A Radiomics Approach to Predict the Emergence of New Hepatocellular Carcinoma in Computed Tomography for High-Risk Patients with Liver Cirrhosis
Abstract
:1. Introduction
2. Materials and Methods
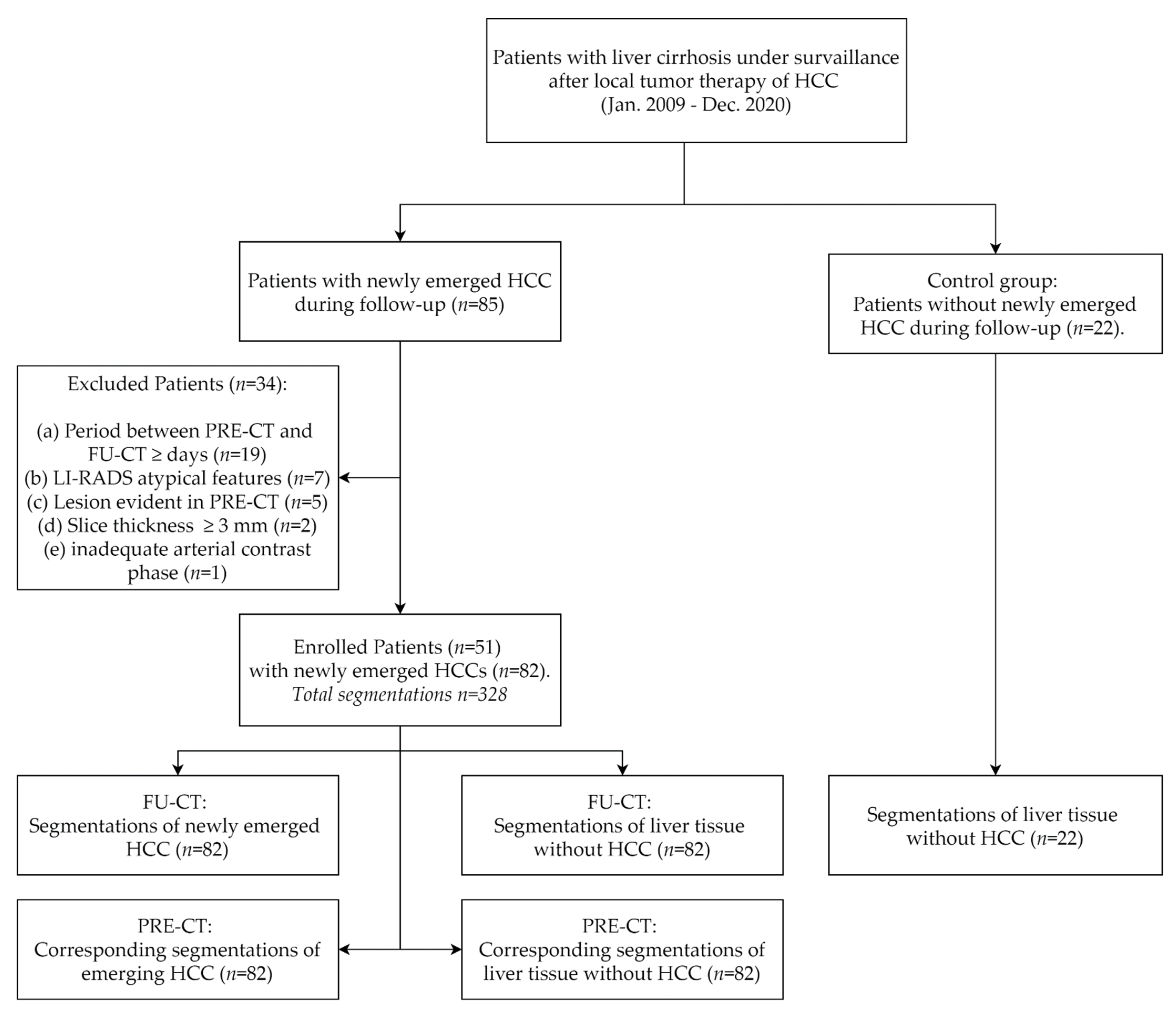
2.1. Study Design and Sample
2.2. CT Parameters
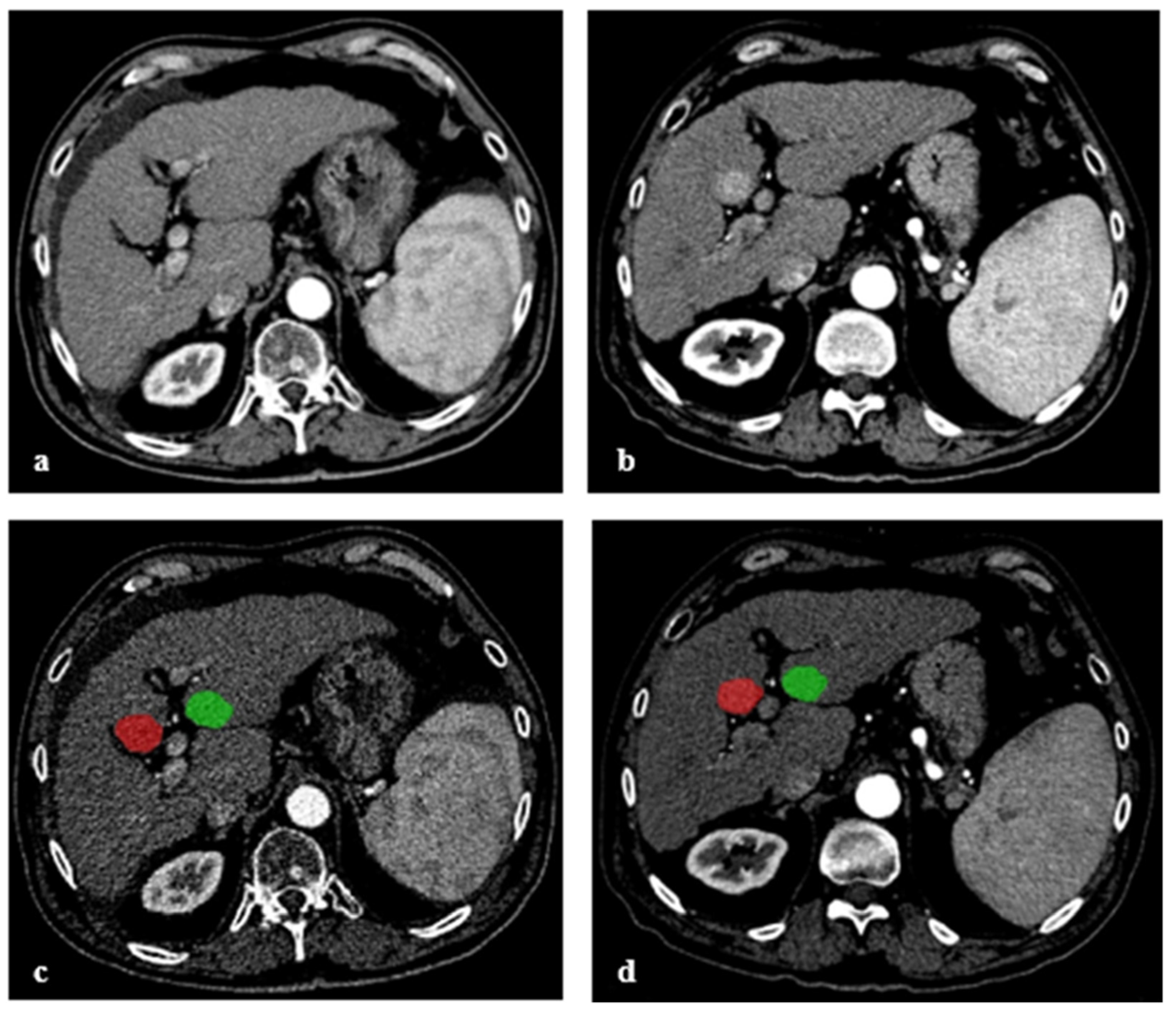
2.3. Image Segmentation
2.4. Radiomic Features
2.5. Development of a Prediction Model
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- GBD 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age–sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015, 385, 117–171. [Google Scholar] [CrossRef]
- Forner, A.; Vilana, R.; Ayuso, C.; Bianchi, L.; Solé, M.; Ayuso, J.R.; Boix, L.; Sala, M.; Varela, M.; Llovet, J.M.; et al. Diagnosis of hepatic nodules 20 mm or smaller in cirrhosis: Prospective validation of the noninvasive diagnostic criteria for hepatocellular carcinoma. Hepatology 2008, 47, 97–104. [Google Scholar] [CrossRef]
- Forner, A.; Llovet, J.M.; Bruix, J. Hepatocellular carcinoma. Lancet 2012, 379, 1245–1255. [Google Scholar] [CrossRef]
- Ioannou, G.N.; Splan, M.F.; Weiss, N.S.; McDonald, G.B.; Beretta, L.; Lee, S.P. Incidence and Predictors of Hepatocellular Carcinoma in Patients With Cirrhosis. Clin. Gastroenterol. Hepatol. 2007, 5, 938–945.e4. [Google Scholar] [CrossRef]
- Kansagara, D.; Papak, J.; Pasha, A.S.; O’Neil, M.; Freeman, M.; Relevo, R.; Quinones, A.; Motu’Apuaka, M.; Jou, J.H. Screening for Hepatocellular Carcinoma in Chronic Liver Disease. Ann. Intern. Med. 2014, 161, 261–269. [Google Scholar] [CrossRef] [Green Version]
- Singal, A.G.; Pillai, A.; Tiro, J. Early Detection, Curative Treatment, and Survival Rates for Hepatocellular Carcinoma Surveillance in Patients with Cirrhosis: A Meta-analysis. PLoS Med. 2014, 11, e1001624. