Focal Parenchymal Atrophy of the Pancreas Is Frequently Observed on Pre-Diagnostic Computed Tomography in Patients with Pancreatic Cancer: A Case-Control Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Subjects
2.3. Evaluation of CT Scans
2.4. Statistical Analysis
3. Results
3.1. Characteristics of Patients Who Underwent CT Examinations between 6 Months and 3 Years before PDAC Diagnosis
3.2. FPA Was Frequently Observed on Pre-Diagnostic CT
3.3. FPA Was Less Frequently Observed in PDAC Located in the Pancreatic Head
3.4. FPA Was More Frequently Observed in Patients with PDAC Than Controls without PDAC on Pre-Diagnostic CT
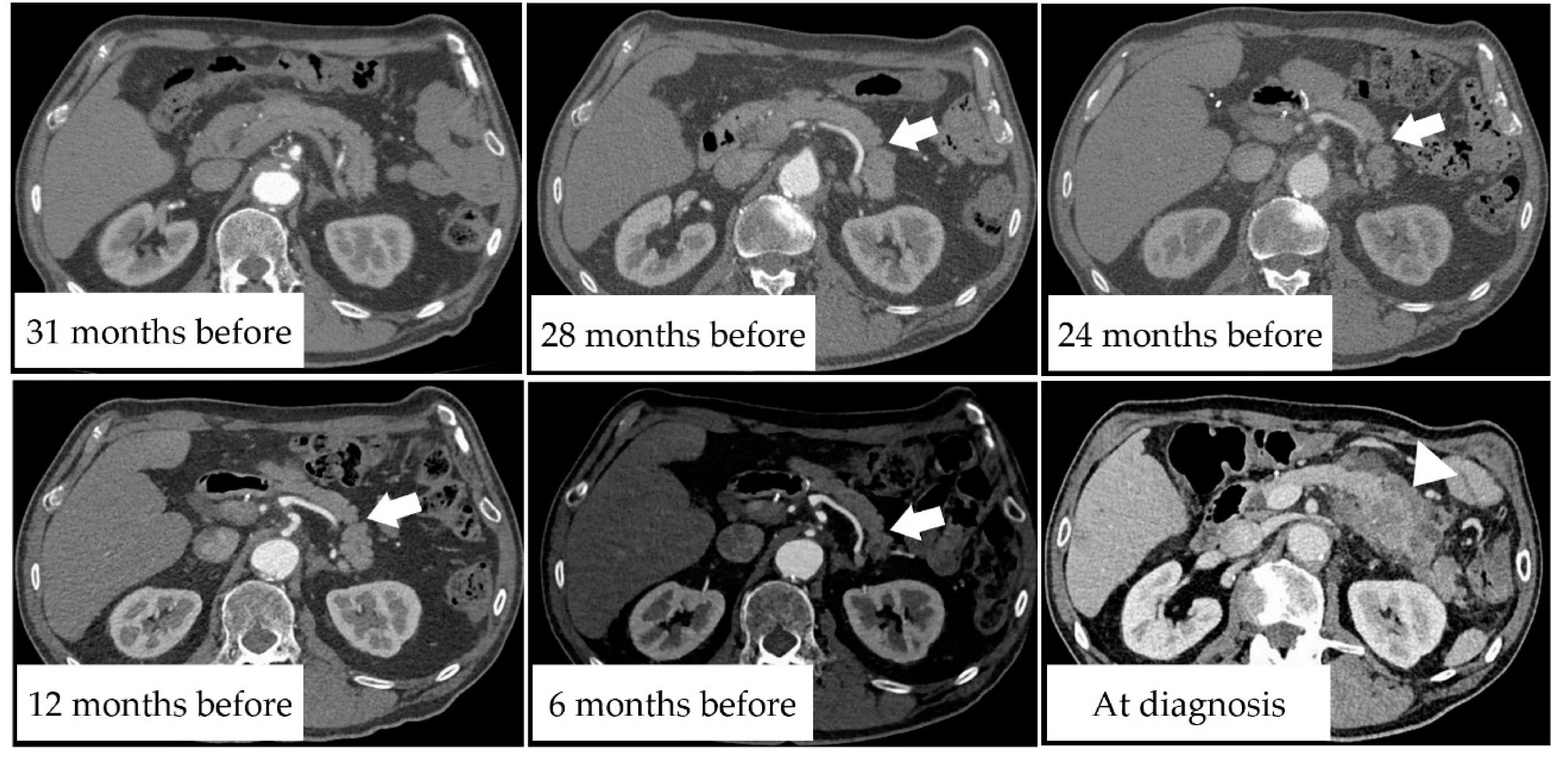
3.5. Case Presentation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Kamisawa, T.; Wood, L.D.; Itoi, T.; Takaori, K. Pancreatic Cancer. Lancet 2016, 388, 73–85. [Google Scholar] [CrossRef]
- Rahib, L.; Smith, B.D.; Aizenberg, R.; Rosenzweig, A.B.; Fleshman, J.M.; Matrisian, L.M. Projecting Cancer Incidence and Deaths to 2030: The Unexpected Burden of Thyroid, Liver, and Pancreas Cancers in the United States. Cancer Res. 2014, 74, 2913–2921. [Google Scholar] [CrossRef] [Green Version]
- Key Statistics for Pancreatic Cancer. Available online: https://www.cancer.org/cancer/pancreatic-cancer/about/key-statistics.html (accessed on 8 July 2021).
