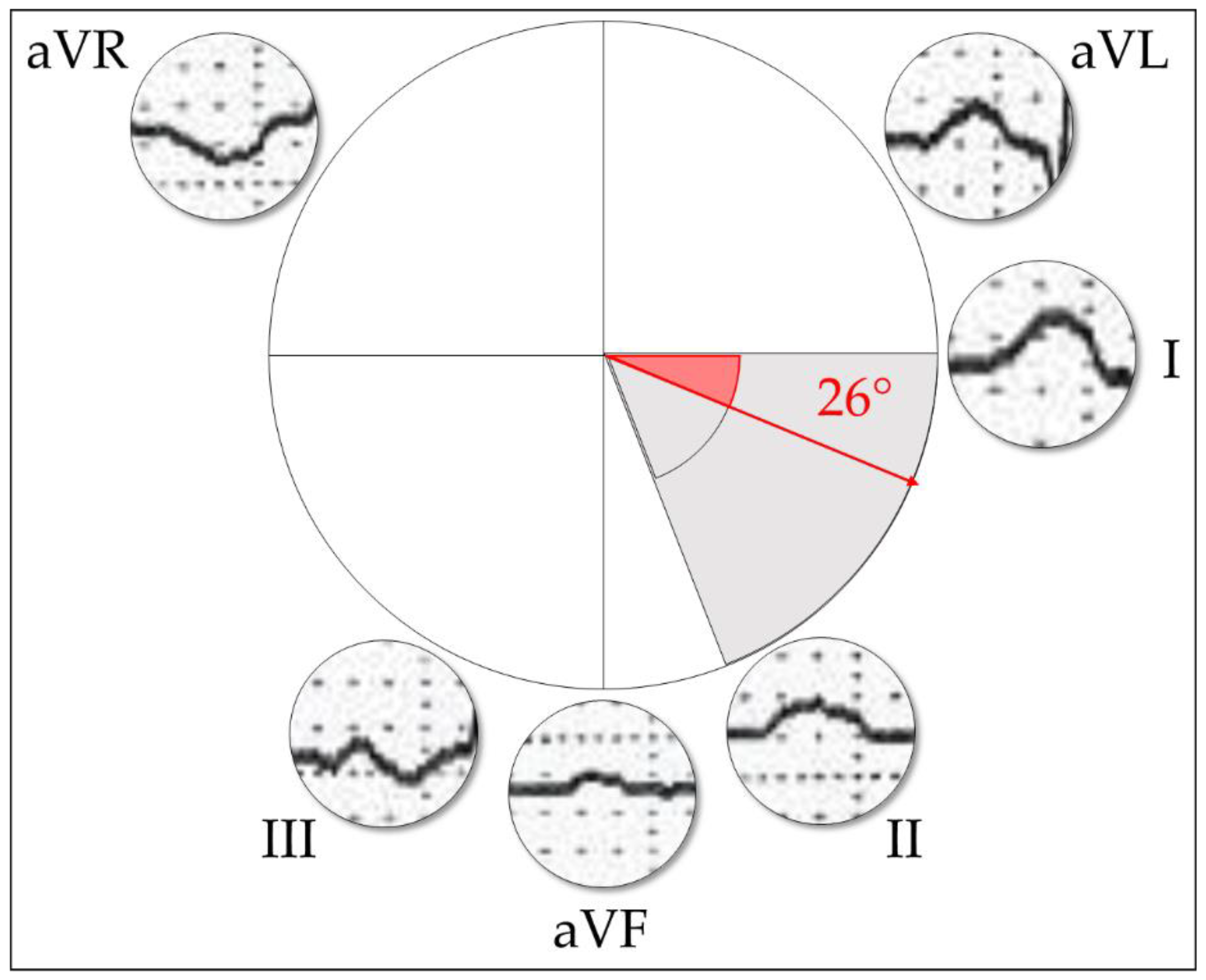
Appendix A.1.5. P-Wave Axis
Appendix A.1.6. P-Wave Voltage in Lead I
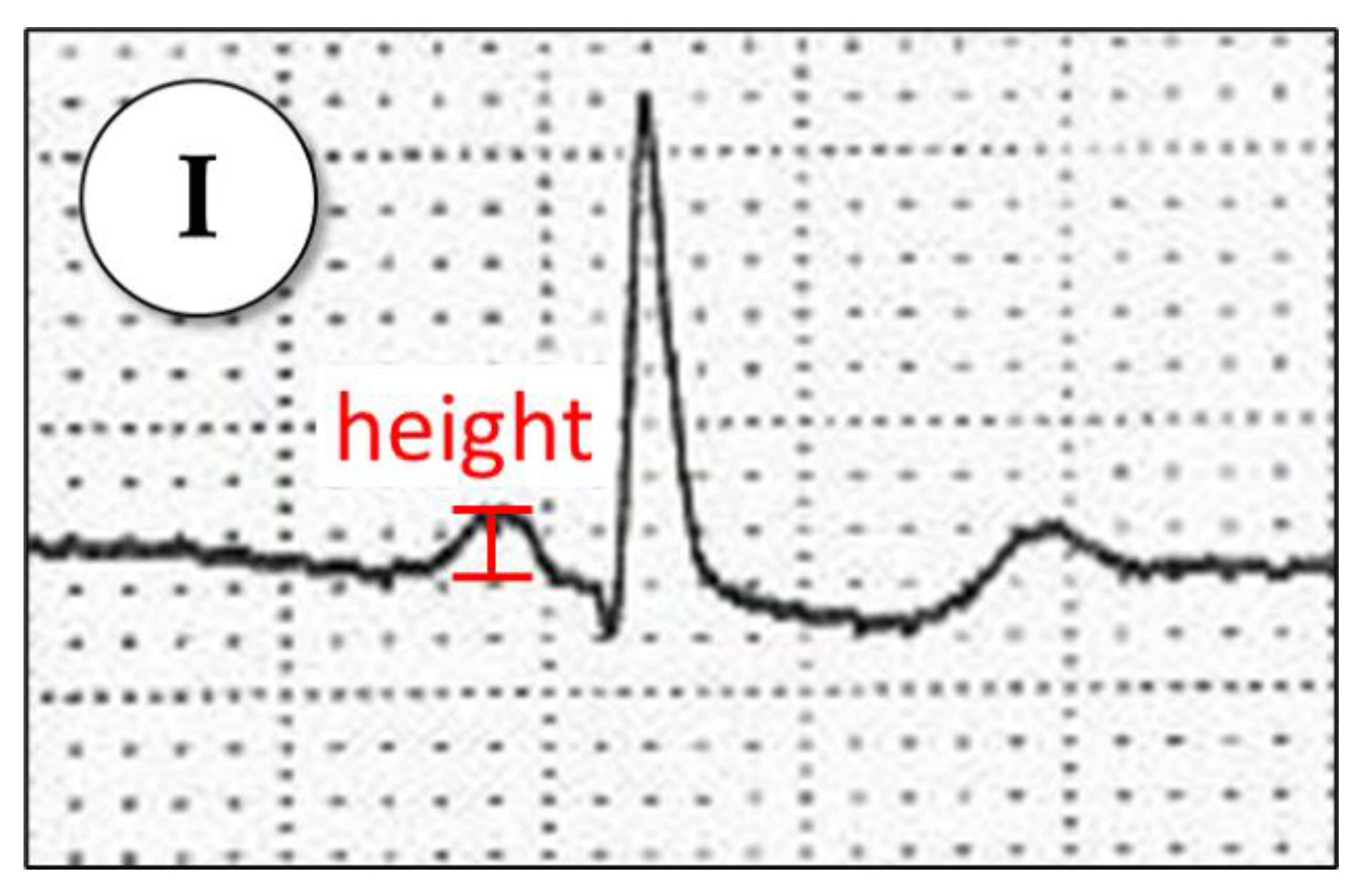
Appendix A.1.7. MVP Score
| Variable | Value | Score |
| Morphology in inferior leads | Nonbiphasic (<120 ms) | 0 |
| Nonbiphasic (≥120 ms) | 1 | |
| Biphasic | 2 | |
| Voltage in lead I | >0.2 mV | 0 |
| 0.10–0.20 mV | 1 | |
| <0.10 mV | 2 | |
| P-wave duration | <120 ms | 0 |
| 120–140 ms | 1 | |
| >140 ms | 2 |
Appendix A.1.8. Orthogonal Types 1, 2, or 3

Appendix A.2. Beat-to-Beat Analysis
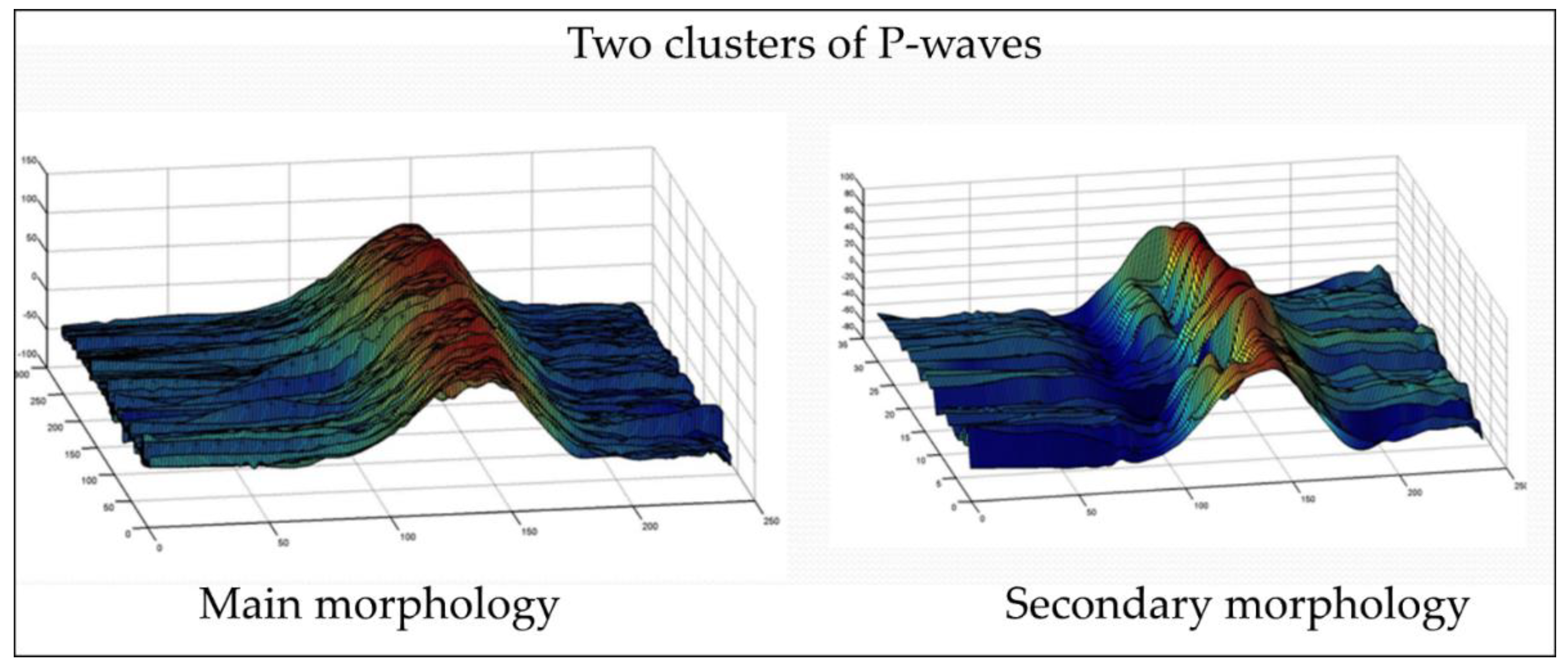
Appendix A.2.1. Main and Secondary Morphologies
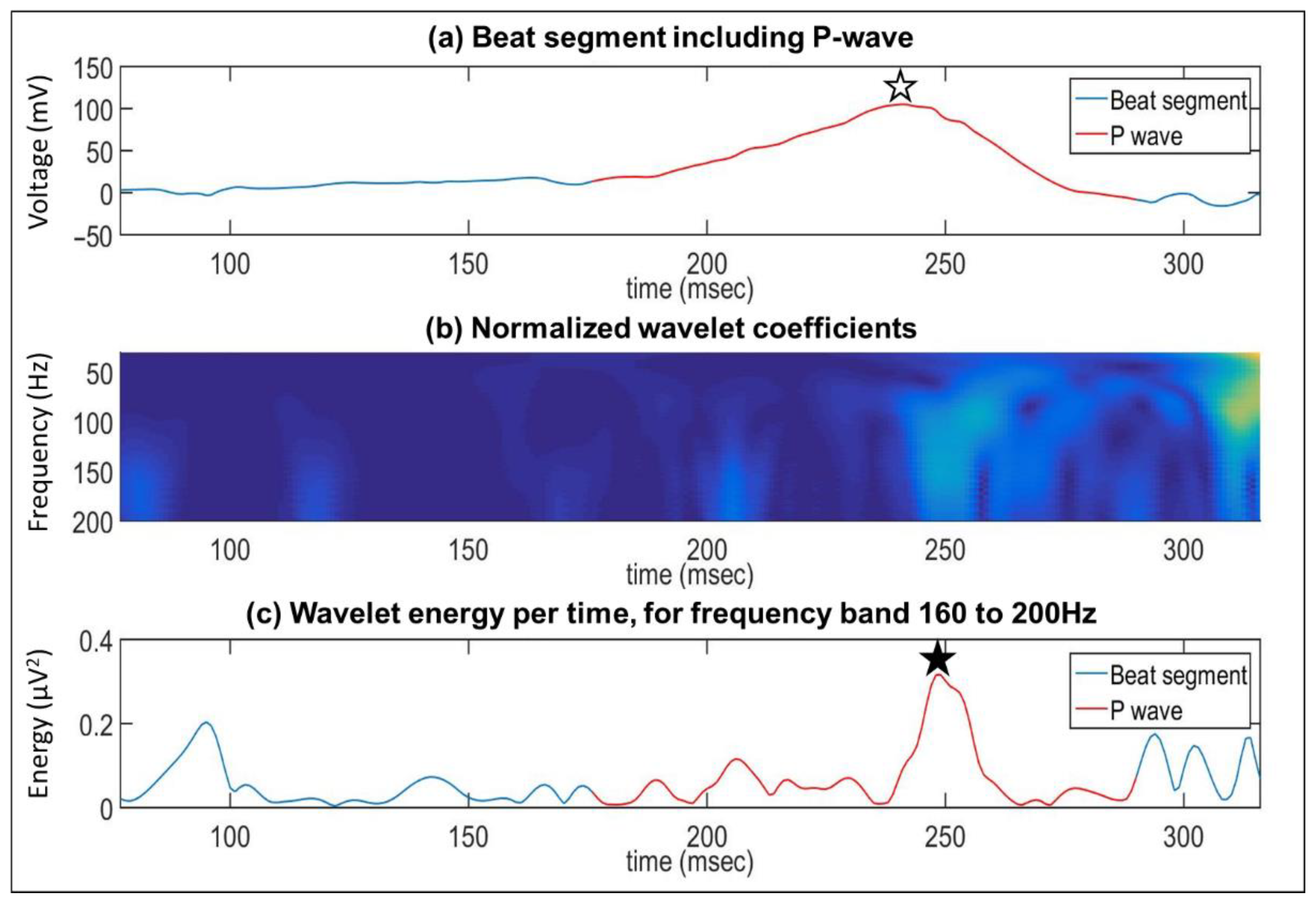
Appendix A.2.2. Wavelet Analysis
References
- Dilaveris, P.E.; Kennedy, H.L. Silent atrial fibrillation: Epidemiology, diagnosis, and clinical impact. Clin. Cardiol. 2017, 40, 413–418. [Google Scholar] [CrossRef]
- Alonso, A.; Norby, F.L. Predicting Atrial Fibrillation and Its Complications. Circ. J. 2016, 80, 1061–1066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- German, D.M.; Kabir, M.M.; Dewland, T.A.; Henrikson, C.A.; Tereshchenko, L. Atrial Fibrillation Predictors: Importance of the Electrocardiogram. Ann. Noninvasive Electrocardiol. 2016, 21, 20–29. [Google Scholar] [CrossRef] [Green Version]
- Saliba, W.; Gronich, N.; Barnett-Griness, O.; Rennert, G. Usefulness of CHADS2 and CHA2DS2-VASc Scores in the Prediction of New-Onset Atrial Fibrillation: A Population-Based Study. Am. J. Med. 2016, 129, 843–849. [Google Scholar] [CrossRef] [Green Version]
- He, J.; Tse, G.; Korantzopoulos, P.; Letsas, K.P.; Ali-Hasan-Al-Saegh, S.; Kamel, H.; Li, G.; Lip, G.Y.; Liu, T. P-Wave Indices and Risk of Ischemic Stroke. Stroke 2017, 48, 2066–2072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez-Sellés, M.; Elosua, R.; Ibarrola, M.; De Andrés, M.; Díez-Villanueva, P.; Bayés-Genis, A.; Baranchuk, A.; Bayés-De-Luna, A. Advanced interatrial block and P-wave duration are associated with atrial fibrillation and stroke in older adults with heart disease: The BAYES registry. Europace 2020, 22, 1001–1008. [Google Scholar] [CrossRef] [PubMed]
- Maheshwari, A.; Norby, F.; Roetker, N.S.; Soliman, E.Z.; Koene, R.J.; Rooney, M.R.; O’Neal, W.T.; Shah, A.M.; Claggett, B.L.; Solomon, S.D.; et al. Refining Prediction of Atrial Fibrillation-Related Stroke Using the P2-CHA2DS2-VASc Score: ARIC and MESA. Circulation 2019, 139, 180–191. [Google Scholar] [CrossRef]
