Magnetic Resonance Imaging for Surveillance of Hepatocellular Carcinoma: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Search
2.3. Data Items
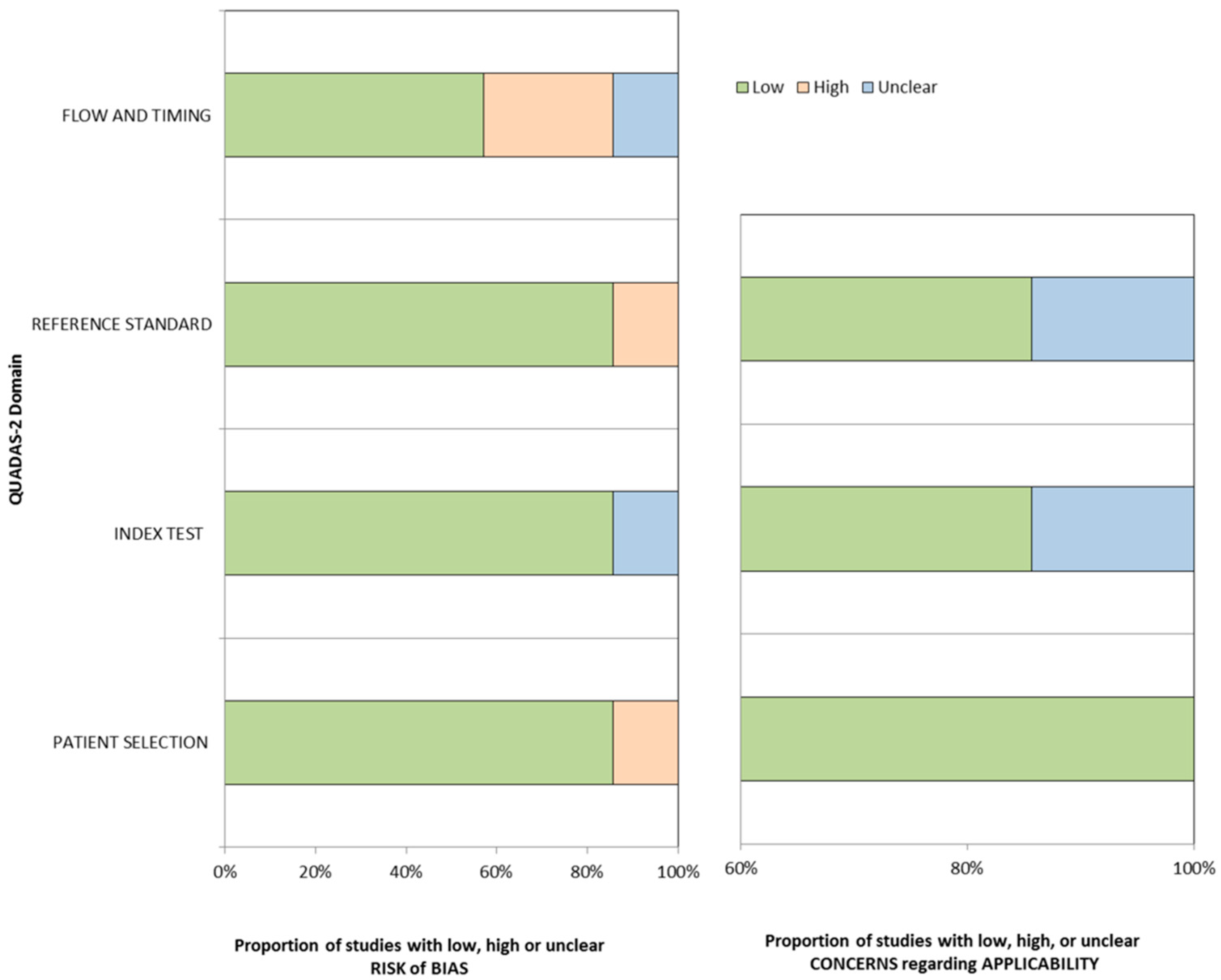
2.4. Risk of Bias in Individual Studies
2.5. Summary Measures
2.6. Synthesis of Results
3. Results
3.1. Literature Search
3.2. Study Quality Assessment
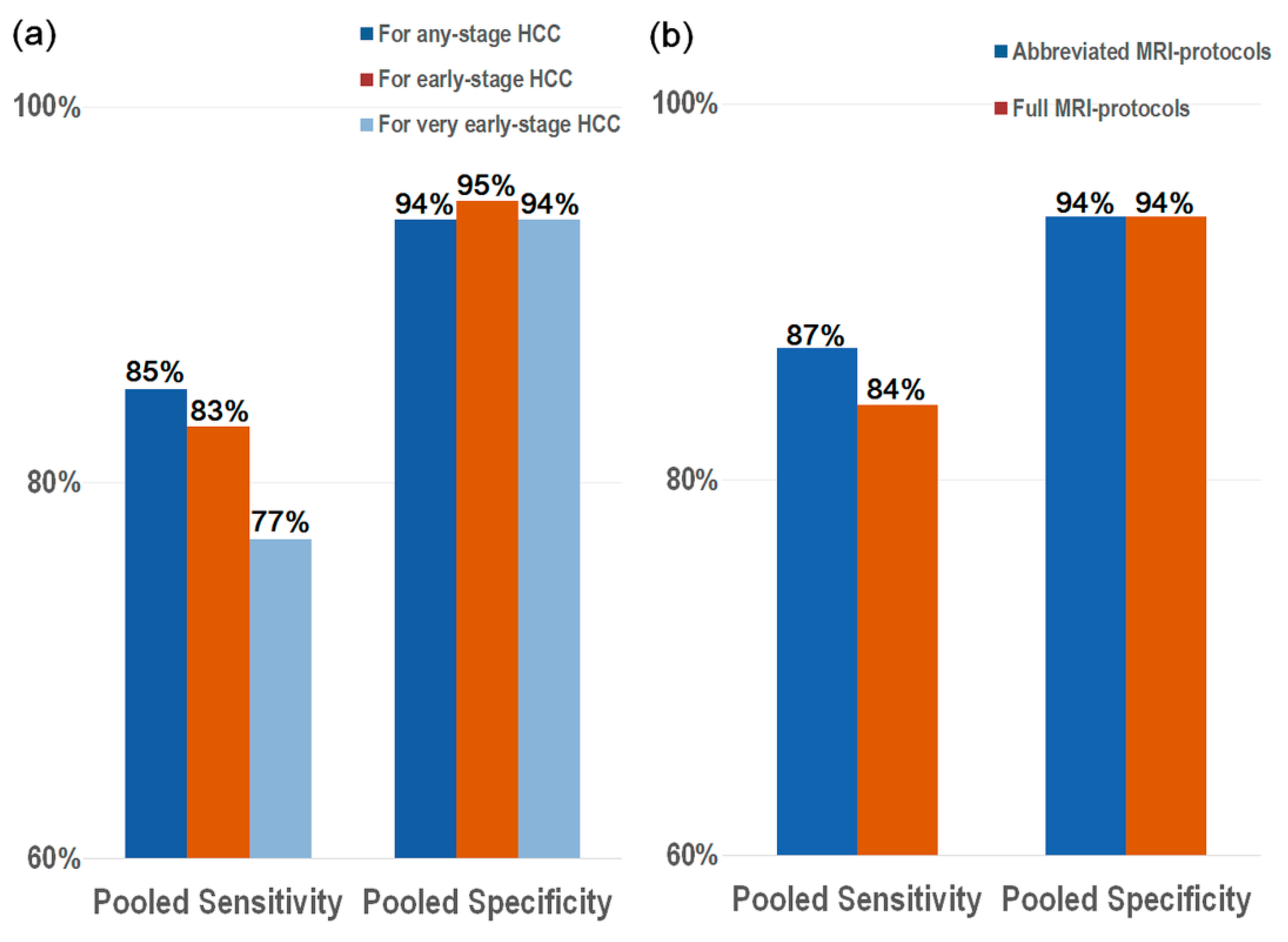
3.3. Diagnostic Performance of sMRI for the Detection of HCC
3.4. Full MRI Protocols vs. Abbreviated MRI Protocols
3.5. Sensitivity Analysis and Meta-Regression Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Akinyemiju, T.; Abera, S.; Ahmed, M.; Alam, N.; Alemayohu, M.A.; Allen, C.; Al-Raddadi, R.; Alvis-Guzmán, N.; Amoako, Y.; Artaman, A.; et al. The Burden of Primary Liver Cancer and Underlying Etiologies from 1990 to 2015 at the Global, Regional, and National Level: Results from the Global Burden of Disease Study 2015. JAMA Oncol. 2017, 3, 1683–1691. [Google Scholar]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marrero, J.A.; Kulik, L.M.; Sirlin, C.B.; Zhu, A.X.; Finn, R.S.; Abecassis, M.M.; Roberts, L.R.; Heimbach, J.K. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2018, 68, 723–750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef] [Green Version]
- Korean Liver Cancer Association; National Cancer Center. 2018 Korean Liver Cancer Association-National Cancer Center Korea Practice Guidelines for the Management of Hepatocellular Carcinoma. Gut Liver 2019, 13, 227–299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, B.H.; Yang, B.H.; Tang, Z.Y. Randomized controlled trial of screening for hepatocellular carcinoma. J. Cancer Res. Clin. Oncol. 2004, 130, 417–422. [Google Scholar] [CrossRef]
- Singal, A.G.; Pillai, A.; Tiro, J. Early detection, curative treatment, and survival rates for hepatocellular carcinoma surveillance in patients with cirrhosis: A meta-analysis. PLoS Med. 2014, 11, e1001624. [Google Scholar] [CrossRef]
- Pocha, C.; Dieperink, E.; McMaken, K.A.; Knott, A.; Thuras, P.; Ho, S.B. Surveillance for hepatocellular cancer with ultrasonography vs. computed tomography: A randomised study. Aliment. Pharmacol. Ther. 2013, 38, 303–312. [Google Scholar] [CrossRef]
- Andersson, K.L.; Salomon, J.A.; Goldie, S.J.; Chung, R.T. Cost effectiveness of alternative surveillance strategies for hepatocellular carcinoma in patients with cirrhosis. Clin. Gastroenterol. Hepatol. 2008, 6, 1418–1424. [Google Scholar] [CrossRef] [Green Version]