- Monitoring of Cancer Incidence in Japan—Survival 2009–2011 Report (Center for Cancer Control and Information Services, National Cancer Center, 2020). Available online: https://ganjoho.jp/reg_stat/statistics/data/dl/index.html#survival (accessed on 8 July 2021).
- Singhi, A.D.; Koay, E.J.; Chari, S.T.; Maitra, A. Early Detection of Pancreatic Cancer: Opportunities and Challenges. Gastroenterology 2019, 156, 2024–2040. [Google Scholar] [CrossRef] [Green Version]
- Pereira, S.P.; Oldfield, L.; Ney, A.; Hart, P.A.; Keane, M.G.; Pandol, S.J.; Li, D.; Greenhalf, W.; Jeon, C.Y.; Koay, E.J.; et al. Early Detection of Pancreatic Cancer. Lancet Gastroenterol. Hepatol. 2020, 5, 698–710. [Google Scholar] [CrossRef]
- Kenner, B.; Chari, S.T.; Kelsen, D.; Klimstra, D.S.; Pandol, S.J.; Rosenthal, M.; Rustgi, A.K.; Taylor, J.A.; Yala, A.; Abul-Husn, N.; et al. Artificial Intelligence and Early Detection of Pancreatic Cancer: 2020 Summative Review. Pancreas 2021, 50, 251–279. [Google Scholar] [CrossRef] [PubMed]
- Egawa, S.; Toma, H.; Ohigashi, H.; Okusaka, T.; Nakao, A.; Hatori, T.; Maguchi, H.; Yanagisawa, A.; Tanaka, M. Japan Pancreatic Cancer Registry; 30th Year Anniversary: Japan Pancreas Society. Pancreas 2012, 41, 985–992. [Google Scholar] [CrossRef]
- Blackford, A.L.; Canto, M.I.; Klein, A.P.; Hruban, R.H.; Goggins, M. Recent Trends in the Incidence and Survival of Stage 1A Pancreatic Cancer: A Surveillance, Epidemiology, and End Results Analysis. J. Natl. Cancer Inst. 2020, 112, 1162–1169. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.S.; Kim, M.J.; Choi, J.Y.; Hong, H.S.; Chung, Y.E.; Lim, J.S. Indicative Findings of Pancreatic Cancer in Prediagnostic CT. Eur. Radiol. 2009, 19, 2448–2455. [Google Scholar] [CrossRef] [PubMed]
- Elbanna, K.Y.; Jang, H.J.; Kim, T.K. Imaging Diagnosis and Staging of Pancreatic Ductal Adenocarcinoma: A Comprehensive Review. Insights Imaging 2020, 11, 58. [Google Scholar] [CrossRef] [Green Version]
- Vasen, H.F.A.; Boekestijn, B.; Ibrahim, I.S.; Inderson, A.; Bonsing, B.A.; de Vos Tot Nederveen Cappel, W.H.; Feshtali, S.; Wasser, M.N. Dilatation of The Main Pancreatic Duct as First Manifestation of Small Pancreatic Ductal Adenocarcinomas Detected in A Hereditary Pancreatic Cancer Surveillance Program. HPB 2019, 21, 1371–1375. [Google Scholar] [CrossRef]
- Miura, S.; Kume, K.; Kikuta, K.; Hamada, S.; Takikawa, T.; Yoshida, N.; Hongo, S.; Tanaka, Y.; Matsumoto, R.; Sano, T.; et al. Focal Parenchymal Atrophy and Fat Replacement Are Clues for Early Diagnosis of Pancreatic Cancer with Abnormalities of the Main Pancreatic Duct. Tohoku J. Exp. Med. 2020, 252, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Yamao, K.; Takenaka, M.; Ishikawa, R.; Okamoto, A.; Yamazaki, T.; Nakai, A.; Omoto, S.; Kamata, K.; Minaga, K.; Matsumoto, I.; et al. Partial Pancreatic Parenchymal Atrophy Is a New Specific Finding to Diagnose Small Pancreatic Cancer (≤10 mm) Including Carcinoma in Situ: Comparison with Localized Benign Main Pancreatic Duct Stenosis Patients. Diagnostics 2020, 10, 445. [Google Scholar] [CrossRef]