- Dakos, G.; Chatzizisis, Y.S.; Konstantinou, D.; Chouvarda, I.; Filos, D.; Paraskevaidis, S.; Mantziari, L.; Maglaveras, N.; Karvounis, H.; Styliadis, I.; et al. Wavelet-based analysis of P waves identifies patients with lone atrial fibrillation: A cross-sectional pilot study. Int. J. Cardiol. 2014, 174, 389–392. [Google Scholar] [CrossRef]
- Filos, D.; Chouvarda, I.; Tachmatzidis, D.; Vassilikos, V.; Maglaveras, N. Beat-to-beat P-wave morphology as a predictor of paroxysmal atrial fibrillation. Comput. Methods Programs Biomed. 2017, 151, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Filos, D.; Tachmatzidis, D.; Maglaveras, N.; Vassilikos, V.; Chouvarda, I. Understanding the Beat-to-Beat Variations of P-Waves Morphologies in AF Patients During Sinus Rhythm: A Scoping Review of the Atrial Simulation Studies. Front. Physiol. 2019, 10, 742. [Google Scholar] [CrossRef]
- Yıldırım, E.; Günay, N.; Bayam, E.; Keskin, M.; Ozturkeri, B.; Selcuk, M. Relationship between paroxysmal atrial fibrillation and a novel electrocardiographic parameter P wave peak time. J. Electrocardiol. 2019, 57, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Zheng, Z.; Wu, B.; Tang, L.; Xie, X.; Dong, R.; Luo, Y.; Li, S.; Zhu, J.; Liu, J. Predictive value of P wave terminal force in lead V1 for atrial fibrillation: A meta-analysis. Ann. Noninvasive Electrocardiol. 2020, 25, e12739. [Google Scholar] [CrossRef] [PubMed]
- Bayés de Luna, A.; Platonov, P.; Cosio, F.G.; Cygankiewicz, I.; Pastore, C.A.; Baranowski, R.; Bayés-Genis, A.; Guindo, J.; Viñolas, X.; Garcia-Niebla, J.; et al. Interatrial blocks. A separate entity from left atrial enlargement: A consensus report. J. Electrocardiol. 2012, 45, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Alexander, B.; Milden, J.; Hazim, B.; Haseeb, S.; Bayes-Genis, A.; Elosua, R.; Martínez-Sellés, M.; Yeung, C.; Ma, W.H.; Baranchuk, A.; et al. New electrocardiographic score for the prediction of atrial fibrillation: The MVP ECG risk score (morphology-voltage-P-wave duration). Ann. Noninvasive Electrocardiol. 2019, 24, e12669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eranti, A.; Carlson, J.; Kenttä, T.; Holmqvist, F.; Holkeri, A.; Haukilahti, M.A.; Kerola, T.; Aro, A.L.; Rissanen, H.; Noponen, K.; et al. Orthogonal P-wave morphology, conventional P-wave indices, and the risk of atrial fibrillation in the general population using data from the Finnish Hospital Discharge Register. Europace 2020, 22, 1173–1181. [Google Scholar] [CrossRef]
- Delong, E.R.; Delong, D.M.; Clarke-Pearson, D.L. Comparing the Areas under Two or More Correlated Receiver Operating Characteristic Curves: A Nonparametric Approach. Biometrics 1988, 44, 837–845. [Google Scholar] [CrossRef]