- Tzartzeva, K.; Obi, J.; Rich, N.E.; Parikh, N.D.; Marrero, J.A.; Yopp, A.; Waljee, A.K.; Singal, A.G. Surveillance Imaging and Alpha Fetoprotein for Early Detection of Hepatocellular Carcinoma in Patients with Cirrhosis: A Meta-analysis. Gastroenterology 2018, 154, 1706–1718.e1. [Google Scholar] [CrossRef] [Green Version]
- Chou, R.; Cuevas, C.; Fu, R.; Devine, B.; Wasson, N.; Ginsburg, A.; Zakher, B.; Pappas, M.; Graham, E.; Sullivan, S.D. Imaging Techniques for the Diagnosis of Hepatocellular Carcinoma: A Systematic Review and Meta-analysis. Ann. Intern. Med. 2015, 162, 697–711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.D.; Lim, Y.S.; Han, S.; An, J.; Kim, G.A.; Kim, S.Y.; Lee, S.J.; Won, H.J.; Byun, H.J. Evaluation of early-stage hepatocellular carcinoma by magnetic resonance imaging with gadoxetic acid detects additional lesions and increases overall survival. Gastroenterology 2015, 148, 1371–1382. [Google Scholar] [CrossRef] [PubMed]
- Brunsing, R.L.; Chen, D.H.; Schlein, A.; Wolfson, T.; Gamst, A.; Mamidipalli, A.; Violi, N.V.; Marks, R.M.; Taouli, B.; Loomba, R.; et al. Gadoxetate-enhanced Abbreviated MRI for Hepatocellular Carcinoma Surveillance: Preliminary Experience. Radiol. Imaging Cancer 2019, 1, e190010. [Google Scholar] [CrossRef] [PubMed]
- Marks, R.M.; Ryan, A.; Heba, E.R.; Tang, A.; Wolfson, T.J.; Gamst, A.C.; Sirlin, C.B.; Bashir, M.R. Diagnostic per-patient accuracy of an abbreviated hepatobiliary phase gadoxetic acid-enhanced MRI for hepatocellular carcinoma surveillance. AJR Am. J. Roentgenol. 2015, 204, 527–535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sutherland, T.; Watts, J.; Ryan, M.; Galvin, A.; Temple, F.; Vuong, J.; Little, A.F. Diffusion-weighted MRI for hepatocellular carcinoma screening in chronic liver disease: Direct comparison with ultrasound screening. J. Med. Imaging Radiat. Oncol. 2017, 61, 34–39. [Google Scholar] [CrossRef] [Green Version]
- Chan, M.V.; McDonald, S.J.; Ong, Y.Y.; Mastrocostas, K.; Ho, E.; Huo, Y.R.; Santhakumar, C.; Lee, A.U.; Yang, J. HCC screening: Assessment of an abbreviated non-contrast MRI protocol. Eur. Radiol. Exp. 2019, 3, 49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shah, T.U.; Semelka, R.C.; Pamuklar, E.; Firat, Z.; Gerber, R.D.; Shrestha, R.; Russo, M.W. The risk of hepatocellular carcinoma in cirrhotic patients with small liver nodules on MRI. Am. J. Gastroenterol. 2006, 101, 533–540. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ 2009, 339, b2700. [Google Scholar] [CrossRef] [Green Version]