- Nakahodo, J.; Kikuyama, M.; Nojiri, S.; Chiba, K.; Yoshimoto, K.; Kamisawa, T.; Horiguchi, S.I.; Honda, G. Focal Parenchymal Atrophy of Pancreas: An Important Sign of Underlying High-Grade Pancreatic Intraepithelial Neoplasia Without Invasive Carcinoma, i.e., Carcinoma in Situ. Pancreatology 2020, 20, 1689–1697. [Google Scholar] [CrossRef] [PubMed]
- Kanno, A.; Masamune, A.; Hanada, K.; Maguchi, H.; Shimizu, Y.; Ueki, T.; Hasebe, O.; Ohtsuka, T.; Nakamura, M.; Takenaka, M.; et al. Japan Study Group on the Early Detection of Pancreatic Cancer (JEDPAC). Multicenter Study of Early Pancreatic Cancer in Japan. Pancreatology 2018, 18, 61–67. [Google Scholar] [CrossRef]
- Singh, D.P.; Sheedy, S.; Goenka, A.H.; Wells, M.; Lee, N.J.; Barlow, J.; Sharma, A.; Kandlakunta, H.; Chandra, S.; Garg, S.K.; et al. Computerized Tomography Scan in Pre-diagnostic Pancreatic Ductal Adenocarcinoma: Stages of Progression and Potential Benefits of Early Intervention: A Retrospective Study. Pancreatology 2020, 20, 1495–1501. [Google Scholar] [CrossRef] [PubMed]
- Gangi, S.; Fletcher, J.G.; Nathan, M.A.; Christensen, J.A.; Harmsen, W.S.; Crownhart, B.S.; Chari, S.T. Time Interval Between Abnormalities Seen on CT and the Clinical Diagnosis of Pancreatic Cancer: Retrospective Review of CT scans Obtained Before Diagnosis. AJR Am. J. Roentgenol. 2004, 182, 897–903. [Google Scholar] [CrossRef]
- Pelaez-Luna, M.; Takahashi, N.; Fletcher, J.G.; Chari, S.T. Resectability of Presymptomatic Pancreatic Cancer and Its Relationship to Onset of Diabetes: A Retrospective Review of CT scans and Fasting Glucose Values Prior to Diagnosis. Am. J. Gastroenterol. 2007, 102, 2157–2163. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.B.; Edge, S.; Greene, F.; Byrd, D.R.; Brookland, R.K.; Washington, M.K.; Gershenwald, J.E.; Compton, C.C.; Hess, K.R.; Sullivan, D.C.; et al. American Joint Committee on Cancer. In AJCC Cancer Staging Manual, 8th ed.; Springer: New York, NY, USA, 2017. [Google Scholar]
- Lee, E.S.; Lee, J.M. Imaging Diagnosis of Pancreatic Cancer: A State-of-the-art Review. World J. Gastroenterol. 2014, 20, 7864–7877. [Google Scholar] [CrossRef]
- Matsuda, Y. Age-Related Morphological Changes in the Pancreas and Their Association with Pancreatic Carcinogenesis. Pathol. Int. 2019, 69, 450–462. [Google Scholar] [CrossRef] [Green Version]
- Syed, A.B.; Mahal, R.S.; Schumm, L.P.; Dachman, A.H. Pancreas Size and Volume on Computed Tomography in Normal Adults. Pancreas 2012, 41, 589–595. [Google Scholar] [CrossRef]