- Peduzzi, P.; Concato, J.; Kemper, E.; Holford, T.R.; Feinstein, A.R. A simulation study of the number of events per variable in logistic regression analysis. J. Clin. Epidemiol. 1996, 49, 1373–1379. [Google Scholar] [CrossRef]
- Magnani, J.W.; Zhu, L.; Lopez, F.; Pencina, M.J.; Agarwal, S.K.; Soliman, E.Z.; Benjamin, E.; Alonso, A. P-wave indices and atrial fibrillation: Cross-cohort assessments from the Framingham Heart Study (FHS) and Atherosclerosis Risk in Communities (ARIC) study. Am. Heart J. 2015, 169, 53–61.e1. [Google Scholar] [CrossRef] [Green Version]
- Park, J.K.; Park, J.; Uhm, J.S.; Joung, B.; Lee, M.H.; Pak, H.N. Low P-Wave Amplitude (<0.1 MV) in Lead I Is Associated with Displaced Inter-Atrial Conduction and Clinical Recurrence of Paroxysmal Atrial Fibrillation after Radiofrequency Catheter Ablation. Europace 2016, 18, 384–391. [Google Scholar] [CrossRef]
- Massó-van Roessel, A.; Escobar-Robledo, L.A.; Dégano, I.; Grau, M.; Sala, J.; Ramos, R.; Marrugat, J.; Bayés de Luna, A.; Elosua, R. Analysis of the Association Between Electrocardiographic P-Wave Characteristics and Atrial Fibrillation in the REGICOR Study. Rev. Esp. Cardiol. 2017, 70, 841–847. [Google Scholar] [CrossRef]
- Conte, G.; Luca, A.; Yazdani, S.; Caputo, M.L.; Regoli, F.; Moccetti, T.; Kappenberger, L.; Vesin, J.-M.; Auricchio, A. Usefulness of P-Wave Duration and Morphologic Variability to Identify Patients Prone to Paroxysmal Atrial Fibrillation. Am. J. Cardiol. 2017, 119, 275–279. [Google Scholar] [CrossRef]
- Censi, F.; Corazza, I.; Reggiani, E.; Calcagnini, G.; Mattei, E.; Triventi, M.; Boriani, G. P-wave Variability and Atrial Fibrillation. Sci. Rep. 2016, 6, 26799. [Google Scholar] [CrossRef]
- Holmqvist, F.; Olesen, M.S.; Tveit, A.; Enger, S.; Tapanainen, J.; Jurkko, R.; Havmöller, R.; Haunsø, S.; Carlson, J.; Svendsen, J.H.; et al. Abnormal atrial activation in young patients with lone atrial fibrillation. Europace 2011, 13, 188–192. [Google Scholar] [CrossRef]
- Engel, T.R.; Vallone, N.; Windle, J. Signal-averaged electrocardiograms in patients with atrial fibrillation or flutter. Am. Heart J. 1988, 115, 592–597. [Google Scholar] [CrossRef]
- Okumura, Y.; Watanabe, I.; Ohkubo, K.; Ashino, S.; Kofune, M.; Hashimoto, K.; Shindo, A.; Sugimura, H.; Nakai, T.; Kasamaki, Y.; et al. Prediction of the Efficacy of Pulmonary Vein Isolation for the Treatment of Atrial Fibrillation by the Signal-Averaged P-Wave Duration. PACE—Pacing Clin. Electrophysiol. 2007, 30, 304–313. [Google Scholar] [CrossRef]