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Di Bisceglie, A.M.; Bruix, J.; Kramer, B.S.; Lencioni, R.; Zhu, A.X.; Sherman, M.; Schwartz, M.; Lotze, M.; Talwalkar, J.; et al. Design and Endpoints of Clinical Trials in Hepatocellular Carcinoma. J. Natl. Cancer Inst. 2008, 100, 698–711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forner, A.; Reig, M.; Bruix, J. Hepatocellular carcinoma. Lancet 2018, 391, 1301–1314. [Google Scholar] [CrossRef]
- Yu, N.C.; Chaudhari, V.; Raman, S.S.; Lassman, C.; Tong, M.J.; Busuttil, R.W.; Lu, D.S. CT and MRI Improve Detection of Hepatocellular Carcinoma, Compared With Ultrasound Alone, in Patients With Cirrhosis. Clin. Gastroenterol. Hepatol. 2011, 9, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Colli, A.; Fraquelli, M.; Casazza, G.; Massironi, S.; Colucci, A.; Conte, D.; Duca, P. Accuracy of Ultrasonography, Spiral CT, Magnetic Resonance, and Alpha-Fetoprotein in Diagnosing Hepatocellular Carcinoma: A Systematic Review. CME. Am. J. Gastroenterol. 2006, 101, 513–523. [Google Scholar] [CrossRef]
- SanGiovanni, A.; Manini, M.A.; Iavarone, M.; Romeo, R.; Forzenigo, L.V.; Fraquelli, M.; Massironi, S.; Della Corte, C.; Ronchi, G.; Rumi, M.; et al. The diagnostic and economic impact of contrast imaging techniques in the diagnosis of small hepatocellular carcinoma in cirrhosis. Gut 2009, 59, 638–644. [Google Scholar] [CrossRef]
- Chernyak, V.; Fowler, K.J.; Kamaya, A.; Kielar, A.Z.; Elsayes, K.M.; Bashir, M.R.; Kono, Y.; Do, R.K.; Mitchell, D.G.; Singal, A.G.; et al. Liver Imaging Reporting and Data System (LI-RADS) Version 2018: Imaging of Hepatocellular Carcinoma in At-Risk Patients. Radiol. 2018, 289, 816–830. [Google Scholar] [CrossRef]
- Semaan, S.; Violi, N.V.; Lewis, S.; Chatterji, M.; Song, C.; Besa, C.; Babb, J.; Fiel, M.I.; Schwartz, M.; Thung, S.; et al. Hepatocellular carcinoma detection in liver cirrhosis: Diagnostic performance of contrast-enhanced CT vs. MRI with extracellular contrast vs. gadoxetic acid. Eur. Radiol. 2020, 30, 1020–1030. [Google Scholar] [CrossRef]