- Eguchi, H.; Yamaue, H.; Unno, M.; Mizuma, M.; Hamada, S.; Igarashi, H.; Kuroki, T.; Satoi, S.; Shimizu, Y.; Tani, M.; et al. Committee of Clinical Research, Japan Pancreas Society. Clinicopathological Characteristics of Young Patients with Pancreatic Cancer: An Analysis of Data from Pancreatic Cancer Registry of Japan Pancreas Society. Pancreas 2016, 45, 1411–1417. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, Y.; Furukawa, T.; Yachida, S.; Nishimura, M.; Seki, A.; Nonaka, K.; Aida, J.; Takubo, K.; Ishiwata, T.; Kimura, W.; et al. The Prevalence and Clinicopathological Characteristics of High-Grade Pancreatic Intraepithelial Neoplasia: Autopsy Study Evaluating the Entire Pancreatic Parenchyma. Pancreas 2017, 46, 658–664. [Google Scholar] [CrossRef]
- Dreyer, S.B.; Jamieson, N.B.; Upstill-Goddard, R.; Bailey, P.J.; McKay, C.J.; Australian Pancreatic Cancer Genome Initiative; Biankin, A.V.; Chang, D.K. Defining the Molecular Pathology of Pancreatic Body and Tail Adenocarcinoma. Br. J. Surg. 2018, 105, e183–e191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Owens, D.K.; Davidson, K.W.; Krist, A.H.; Barry, M.J.; Cabana, M.; Caughey, A.B.; Curry, S.J.; Doubeni, C.A.; Epling, J.W.; Jr Kubik, M.; et al. US Preventive Services Task Force. Screening for Pancreatic Cancer: US Preventive Services Task Force Reaffirmation Recommendation Statement. JAMA 2019, 322, 438–444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aslanian, H.R.; Lee, J.H.; Canto, M.I. AGA Clinical Practice Update on Pancreas Cancer Screening in High-Risk Individuals: Expert Review. Gastroenterology 2020, 159, 358–362. [Google Scholar] [CrossRef]
- Takikawa, T.; Kikuta, K.; Kume, K.; Hamada, S.; Miura, S.; Yoshida, N.; Hongo, S.; Tanaka, Y.; Matsumoto, R.; Sano, T.; et al. New-Onset or Exacerbation of Diabetes Mellitus Is a Clue to the Early Diagnosis of Pancreatic Cancer. Tohoku J. Exp. Med. 2020, 252, 353–364. [Google Scholar] [CrossRef]
- Takeda, Y.; Saiura, A.; Takahashi, Y.; Inoue, Y.; Ishizawa, T.; Mise, Y.; Matsumura, M.; Ichida, H.; Matsuki, R.; Tanaka, M.; et al. Asymptomatic Pancreatic Cancer: Does Incidental Detection Impact Long-term Outcomes? J. Gastrointest. Surg. 2017, 21, 1287–1295. [Google Scholar] [CrossRef]
- Brune, K.; Abe, T.; Canto, M.; O’Malley, L.; Klein, A.P.; Maitra, A.; Volkan Adsay, N.; Fishman, E.K.; Cameron, J.L.; Yeo, C.J.; et al. Multifocal Neoplastic Precursor Lesions Associated with Lobular Atrophy of the Pancreas in Patients Having a Strong Family History of Pancreatic Cancer. Am. J. Surg. Pathol. 2006, 30, 1067–1076. [Google Scholar]
- Hamada, S.; Shimosegawa, T.; Taguchi, K.; Nabeshima, T.; Yamamoto, M.; Masamune, A. Simultaneous K-ras Activation and Keap1 Deletion Cause Atrophy of Pancreatic Parenchyma. Am. J. Physiol. Gastrointest. Liver Physiol. 2018, 314, G65–G74. [Google Scholar] [CrossRef]