- Savelieva, I.; Aytemir, K.; Hnatkova, K.; Camm, A.J.; Malik, M. Short-, mid-, and long-term reproducibility of the atrial signal-averaged electrocardiogram in healthy subjects: Comparison with the conventional ventricular signal-averaged electrocardiogram. PACE—Pacing Clin. Electrophysiol. 2000, 23, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Dhala, A.; Underwood, D.; Leman, R.; Madu, E.; Baugh, D.; Ozawa, Y.; Kasamaki, Y.; Xue, Q.; Reddy, S. Signal-Averaged P-Wave Analysis of Normal Controls and Patients with Paroxysmal Atrial Fibrillation: A Study in Gender Differences, Age Dependence, and Reproducibility. Clin. Cardiol. 2002, 25, 525–531. [Google Scholar] [CrossRef]
- Aytemir, K.; Amasyali, B.; Abali, G.; Kose, S.; Kilic, A.; Onalan, O.; Tokgozoglu, L.; Kabakci, G.; Ozkutlu, H.; Nazli, N.; et al. The signal-averaged P-wave duration is longer in hypertensive patients with history of paroxysmal atrial fibrillation as compared to those without. Int. J. Cardiol. 2005, 103, 37–40. [Google Scholar] [CrossRef] [PubMed]
- Koide, Y.; Yotsukura, M.; Sakata, K.; Yoshino, H.; Ishikawa, K. Investigation of the predictors of transition to persistent atrial fibrillation in patients with paroxysmal atrial fibrillation. Clin. Cardiol. 2002, 25, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Budeus, M.; Felix, O.; Hennersdorf, M.; Wieneke, H.; Erbel, R.; Sack, S. Prediction of Conversion from Paroxysmal to Permanent Atrial Fibrillation. PACE—Pacing Clin. Electrophysiol. 2007, 30, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Platonov, P.; Mitrofanova, L.B.; Orshanskaya, V.; Ho, S.Y. Structural Abnormalities in Atrial Walls Are Associated With Presence and Persistency of Atrial Fibrillation But Not With Age. J. Am. Coll. Cardiol. 2011, 58, 2225–2232. [Google Scholar] [CrossRef] [Green Version]
- Schreiber, T.; Kähler, N.; Tscholl, V.; Nagel, P.; Blaschke, F.; Landmesser, U.; Attanasio, P.; Huemer, M. Correlation of P-wave properties with the size of left atrial low voltage areas in patients with atrial fibrillation. J. Electrocardiol. 2019, 56, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Nagel, C.; Luongo, G.; Azzolin, L.; Schuler, S.; Dössel, O.; Loewe, A. Non-Invasive and Quantitative Estimation of Left Atrial Fibrosis Based on P Waves of the 12-Lead ECG—A Large-Scale Computational Study Covering Anatomical Variability. J. Clin. Med. 2021, 10, 1797. [Google Scholar] [CrossRef] [PubMed]