- Roberts, L.R.; Sirlin, C.B.; Zaiem, F.; Almasri, J.; Prokop, L.J.; Heimbach, J.K.; Murad, M.H.; Mohammed, K. Imaging for the diagnosis of hepatocellular carcinoma: A systematic review and meta-analysis. Hepatology 2018, 67, 401–421. [Google Scholar] [CrossRef] [Green Version]
- Lambin, P.; Leijenaar, R.T.H.; Deist, T.M.; Peerlings, J.; de Jong, E.E.C.; van Timmeren, J.; Sanduleanu, S.; Larue, R.T.H.M.; Even, A.J.G.; Jochems, A.; et al. Radiomics: The bridge between medical imaging and personalized medicine. Nat. Rev. Clin. Oncol. 2017, 14, 749–762. [Google Scholar] [CrossRef]
- Lambin, P.; Rios-Velazquez, E.; Leijenaar, R.; Carvalho, S.; van Stiphout, R.G.P.M.; Granton, P.; Zegers, C.M.L.; Gillies, R.; Boellard, R.; Dekker, A.; et al. Radiomics: Extracting more information from medical images using advanced feature analysis. Eur. J. Cancer 2012, 48, 441–446. [Google Scholar] [CrossRef] [Green Version]
- Thüring, J.; Rippel, O.; Haarburger, C.; Merhof, D.; Schad, P.; Bruners, P.; Kuhl, C.K.; Truhn, D. Multiphase CT-based prediction of Child-Pugh classification: A machine learning approach. Eur. Radiol. Exp. 2020, 4, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wakabayashi, T.; Ouhmich, F.; Gonzalez-Cabrera, C.; Felli, E.; Saviano, A.; Agnus, V.; Savadjiev, P.; Baumert, T.F.; Pessaux, P.; Marescaux, J.; et al. Radiomics in hepatocellular carcinoma: A quantitative review. Hepatol. Int. 2019, 13, 546–559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khobragade, P.; Rupcich, F.; Fan, J.; Crotty, D.J.; Kulkarni, N.M.; O’Connor, S.; Foley, W.D.; Schmidt, T.G. CT automated exposure control using a generalized detectability index. Med. Phys. 2018, 46, 140–151. [Google Scholar] [CrossRef] [PubMed]
- Elsayes, K.M.; Hooker, J.C.; Agrons, M.M.; Kielar, A.Z.; Tang, A.; Fowler, K.; Chernyak, V.; Bashir, M.R.; Kono, Y.; Do, R.K.; et al. 2017 Version of LI-RADS for CT and MR Imaging: An Update. Radiographics 2017, 37, 1994–2017. [Google Scholar] [CrossRef]