- Masamune, A.; Hamada, S.; Yoshida, N.; Nabeshima, T.; Shimosegawa, T. Pyruvate Kinase Isozyme M2 Plays a Critical Role in the Interactions Between Pancreatic Stellate Cells and Cancer Cells. Dig. Dis. Sci. 2018, 63, 1868–1877. [Google Scholar] [CrossRef]
- Takehara, M.; Sato, Y.; Kimura, T.; Noda, K.; Miyamoto, H.; Fujino, Y.; Miyoshi, J.; Nakamura, F.; Wada, H.; Bando, Y.; et al. Cancer-associated Adipocytes Promote Pancreatic Cancer Progression through SAA1 Expression. Cancer Sci. 2020, 111, 2883–2894. [Google Scholar] [CrossRef]
- Takahashi, M.; Hori, M.; Ishigamori, R.; Mutoh, M.; Imai, T.; Nakagama, H. Fatty Pancreas: A Possible Risk Factor for Pancreatic Cancer in Animals and Humans. Cancer Sci. 2018, 109, 3013–3023. [Google Scholar] [CrossRef] [PubMed]
- Desai, V.; Patel, K.; Sheth, R.; Barlass, U.; Chan, Y.M.; Sclamberg, J.; Bishehsari, F. Pancreatic Fat Infiltration is Associated with a Higher Risk of Pancreatic Ductal Adenocarcinoma. Visc. Med. 2020, 36, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Toshima, F.; Watanabe, R.; Inoue, D.; Yoneda, N.; Yamamoto, T.; Sasahira, N.; Sasaki, T.; Matsuyama, M.; Minehiro, K.; Tateishi, U.; et al. CT Abnormalities of the Pancreas Associated with the Subsequent Diagnosis of Clinical Stage I Pancreatic Ductal Adenocarcinoma More Than One Year Later: A Case-Control Study. AJR Am. J. Roentgenol. 2021. [Google Scholar] [CrossRef]
- Yachida, S.; Jones, S.; Bozic, I.; Antal, T.; Leary, R.; Fu, B.; Kamiyama, M.; Hruban, R.H.; Eshleman, J.R.; Nowak, M.A.; et al. Distant Metastasis Occurs Late During the Genetic Evolution of Pancreatic cancer. Nature 2010, 467, 1114–1117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Canto, M.I.; Almario, J.A.; Schulick, R.D.; Yeo, C.J.; Klein, A.; Blackford, A.; Shin, E.J.; Sanyal, A.; Yenokyan, G.; Lennon, A.M.; et al. Risk of Neoplastic Progression in Individuals at High Risk for Pancreatic Cancer Undergoing Long-term Surveillance. Gastroenterology 2018, 155, 740–751.e2. [Google Scholar] [CrossRef] [Green Version]


| PDAC (N = 76) | |
|---|---|
| Male, N (%) | 43 (56.6) |
| Age at PDAC Diagnosis, Median (Range) | 72 (49–85) |
| Location of PDAC, N (%) | |
| Head | 27 (35.5) |
| Body | 30 (39.5) |
| Tail | 19 (25.0) |
| Pancreatic resection, N (%) | 45 (59.2) |
| Mass diameter, mm, median (range) | 21.5 (0–80.0) |
| UICC Stage, N (%) | |
| Stage 0 | 1 (1.3) |
| Stage IA | 15 (19.7) |
| Stage IB | 10 (13.2) |
| Stage IIA | 4 (5.3) |
| Stage IIB | 16 (21.1) |
| Stage III | 8 (10.5) |
| Stage IV | 22 (28.9) |
| Reason for pre-diagnostic CT, N (%) | |
| Follow-up for pancreatic disease | 32 (42.1) |
| Follow-up for various cancers | 27 (35.5) |
| Follow-up for liver disease | 4 (5.3) |
| Follow-up for cardiovascular disease | 3 (3.9) |