- Filos, D.; Korosoglou, P.; Tachmatzidis, D.; Maglaveras, N.; Vassilikos, V.; Chouvarda, I. Multiple P-Wave Morphologies in Paroxysmal Atrial Fibrillation Patients During Sinus Rhythm: A Simulation Study. In Proceedings of the 2018 Computing in Cardiology Conference (Cinc), Maastricht, The Netherlands, 23–26 September 2018; IEEE Computer Society: Maastricht, The Netherlands, 2018; pp. 1–4. [Google Scholar]
- Pezzuto, S.; Gharaviri, A.; Schotten, U.; Potse, M.; Conte, G.; Caputo, M.L.; Regoli, F.; Krause, R.; Auricchio, A. Beat-to-beat P-wave morphological variability in patients with paroxysmal atrial fibrillation: Anin silicostudy. Europace 2018, 20, iii26–iii35. [Google Scholar] [CrossRef] [PubMed]
| Patient Parameters | PAF (n = 33) | Healthy (n = 56) | p Value |
|---|---|---|---|
| Age | 55.4 ± 12.6 | 55.2 ± 5.7 | 0.933 |
| Male sex (%) | 24 (72.7) | 38 (67.9) | 0.812 |
| Body mass index (kg/m2) | 28.2 ± 4.6 | 26.3 ± 5.9 | 0.756 |
| Hypertension (%) | 14 (42.4) | 12 (21.4) | 0.053 |
| Diabetes (%) | 0 (0.0) | 1 (1.8) | 1.000 |
| Dyslipidemia (%) | 10 (30.3) | 14 (25.0) | 0.626 |
| CHA2DS2-VASc score | 1.2 ± 1.3 | 0.7 ± 0.8 | 0.123 |
| Beat-to-Beat Analysis Features | Leads | Freq. Zones | Mean/cv | Subtotal |
|---|---|---|---|---|
| Percentage of P-waves matching main morphology | 3 | - | 1 | 3 |
| Percentage of P-waves matching secondary morphology | 3 | - | 1 | 3 |
| Secondary to main P-waves ratio | 3 | - | 1 | 3 |
| Percentage of P-waves matching main morphology in all axes at the same time | 1 | - | 1 | 1 |
| Distance between P-wave onset to R-wave | 3 | - | 2 | 6 |
| Distance between P-wave onset to Q-wave | 3 | - | 2 | 6 |
| Distance between P-wave peak to R-wave | 3 | - | 2 | 6 |
| Distance between P-wave peak to Q-wave | 3 | - | 2 | 6 |
| Distance between P-wave offset to R-wave | 3 | - | 2 | 6 |
| Distance between P-wave offset to Q-wave | 3 | - | 2 | 6 |
| Distance between P-wave onset to P-wave peak | 3 | - | 2 | 6 |
| Distance between P-wave onset to P-wave peak/P-wave duration | 3 | - | 2 | 6 |
| Distance between P-wave peak to P-wave offset | 3 | - | 2 | 6 |
| Distance between P-wave onset and maximum energy location | 3 | 3 | 2 | 18 |
| Distance between P-wave onset and maximum energy location normalized to P-wave duration | 3 | 3 | 2 | 18 |
| Distance between maximum energy location and P-wave offset | 3 | 3 | 2 | 18 |
| Distance between maximum energy location and R-wave | 3 | 3 | 2 | 18 |
| Distance between maximum energy location and Q-wave | 3 | 3 | 2 | 18 |
| Distance between P-wave peak and maximum energy location | 3 | 3 | 2 | 18 |
| Maximum energy value | 3 | 3 | 2 | 18 |
| Mean energy value | 3 | 3 | 2 | 18 |