- Whiting, P.F.; Rutjes, A.W.; Westwood, M.E.; Mallett, S.; Deeks, J.J.; Reitsma, J.B.; Leeflang, M.M.; Sterne, J.A.; Bossuyt, P.M.; QUADAS-2 Group. QUADAS-2: A revised tool for the quality assessment of diagnostic accuracy studies. Ann. Intern. Med. 2011, 155, 529–536. [Google Scholar] [CrossRef]
- Llovet, J.M.; Bru, C.; Bruix, J. Prognosis of hepatocellular carcinoma: The BCLC staging classification. Semin. Liver Dis. 1999, 19, 329–338. [Google Scholar] [CrossRef]
- Kim, S.Y.; An, J.; Lim, Y.S.; Han, S.; Lee, J.Y.; Byun, J.H.; Won, H.J.; Lee, S.J.; Lee, H.C.; Lee, Y.S. MRI with Liver-Specific Contrast for Surveillance of Patients with Cirrhosis at High Risk of Hepatocellular Carcinoma. JAMA Oncol. 2017, 3, 456–463. [Google Scholar] [CrossRef]
- Demirtas, C.O.; Gunduz, F.; Tuney, D.; Baltacioglu, F.; Kani, H.T.; Bugdayci, O.; Alahdab, Y.O.; Ozdogan, O.C. Annual contrast-enhanced magnetic resonance imaging is highly effective in the surveillance of hepatocellular carcinoma among cirrhotic patients. Eur. J. Gastroenterol. Hepatol. 2020, 32, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Omata, M.; Cheng, A.L.; Kokudo, N.; Kudo, M.; Lee, J.M.; Jia, J.; Tateishi, R.; Han, K.H.; Chawla, Y.K.; Shiina, S.; et al. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: A 2017 update. Hepatol. Int. 2017, 11, 317–370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kokudo, N.; Takemura, N.; Hasegawa, K.; Takayama, T.; Kubo, S.; Shimada, M.; Nagano, H.; Hatano, E.; Izumi, N.; Kaneko, S.; et al. Clinical practice guidelines for hepatocellular carcinoma: The Japan Society of Hepatology 2017 (4th JSH-HCC guidelines) 2019 update. Hepatol. Res. 2019, 49, 1109–1113. [Google Scholar] [CrossRef]
- Del Poggio, P.; Olmi, S.; Ciccarese, F.; Marco, M.D.; Rapaccini, G.L.; Benvegnù, L.; Borzio, F.; Farinati, F.; Zoli, M.; Giannini, E.G.; et al. Factors that affect efficacy of ultrasound surveillance for early stage hepatocellular carcinoma in patients with cirrhosis. Clin. Gastroenterol. Hepatol. 2014, 12, 1927–1933.e2. [Google Scholar] [CrossRef]