| Follow-up for gastrointestinal disease | 2 (2.6) |
| Follow-up for respiratory disease | 2 (2.6) |
| Other | 6 (7.9) |
| At Diagnosis (N = 76) | 6 Months–1 Year before (N = 44) | 1–2 Years before (N = 51) | 2–3 Years before (N = 41) | |
|---|---|---|---|---|
| Time interval before the diagnosis, years, median (range) | - | 0.64(0.50–0.99) | 1.27 (1.00–1.96) | 2.20 (2.00–2.99) |
| Contrast-medium use, N (%) | 76 (100) | 35 (79.5) | 42 (82.4) | 33 (80.5) |
| Mass, N (%) | 63 (82.9) | 10 (22.7) | 0 (0) | 0 (0) |
| Mass diameter, mm, median (range) | 21.5 (0–80.0) | 17.5 (11.0–26.0) | 0 (0) | 0 (0) |
| MPD cut-off/dilatation, N (%) | 58 (76.3) | 12 (27.3) | 7 (13.7) | 1 (2.4) |
| Pancreatic cyst within 2 cm of tumor, N (%) | 22 (28.9) | 12 (27.3) | 18 (35.3) | 12 (29.3) |
| Pancreatic cyst beyond 2 cm of tumor, N (%) | 28 (36.8) | 14 (31.8) | 13 (25.5) | 11 (26.8) |
| Focal parenchymal atrophy, N (%) | 6 (7.9) | 14 (31.8) | 14 (27.5) | 9 (22.0) |
| Location of PDAC, N (%) | ||||
| Head | 27 (35.5) | 18 (40.9) | 18 (35.3) | 14 (34.1) |
| Body | 30 (39.5) | 15 (34.1) | 18 (35.3) | 15 (36.6) |
| Tail | 19 (25.0) | 11 (25.0) | 15 (29.4) | 12 (29.3) |
| UICC Stage at diagnosis, N (%) | ||||
| Stage 0 | 1 (1.3) | 1 (2.3) | 0 (0) | 0 (0) |
| Stage IA | 15 (19.7) | 6 (13.6) | 13 (25.5) | 9 (22.0) |
| Stage IB | 10 (13.2) | 4 (9.1) | 7 (13.7) | 5 (12.2) |
| Stage IIA | 4 (5.3) | 2 (4.5) | 2 (3.9) | 2 (4.9) |
| Stage IIB | 16 (21.1) | 13 (29.5) | 7 (13.7) | 8 (19.5) |
| Stage III | 8 (10.5) | 6 (13.6) | 6 (11.8) | 5 (12.2) |
| Stage IV | 22 (28.9) | 12 (27.3) | 16 (31.4) | 12 (29.3) |
| At Diagnosis (N = 6) | 6 Months–1 Year before (N = 14) | 1–2 Years before (N = 14) | 2–3 Years before (N = 9) | |
|---|---|---|---|---|
| Time interval before the diagnosis, years, median (range) | - | 0.62 (0.50–0.99) | 1.33 (1.00–1.70) | 2.61 (2.05–2.99) |
| Contrast-medium use, N (%) | 6 (100) | 12 (85.7) | 10 (71.4) | 8 (88.9) |
| Mass, N (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| MPD cut-off/dilatation, N (%) | 5 (83.3) | 5 (35.7) | 1 (7.1) | 0 (0) |
| Pancreatic cyst within 2 cm of tumor, N (%) | 1 (16.7) | 4 (28.6) | 5 (35.7) | 2 (22.2) |
| Pancreatic cyst beyond 2 cm of tumor, N (%) | 2 (33.3) | 2 (14.3) | 4 (28.5) | 4 (44.4) |
| Location of PDAC, N (%) | ||||
| Head | 1 (16.7) | 1 (7.1) | 2 (14.2) | 1 (11.1) |
| Body | 4 (66.7) | 8 (57.1) | 8 (57.1) | 5 (55.5) |
| Tail | 1 (16.7) | 5 (35.7) | 4 (28.6) | 3 (33.3) |
| UICC Stage at diagnosis, N (%) | ||||
| Stage 0 | 1 (16.7) | 1 (7.1) | 0 (0) | 0 (0) |
| Stage IA | 4 (66.6) | 1 (7.1) | 4 (28.6) | 1 (11.1) |
| Stage IB | 0 (0) | 2 (14.3) | 3 (21.4) | 3 (33.3) |
| Stage IIA | 0 (0) | 0 (0) | 0 (0) | 1 (11.1) |
| Stage IIB | 1 (16.7) | 3 (21.4) | 2 (14.3) | 1 (11.1) |
| Stage III | 0 (0) | 4 (28.6) | 1 (7.1) | 1 (11.1) |