| Mean energy value normalized to P-wave duration | 3 | 3 | 2 | 18 |
| Median energy value | 3 | 3 | 2 | 18 |
| Median energy value normalized to P-wave duration | 3 | 3 | 2 | 18 |
| Total | 262 |
| Features | Healthy (n = 56) | AF (n = 33) | p Value |
|---|---|---|---|
| Standard P-wave indices | |||
| P-wave area (ms × mV) | 11.1 ± 4.5 | 8.7 ± 6.6 | 0.025 |
| P-wave duration (ms) | 112.2 ± 16.2 | 122.6 ± 19.5 | 0.006 |
| Partial Interatrial Block | 8 (14.3%) | 14 (42.4%) | 0.003 |
| P-wave voltage in lead I (mV × 10−3) | 145.3 ± 50.5 | 110.4 ± 56.1 | 0.005 |
| MVP ECG score | 2.036 ± 1.436 | 3.000 ± 1.561 | 0.005 |
| PR duration (ms) | 182.6 ± 18.8 | 183.9 ± 27.5 | 0.763 |
| P-wave dispersion (ms) | 25.1 ± 14.5 | 24.2 ± 13.2 | 0.799 |
| P-wave peak time | 67.9 ± 9.7 | 69.1 ± 20.6 | 0.349 |
| P-wave axis | 47.1 ± 31.5 | 53.6 ± 22.3 | 0.075 |
| P-wave Terminal Force in lead V1 (ms × mV) | 5.1 ± 4.5 | 5.2 ± 5.5 | 0.555 |
| P-wave Terminal Force in lead V1 > 4 ms × mV | 18 (32.1%) | 10 (30.3%) | 0.857 |
| Advanced Interatrial Block | 11 (19.6%) | 6 (18.2%) | 0.866 |
| Orthogonal type 1 | 14 (25.0%) | 6 (18.2%) | 0.457 |
| Orthogonal type 2 | 39 (69.6%) | 22 (66.7%) | 0.770 |
| Orthogonal type 3 | 3 (5.4%) | 2 (6.1%) | 0.889 |
| Beat-to-beat analysis | |||
| Morphologies (%) | |||
| P-waves matching main morphology–X | 99.7 ± 0.8 | 96.6 ± 6.4 | <0.001 |
| P-waves matching main morphology–Y | 99.0 ± 3.8 | 95.8 ± 8.0 | <0.001 |
| P-waves matching secondary morphology–X | 0.2 ± 0.6 | 1.5 ± 3.1 | <0.001 |
| P-waves matching secondary morphology–Y | 0.4 ± 1.4 | 2.0 ± 4.6 | 0.017 |
| P-waves matching main morphology–all leads simultaneously | 95.2 ± 7.7 | 89.1 ± 12.3 | 0.041 |
| Secondary to main P-waves ratio–X | 0.002 ± 0.006 | 0.02 ± 0.04 | <0.001 |
| Secondary to main P-waves ratio–Y | 0.005 ± 0.02 | 0.03 ± 0.07 | 0.016 |
| Time domain (ms) | |||
| P-wave peak to R-wave–X, cv | 0.08 ± 0.2 | 0.1 ± 0.08 | <0.001 |
| P-wave peak to Q-wave–X, cv | 0.03 ± 0.4 | 0.2 ± 0.2 | <0.001 |
| P-wave onset to R-wave–X, cv | 0.03 ± 0.02 | 0.05 ± 0.04 | 0.004 |
| P-wave onset to Q-wave–X, cv | 0.04 ± 0.02 | 0.08 ± 0.06 | 0.004 |
| P-wave onset to P-wave peak (peak time)–X, cv | 0.05 ± 0.03 | 0.08 ± 0.06 | <0.001 |
| P-wave peak to P-wave offset–X, cv | 0.1 ± 0.5 | 0.09 ± 0.06 | <0.001 |
| P-wave peak to P-wave offset–Y, cv | 0.05 ± 0.03 | 0.1 ± 0.2 | 0.041 |
| Time-frequency domain (ms) | |||
| Maximum energy location to P-wave offset–mid band, Z, mean | 61.1 ± 18.6 | 53.8 ± 20.1 | 0.044 |