- Simmons, O.; Fetzer, D.T.; Yokoo, T.; Marrero, J.A.; Yopp, A.; Kono, Y.; Parikh, N.D.; Browning, T.; Singal, A.G. Predictors of adequate ultrasound quality for hepatocellular carcinoma surveillance in patients with cirrhosis. Aliment. Pharmacol. Ther. 2017, 45, 169–177. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.C.; Lim, J.H.; Park, C.K.; Kim, M.J.; Kim, S.H.; Lee, S.J.; Lee, W.J.; Lim, H.K. Poor sensitivity of sonography in detection of hepatocellular carcinoma in advanced liver cirrhosis: Accuracy of pretransplantation sonography in 118 patients. Eur. Radiol. 2003, 13, 1693–1698. [Google Scholar] [CrossRef]
- Fitzmorris, P.; Singal, A.K. Surveillance and Diagnosis of Hepatocellular Carcinoma. Gastroenterol. Hepatol. 2015, 11, 38–46. [Google Scholar]
- Kanwal, F.; Singal, A.G. Surveillance for Hepatocellular Carcinoma: Current Best Practice and Future Direction. Gastroenterology 2019, 157, 54–64. [Google Scholar] [CrossRef] [Green Version]
- Park, H.J.; Jang, H.Y.; Kim, S.Y.; Lee, S.J.; Won, H.J.; Byun, J.H.; Choi, S.H.; Lee, S.S.; An, J.; Lim, Y.S. Non-enhanced magnetic resonance imaging as a surveillance tool for hepatocellular carcinoma: Comparison with ultrasound. J. Hepatol. 2020, 72, 718–724. [Google Scholar] [CrossRef] [PubMed]
- Besa, C.; Lewis, S.; Pandharipande, P.V.; Chhatwal, J.; Kamath, A.; Cooper, N.; Knight-Greenfield, A.; Babb, J.S.; Boffetta, P.; Padron, N.; et al. Hepatocellular carcinoma detection: Diagnostic performance of a simulated abbreviated MRI protocol combining diffusion-weighted and T1-weighted imaging at the delayed phase post gadoxetic acid. Abdom. Radiol. 2017, 42, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Goossens, N.; Singal, A.G.; King, L.Y.; Andersson, K.L.; Fuchs, B.C.; Besa, C.; Taouli, B.; Chung, R.T.; Hoshida, Y. Cost-Effectiveness of Risk Score-Stratified Hepatocellular Carcinoma Screening in Patients with Cirrhosis. Clin. Transl. Gastroenterol. 2017, 8, e101. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Lee, J.M.; Lee, J.S.; Lee, H.Y.; Park, B.H.; Kim, Y.H.; Han, J.K.; Choi, B.I. Hepatocellular carcinoma: Diagnostic performance of multidetector CT and MR imaging-a systematic review and meta-analysis. Radiology 2015, 275, 97–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, S.H.; Byun, J.H.; Kwon, H.J.; Ha, H.I.; Lee, S.J.; Kim, S.Y.; Won, H.J.; Kim, P.-N. The usefulness of gadoxetic acid-enhanced dynamic magnetic resonance imaging in hepatocellular carcinoma: Toward improved staging. Ann. Surg. Oncol. 2015, 22, 819–825. [Google Scholar] [CrossRef]