- Van Griethuysen, J.J.M.; Fedorov, A.; Parmar, C.; Hosny, A.; Aucoin, N.; Narayan, V.; Beets-Tan, R.G.H.; Fillion-Robin, J.-C.; Pieper, S.; Aerts, H.J.W.L. Computational Radiomics System to Decode the Radiographic Phenotype. Cancer Res. 2017, 77, e104–e107. [Google Scholar] [CrossRef] [Green Version]
- Zwanenburg, A.; Vallières, M.; Abdalah, M.A.; Aerts, H.J.W.L.; Andrearczyk, V.; Apte, A.; Ashrafinia, S.; Bakas, S.; Beukinga, R.J.; Boellaard, R.; et al. The Image Biomarker Standardization Initiative: Standardized Quantitative Radiomics for High-Throughput Image-based Phenotyping. Radiology 2020, 295, 328–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pedregosa, F.; Varoquaux, G.; Gramfort, A.; Michel, V.; Thirion, B.; Grisel, O.; Blondel, M.; Müller, A.; Nothman, J.; Louppe, G.; et al. Scikit-learn: Machine Learning in Python. J. Mach. Learn. Res. 2011, 12, 2825–2830. [Google Scholar]
- Haralick, R.M.; Shanmugam, K.; Dinstein, I.H. Textural Features for Image Classification. IEEE Trans. Syst. Man Cybern. 1973, 6, 610–621. [Google Scholar] [CrossRef] [Green Version]
- Kokoska, S.; Zwillinger, D. CRC Standard Probability and Statistics Tables and Formulae, 1st ed.; CRC Press: Boca Raton, FL, USA, 2000; ISBN 9780429181467. [Google Scholar]
- Mokrane, F.-Z.; Lu, L.; Vavasseur, A.; Otal, P.; Peron, J.-M.; Luk, L.; Yang, H.; Ammari, S.; Saenger, Y.; Rousseau, H.; et al. Radiomics machine-learning signature for diagnosis of hepatocellular carcinoma in cirrhotic patients with indeterminate liver nodules. Eur. Radiol. 2019, 30, 558–570. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; Gu, D.; Wang, H.; Wei, J.; Wang, Z.; Hao, X.; Ji, Q.; Cao, S.; Song, Z.; Jiang, J.; et al. Radiomics analysis enables recurrence prediction for hepatocellular carcinoma after liver transplantation. Eur. J. Radiol. 