| Maximum energy location to P-wave onset, normalized to P-wave duration–high band, Z, mean | 0.6 ± 0.1 | 0.6 ± 0.09 | 0.049 |
| Maximum energy location to P-wave onset, normalized to P-wave duration–mid band, Z, mean | 0.6 ± 0.1 | 0.6 ± 0.1 | 0.034 |
| Frequency domain (μV2) | |||
| Maximum energy–high band, Y, mean | 0.2 ± 0.1 | 0.1 ± 0.09 | 0.003 |
| Maximum energy–high band, Y, cv | 0.5 ± 0.1 | 0.4 ± 0.2 | 0.028 |
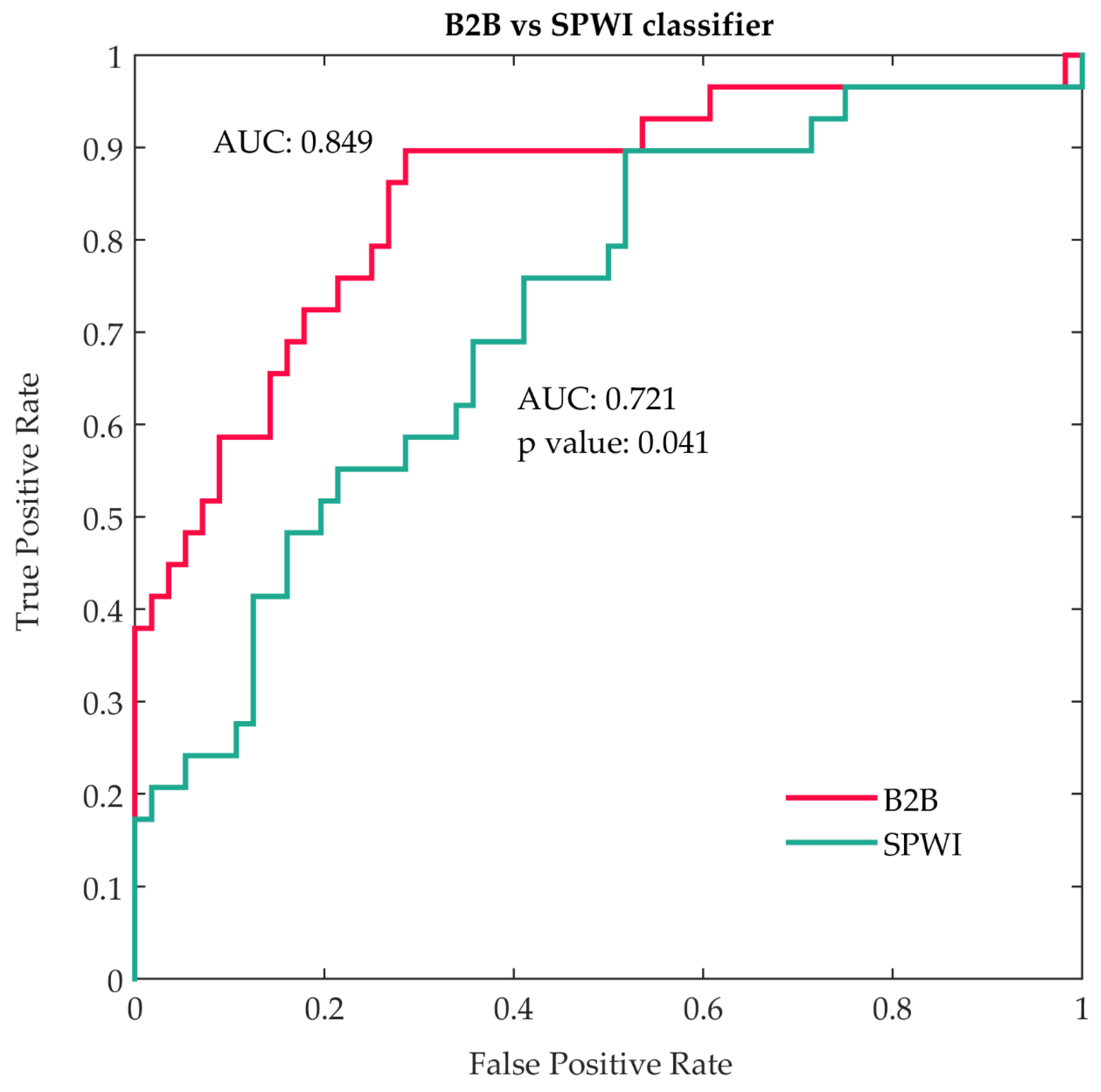
| Rank | Feature | Type | AUC | AUC Limits | OR | 95% CI | p Value |
|---|---|---|---|---|---|---|---|
| 1 | Peak time–X, cv | B2B | 0.726 | 0.606–0.846 | 2.672 | 1.463–4.880 | 0.001 |
| 2 | P-wave peak to Q-wave–X, cv | B2B | 0.725 | 0.614–0.836 | 33.513 | 3.904–287.692 | 0.001 |
| 3 | Main morphology %–Y | B2B | 0.723 | 0.616–0.830 | 0.553 | 0.307–0.996 | 0.048 |
| 4 | P-wave peak to R-wave–X, cv | B2B | 0.716 | 0.598–0.834 | 1.130 | 0.733–1.742 | 0.581 |
| 5 | P-wave peak to P-wave offset–X, cv | B2B | 0.710 | 0.590–0.830 | 0.935 | 0.574–1.523 | 0.788 |
| 6 | Main morphology %–X | B2B | 0.698 | 0.582–0.813 | 0.081 | 0.015–0.434 | 0.003 |
| 7 | Max energy–high band, Y, mean | B2B | 0.681 | 0.562–0.799 | 0.550 | 0.326–0.930 | 0.026 |
| 8 | P-wave onset to Q-wave–X, cv | B2B | 0.679 | 0.562–0.796 | 2.542 | 1.421–4.547 | 0.002 |
| 9 | P-wave voltage in lead I | SPWI | 0.679 | 0.558–0.800 | 0.493 | 0.300–0.810 | 0.005 |
| 10 | * Secondary to main morphology ratio %–X | B2B | 0.679 | 0.586–0.771 | 7.525 | 1.638–34.562 | 0.009 |
| 11 | * Secondary morphology %–X | B2B | 0.678 | 0.585–0.771 | 4.897 | 1.503–15.959 | 0.008 |
| 12 | * P-wave onset to R-wave–X, cv | B2B | 0.676 | 0.556–0.796 | 2.399 | 1.331–4.324 | 0.004 |
| 13 | P-wave duration | SPWI | 0.675 | 0.555–0.795 | 1.842 | 1.150–2.950 | 0.011 |
| 14 | * MVP score | SPWI | 0.673 | 0.560–0.787 | 1.921 | 1.209–3.052 | 0.006 |
| 15 | P-wave area | SPWI | 0.653 | 0.528–0.779 | 0.587 | 0.357–0.964 | 0.035 |
| 16 | Partial Interatrial Block | SPWI | 0.641 | 0.543–0.738 | 1.906 | 1.225–2.964 | 0.004 |
| 17 | Max energy–high band–Y, cv | B2B | 0.640 | 0.510–0.770 | 0.704 | 0.438–1.130 | 0.146 |
| 18 | Max energy location to Pdur.–mid band, Z, mean | B2B | 0.633 | 0.510–0.756 | 1.680 | 1.024–2.755 | 0.040 |
| 19 | Main morphology %–all axes | B2B | 0.631 | 0.500–0.761 | 0.527 | 0.326–0.851 | 0.009 |
| 20 | Max energy location to P offset–mid band, Z, mean | B2B | 0.630 | 0.506–0.755 | 0.668 | 0.420–1.062 | 0.088 |
| 21 | Max energy location to Pdur.–high band, Z, mean | B2B | 0.624 | 0.502–0.746 | 1.575 | 0.973–2.551 | 0.065 |