- Ng, J.; Wu, J. Hepatitis B- and hepatitis C-related hepatocellular carcinomas in the United States: Similarities and differences. Hepat. Mon. 2012, 12, e7635. [Google Scholar] [CrossRef] [Green Version]


| Author (Year of Publication) | Study Design | Study Location (Period) | No. of Patients (% Male) | Percentage of Cirrhotic Patients (%) | Most Common Etiology of Liver Disease (%) | No. of Patients with HCC (% Early HCC) | Patient Age, Year * | MRI Magnet | MRI Protocols | MRI Contrast Agent | Reference Standards for HCC (%) | Surveillance Interval (f/u Period), Month |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Shah TU (2006) [17] | Prospective | United States (2001–2004) | 310 (66.5) | 100 | Hepatitis C (25.5) | 22 (100) | 52.4, mean | 1.5-T | T1WI, T2WI, DCE | Extracellular contrast agent | Pathology (42.9) or multiphase MRI (57.1) | 3–12 (f/u: mean, 22.1) |
| Marks RM (2015) [14] | Retrospective | United States (2008–2012) | 298 (56.4) | NR | Hepatitis C (50.7) | 49 (NR) | 55.9 ± 10.9 | 1.5 or 3.0-T | T2WI, HBP, DWI | Hepatocyte-specific contrast agent | Pathology (NR), multiphase CT or MRI (NR) | NR (f/u: range, 6–13) |
| Kim SY (2017) [21] | Prospective | Korea (2011–2014) | 407 (56.5) | 100 | Hepatitis B (70.8) | 43 (97.7) | 56 (52–62), median (IQR) | 1.5-T | T2WI, DWI, Dual-GRE, DCE, HBP | Hepatocyte-specific contrast agent | Pathology (9.3) or multiphase CT (90.7) | 6 (f/u: median, 18) |
| Sutherland T (2017) [15] | Prospective | Australia (NR) | 192 (72.4) | NR | Hepatitis B (56.3) | 6 (12.2) | 58 (22–80), mean (range) | NR | DWI | NA | Pathology (NR), multiphase CT or MRI (NR) | NR (f/u: > 6) |
| Brunsing RL (2019) [13] | Retrospective | United States (2014–2016) | 141 (54.6) | 92.9 | Hepatitis C (37.9) | 12 (66.7) | 59.1 ± 11.5 | 1.5 or 3.0-T | T2WI, HBP, DWI | Hepatocyte-specific contrast agent | Pathology (NR), multiphase CT or MRI (NR) | 5.0–8.8 (f/u: range, 8.8–26) |
| Chan MV (2019) [16] | Retrospective | Australia (2015–2018) | 188 (49.5) | 23.4 | Hepatitis B (14.9) | 28 (NR) | 63 ± 13 | 3.0-T | T2WI, DWI, Dual-GRE | NA | Multiphase MRI (100) | 6 (f/u: NR) |
| Demirtas CO (2020) [22] | Retrospective | Turkey (2008–2017) | 294 (37.1) | 100 | Hepatitis B (41.5) | 35 (85.7) | 60 (29–86), median (IQR) | 3.0-T | NR † | Extracellular contrast agent | Pathology (NR), multiphase CT or MRI (NR) | 12 (f/u: mean, 40.3) |
| Any-Stage HCC | Early-Stage HCC | Very Early-Stage HCC | ||||||