2019, 117, 33–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kitzing, Y.X.; Ng, B.H.K.; Kitzing, B.; Waugh, R.; Kench, J.; Strasser, S.I.; McCormack, S. Washout of hepatocellular carcinoma on portal venous phase of multidetector computed tomography in a pre-transplant population. J. Med. Imaging Radiat. Oncol. 2015, 59, 673–680. [Google Scholar] [CrossRef]
- Bird, T.G.; Dimitropoulou, P.; Turner, R.M.; Jenks, S.J.; Cusack, P.; Hey, S.; Blunsum, A.; Kelly, S.; Sturgeon, C.; Hayes, P.C.; et al. Alpha-Fetoprotein Detection of Hepatocellular Carcinoma Leads to a Standardized Analysis of Dynamic AFP to Improve Screening Based Detection. PLoS ONE 2016, 11, e0156801. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Choi, S.J.; Lee, S.-H.; Lee, H.Y.; Park, H. Predicting Survival Using Pretreatment CT for Patients With Hepatocellular Carcinoma Treated With Transarterial Chemoembolization: Comparison of Models Using Radiomics. Am. J. Roentgenol. 2018, 211, 1026–1034. [Google Scholar] [CrossRef]


| Epidemiologic and Clinical Characteristics | Cirrhotic Patients with HCC Lesions in the FU-CT | Cirrhotic Patients without HCC Lesions under Surveillance |
|---|---|---|
| Study size | 51 | 22 |
| Age (years), median (IQR) | 69 (63–76) | 55 (51–63) |
| Gender, no. (%) | ||
| Male | 39 (76) | 14 (64) |
| Female | 12 (24) | 8 (36) |
| Total number of segmentations with HCC/without HCC | 82/82 | 0/22 |
| MTD (cm) in FU-CT, median (IQR) | 1.2 (0.9–1.5) | |
| Tumor location in FU-CT, no. (%) | ||
| Left lobe | 41 (50) | |
| Right lobe | 41 (50) | |
| Time interval between PRE-CT and FU-CT (days), median (IQR) | 121 (94–165) | |
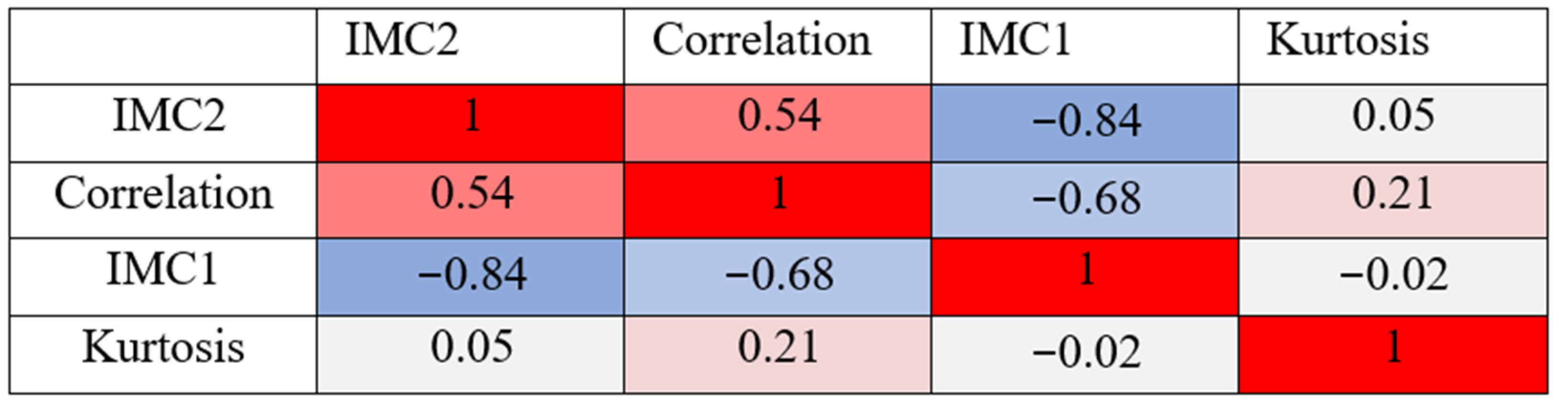
| Feature | Univariate | Multivariate | |||
|---|---|---|---|---|---|
| Correlation | IMC1 | IMC2 | Kurtosis | All 4 Features | |
| p-value | <0.000000001 | <0.00000021 | <0.000000001 | <0.00007 | |