| 22 | P-wave peak to P-wave offset time–Y, cv | B2B | 0.620 | 0.496–0.743 | 6.590 | 0.953–45.565 | 0.056 |
| 23 | Secondary to main morphology ratio %–Y | B2B | 0.600 | 0.505–0.695 | 1.933 | 0.868–4.306 | 0.107 |
| 24 | Secondary morphology %–Y | B2B | 0.600 | 0.504–0.695 | 1.830 | 0.928–3.611 | 0.081 |
| Classifier | Variables | OR | 95% CI | p Value |
|---|---|---|---|---|
| Beat-to-beat analysis | P-wave peak to Q-wave–X, cv | 1.541 | 0.993–2.394 | 0.054 |
| Main morphology %–X | 0.786 | 0.624–0.991 | 0.042 | |
| Max energy–high frequency band, Y, mean | 0.443 | 0.187–1.048 | 0.064 | |
| Standard P-wave indices | Partial Interatrial Block | 1.979 | 0.550–7.122 | 0.296 |
| Voltage in lead I | 0.435 | 0.203–0.931 | 0.032 | |
| P-wave duration | 1.736 | 0.828–3.640 | 0.144 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tachmatzidis, D.; Filos, D.; Chouvarda, I.; Tsarouchas, A.; Mouselimis, D.; Bakogiannis, C.; Lazaridis, C.; Triantafyllou, K.; Antoniadis, A.P.; Fragakis, N.; et al. Beat-to-Beat P-Wave Analysis Outperforms Conventional P-Wave Indices in Identifying Patients with a History of Paroxysmal Atrial Fibrillation during Sinus Rhythm. Diagnostics 2021, 11, 1694. https://doi.org/10.3390/diagnostics11091694
Tachmatzidis D, Filos D, Chouvarda I, Tsarouchas A, Mouselimis D, Bakogiannis C, Lazaridis C, Triantafyllou K, Antoniadis AP, Fragakis N, et al. Beat-to-Beat P-Wave Analysis Outperforms Conventional P-Wave Indices in Identifying Patients with a History of Paroxysmal Atrial Fibrillation during Sinus Rhythm. Diagnostics. 2021; 11(9):1694. https://doi.org/10.3390/diagnostics11091694
Chicago/Turabian StyleTachmatzidis, Dimitrios, Dimitrios Filos, Ioanna Chouvarda, Anastasios Tsarouchas, Dimitrios Mouselimis, Constantinos Bakogiannis, Charalampos Lazaridis, Konstantinos Triantafyllou, Antonios P. Antoniadis, Nikolaos Fragakis, and et al. 2021. "Beat-to-Beat P-Wave Analysis Outperforms Conventional P-Wave Indices in Identifying Patients with a History of Paroxysmal Atrial Fibrillation during Sinus Rhythm" Diagnostics 11, no. 9: 1694. https://doi.org/10.3390/diagnostics11091694
APA StyleTachmatzidis, D., Filos, D., Chouvarda, I., Tsarouchas, A., Mouselimis, D., Bakogiannis, C., Lazaridis, C., Triantafyllou, K., Antoniadis, A. P., Fragakis, N., Efthimiadis, G., Maglaveras, N., Tsalikakis, D. G., & Vassilikos, V. P. (2021). Beat-to-Beat P-Wave Analysis Outperforms Conventional P-Wave Indices in Identifying Patients with a History of Paroxysmal Atrial Fibrillation during Sinus Rhythm. Diagnostics, 11(9), 1694. https://doi.org/10.3390/diagnostics11091694