|---|---|---|---|---|---|---|---|---|
| Author (Year) | Sensitivity (95% CI) | Specificity (95% CI) | Author | Sensitivity (95% CI) | Specificity (95% CI) | Author | Sensitivity (95% CI) | Specificity (95% CI) |
| Shah TU (2006) [17] | 77% (55, 92) | 83% (78, 87) | Shah TU [17] | 77% (55, 92) | 83% (78, 87) | Shah TU [17] | 77% (55, 92) | 83% (78, 87) |
| Marks RM (2015) [14] | 88% (75, 95) | 91% (87, 94) | Kim SY [21] | 86% (71, 95) | 97% (96, 98) | Kim SY [21] | 84% (67, 95) | 97% (96, 98) |
| Kim SY (2017) [21] | 86% (71, 94) | 97% (96, 98) | Sutherland T [15] | 80% (28, 99) | 98% (95, 100) | Chan MV [16] | 59% (33, 82) | 95% (90, 98) |
| Sutherland T (2017) [15] | 83% (42, 99) | 98% (95, 100) | Brunsing RL [13] | 88% (47, 100) | 91% (85, 95) | Demirtas CO [22] | 80% (52, 96) | 96% (93, 98) |
| Brunsing RL (2019) [13] | 92% (60, 100) | 91% (84, 95) | Demirtas CO [22] | 83% (65, 94) | 95% (92, 98) | |||
| Chan MV (2019) [16] | 82% (62, 93) | 94% (89, 97) | ||||||
| Demirtas CO (2020) [22] | 86% (69, 95) | 95% (92, 98) | ||||||
| Meta-analytic pooled estimations | 85% (79, 90) | 94% (90, 97) | 83% (74, 89) | 95% (89, 97) | 77% (66, 85) | 94% (88, 97) | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.H.; Choi, S.H.; Shim, J.H.; Kim, S.Y.; Lee, S.S.; Byun, J.H.; Kim, K.W.; Choi, J.-I. Magnetic Resonance Imaging for Surveillance of Hepatocellular Carcinoma: A Systematic Review and Meta-Analysis. Diagnostics 2021, 11, 1665. https://doi.org/10.3390/diagnostics11091665
Kim DH, Choi SH, Shim JH, Kim SY, Lee SS, Byun JH, Kim KW, Choi J-I. Magnetic Resonance Imaging for Surveillance of Hepatocellular Carcinoma: A Systematic Review and Meta-Analysis. Diagnostics. 2021; 11(9):1665. https://doi.org/10.3390/diagnostics11091665
Chicago/Turabian StyleKim, Dong Hwan, Sang Hyun Choi, Ju Hyun Shim, So Yeon Kim, Seung Soo Lee, Jae Ho Byun, Kyung Won Kim, and Joon-Il Choi. 2021. "Magnetic Resonance Imaging for Surveillance of Hepatocellular Carcinoma: A Systematic Review and Meta-Analysis" Diagnostics 11, no. 9: 1665. https://doi.org/10.3390/diagnostics11091665
APA StyleKim, D. H., Choi, S. H., Shim, J. H., Kim, S. Y., Lee, S. S., Byun, J. H., Kim, K. W., & Choi, J.-I. (2021). Magnetic Resonance Imaging for Surveillance of Hepatocellular Carcinoma: A Systematic Review and Meta-Analysis. Diagnostics, 11(9), 1665. https://doi.org/10.3390/diagnostics11091665