| Sensitivity (%) | 57 (44–90) | 65 (51–86) | 81 (65–90) | 33 (21–79) | 72 (57–83) |
| Specificity (%) | 89 (51–97) | 76 (52–86) | 71 (59–83) | 96 (47–100) | 86 (76–96) |
| Threshold | 0.22 (0.15–0.24) | 0.04 (0.03–0.05) | 0.29 (0.28–0.33) | 3.36 (2.97–3.71) | 0.45 (0.39–0.61) |
| AUC (%) | 76 (68–83) | 72 (64–79) | 78 (72–85) | 62 (54–70) | 81 (74–87) |
| Weighting in multi-variate model (%) | 23.5 | 26.2 | 35.2 | 15.1 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tietz, E.; Truhn, D.; Müller-Franzes, G.; Berres, M.-L.; Hamesch, K.; Lang, S.A.; Kuhl, C.K.; Bruners, P.; Schulze-Hagen, M. A Radiomics Approach to Predict the Emergence of New Hepatocellular Carcinoma in Computed Tomography for High-Risk Patients with Liver Cirrhosis. Diagnostics 2021, 11, 1650. https://doi.org/10.3390/diagnostics11091650
Tietz E, Truhn D, Müller-Franzes G, Berres M-L, Hamesch K, Lang SA, Kuhl CK, Bruners P, Schulze-Hagen M. A Radiomics Approach to Predict the Emergence of New Hepatocellular Carcinoma in Computed Tomography for High-Risk Patients with Liver Cirrhosis. Diagnostics. 2021; 11(9):1650. https://doi.org/10.3390/diagnostics11091650
Chicago/Turabian StyleTietz, Eric, Daniel Truhn, Gustav Müller-Franzes, Marie-Luise Berres, Karim Hamesch, Sven Arke Lang, Christiane Katharina Kuhl, Philipp Bruners, and Maximilian Schulze-Hagen. 2021. "A Radiomics Approach to Predict the Emergence of New Hepatocellular Carcinoma in Computed Tomography for High-Risk Patients with Liver Cirrhosis" Diagnostics 11, no. 9: 1650. https://doi.org/10.3390/diagnostics11091650
APA StyleTietz, E., Truhn, D., Müller-Franzes, G., Berres, M.-L., Hamesch, K., Lang, S. A., Kuhl, C. K., Bruners, P., & Schulze-Hagen, M. (2021). A Radiomics Approach to Predict the Emergence of New Hepatocellular Carcinoma in Computed Tomography for High-Risk Patients with Liver Cirrhosis. Diagnostics, 11(9), 1650. https://doi.org/10.3390/diagnostics11091650