| Stage IV | 0 (0) | 3 (21.4) | 4 (28.6) | 2 (22.2) |
| With FPA (N = 26) | Without FPA (N = 50) | p Value | |
|---|---|---|---|
| Number of pre-diagnostic CT, N (%) | 0.50 | ||
| 1 | 13 (50.0) | 21 (42.0) | |
| 2 | 9 (34.6) | 15 (30.0) | |
| 3 | 4 (15.4) | 14 (28.0) | |
| Male, N (%) | 13 (50.0) | 30 (60.0) | 0.47 |
| Age at PDAC diagnosis, median (range) | 73 (65-80) | 71 (49-85) | 0.38 |
| Mass, N (%) | 21 (80.8) | 42 (84.0) | 0.75 |
| Mass diameter, mm, median (range) | 22.5 (0-80.0) | 19.5 (0-66.0) | 0.53 |
| MPD cut-off/dilatation, N (%) | 22 (84.6) | 36 (72.0) | 0.27 |
| Pancreatic cyst within 2cm of tumor, N (%) | 6 (23.1) | 16 (32.0) | 0.59 |
| Pancreatic cyst beyond 2 cm of tumor, N (%) | 9 (34.6) | 19 (38.0) | 0.80 |
| Location of PDAC, N (%) | 0.007 | ||
| Head | 3 (11.5) | 24 (48.0) | |
| Body | 14 (53.8) | 16 (32.0) | |
| Tail | 9 (34.6) | 10 (20.0) | |
| UICC Stage at diagnosis, N (%) | 0.81 | ||
| Stage 0 | 1 (3.8) | 0 (0) | |
| Stage IA | 4 (15.4) | 11 (22.0) | |
| Stage IB | 4 (15.4) | 6 (12.0) | |
| Stage IIA | 1 (3.8) | 3 (6.0) | |
| Stage IIB | 5 (19.2) | 11 (22.0) | |
| Stage III | 4 (15.4) | 4 (8.0) | |
| Stage IV | 7 (26.9) | 15 (30.0) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miura, S.; Takikawa, T.; Kikuta, K.; Hamada, S.; Kume, K.; Yoshida, N.; Tanaka, Y.; Matsumoto, R.; Ikeda, M.; Kataoka, F.; et al. Focal Parenchymal Atrophy of the Pancreas Is Frequently Observed on Pre-Diagnostic Computed Tomography in Patients with Pancreatic Cancer: A Case-Control Study. Diagnostics 2021, 11, 1693. https://doi.org/10.3390/diagnostics11091693
Miura S, Takikawa T, Kikuta K, Hamada S, Kume K, Yoshida N, Tanaka Y, Matsumoto R, Ikeda M, Kataoka F, et al. Focal Parenchymal Atrophy of the Pancreas Is Frequently Observed on Pre-Diagnostic Computed Tomography in Patients with Pancreatic Cancer: A Case-Control Study. Diagnostics. 2021; 11(9):1693. https://doi.org/10.3390/diagnostics11091693
Chicago/Turabian StyleMiura, Shin, Tetsuya Takikawa, Kazuhiro Kikuta, Shin Hamada, Kiyoshi Kume, Naoki Yoshida, Yu Tanaka, Ryotaro Matsumoto, Mio Ikeda, Fumiya Kataoka, and et al. 2021. "Focal Parenchymal Atrophy of the Pancreas Is Frequently Observed on Pre-Diagnostic Computed Tomography in Patients with Pancreatic Cancer: A Case-Control Study" Diagnostics 11, no. 9: 1693. https://doi.org/10.3390/diagnostics11091693
APA StyleMiura, S., Takikawa, T., Kikuta, K., Hamada, S., Kume, K., Yoshida, N., Tanaka, Y., Matsumoto, R., Ikeda, M., Kataoka, F., Sasaki, A., Hatta, W., Inoue, J., & Masamune, A. (2021). Focal Parenchymal Atrophy of the Pancreas Is Frequently Observed on Pre-Diagnostic Computed Tomography in Patients with Pancreatic Cancer: A Case-Control Study. Diagnostics, 11(9), 1693. https://doi.org/10.3390/diagnostics11091693








