Abstract
Due to its high prevalence, screening for hepatic fibrosis in the low-risk population is called for action in the primary care clinic. However, current guidelines provide conflicting recommendations on populations to be screened. We aimed to identify the target populations that would most benefit from screening for hepatic fibrosis in clinical practice. This study examined 1288 subjects who underwent magnetic resonance elastography. The diagnostic performance of the Fibrosis-4 (FIB-4) index and NAFLD fibrosis score was compared in the following groups: (1) ultrasonography (USG)-diagnosed NAFLD, (2) elevated liver enzyme, (3) metabolic syndrome, (4) impaired fasting glucose, and (5) type 2 diabetes regardless of fatty liver. Decision curve analysis was performed to express the net benefit of groups over a range of probability thresholds (Pts). The diabetes group showed a better area under the receiver operating characteristic curve (AUROC: 0.69) compared with subjects in the USG-diagnosed NAFLD (AUROC: 0.57) and elevated liver enzyme (AUROC: 0.55) groups based on the FIB-4 index. In decision curve analysis, the diabetes group showed the highest net benefit for the detection of significant fibrosis across a wide range of Pts. Patients with diabetes, even in the absence of fatty liver, would be preferable for hepatic fibrosis screening in low-risk populations.
1. Introduction
Chronic liver disease is common worldwide and has become a major global issue. The overall mortality rate increases as liver fibrosis progresses, up to 1.36-fold with greater than significant hepatic fibrosis [1]. Therefore, diagnosis should be achieved in early stages when liver fibrosis is mild-to-moderate. In addition, significant hepatic fibrosis has a prevalence of 5–7% in unselected populations [2,3]. Due to its high prevalence, screening for significant hepatic fibrosis in the low-risk population is called for action in the primary care clinic. However, screening for hepatic fibrosis in the general population is difficult in practice.
A major obstacle for liver fibrosis screening in the general population includes the identification of groups to be screened. Although screening is not universally recommended, targeted screening in groups deemed at high risk for fibrosis remains a contentious issue, with conflicting guidance from major international societies. In most primary care settings, screening tests for liver fibrosis are focused on patients with suspicion of non-alcoholic fatty liver disease (NAFLD). However, only approximately 15–20% of patients with NAFLD develop non-alcoholic steatohepatitis (NASH) [4], the progressive form of liver disease. Therefore, the high prevalence and relatively low severity of NAFLD require the triage of patients for liver fibrosis screening. Meanwhile, despite the high prevalence of advanced fibrosis in patients with diabetes [3,5,6], consensus on the need for the screening of significant hepatic fibrosis in all diabetic patients is unclear, with a lack of evidence demonstrating benefit from screening. To the best of our knowledge, the cost-effectiveness of a screening strategy for NASH or advanced fibrosis in patients with diabetes has been evaluated in only two studies, which showed conflicting results [7,8]. Furthermore, in the screening strategies used in those studies, all patients first underwent ultrasonography (USG) to detect fatty liver. Whether fibrosis screening tests should be performed in all diabetic patients regardless of fatty liver remains unclear.
Therefore, in the present study, the diagnostic performance of non-invasive tests for screening liver fibrosis based on various conditions including diabetes with and without fatty liver was evaluated to identify target groups that might most benefit in clinical practice.
2. Materials and Methods
2.1. Study Design
This was a cross-sectional study and included a subset of the Kangbuk Samsung Health Study (KSHS, Seoul, Korea) participants 18 years of age or older who underwent health screening examinations including magnetic resonance elastography (MRE) between January 2015 and May 2018 (n = 2149). The KSHS is a Korean cohort study including individuals who have participated in a comprehensive health screening examination annually or biannually at Kangbuk Samsung Hospital Total Healthcare Centers in Seoul and Suwon, South Korea. More than 80% of participants were employees of various companies or their family members. In South Korea, the Industrial Safety and Health Act requires health examinations for all employees. Examinations are offered free of charge, and MRE is voluntarily chosen by employees and paid for by their employer. This study was approved by the Kangbuk Samsung Hospital Institutional Review Board (IRB No. KBSMC 2020-11-029, approved on 26 November 2020).
2.2. Inclusion and Exclusion Criteria
To provide data on applicability of non-invasive tests in low-risk populations, participants were excluded if they had positive viral hepatitis serology (hepatitis B surface antigen (n = 167) or hepatitis C antibody (n = 12)), chronic liver disease caused by significant alcohol consumption (>20 g/day, n = 642) [9,10], or liver cirrhosis on USG (n = 27). Subjects who had incomplete data were also excluded (n = 13) (Figure 1).
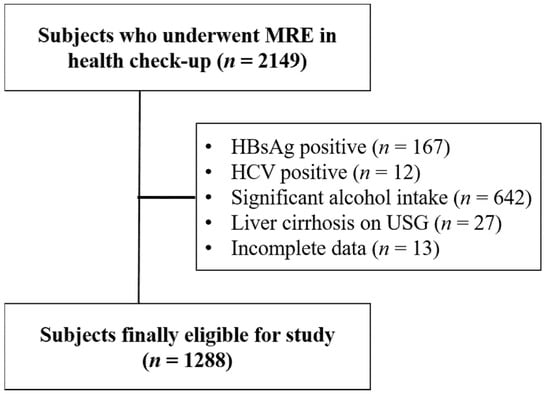
Figure 1.
Flow chart of study participants. MRE, magnetic resonance elastography.
2.3. MRE Measurement
MRE was used as the reference standard for fibrosis. Although liver biopsy is the gold standard method to assess fibrosis, MRE is non-invasive, has no significant side effects, and is used in clinical practice as the reference standard to avoid liver biopsy. MREs were performed using 1.5T MRI systems (Signa HDxT; GE Healthcare in Seoul, and Optima 360 Advance, GE Healthcare in Suwon, Korea). Measurements were performed according to the manufacturer’s instructions. A standard 60-Hz shear-wave was generated by an acoustic passive driver attached to the body wall anterior to the liver and coupled with an acoustic active driver outside the scanning room. A two-dimensional gradient-recalled echo pulse sequence synchronized to the shear-wave frequency was acquired to obtain three noncontiguous axial slices (8-mm-thick sections). The wave images from each slice location were processed automatically using an inversion algorithm to generate axial liver stiffness maps. The image analyst drew regions of interest in parts of the liver showing wave propagation on the images, avoiding large blood vessels and artifacts. The mean stiffness value was calculated by averaging all pixel values, and the results were displayed automatically on each map in units of kilopascal (kPa). The fibrosis stages were defined as ≥ F2 (significant fibrosis) and ≥ F3 (advanced fibrosis), with thresholds of 3.0 and 3.6 kPa, respectively [11].
2.4. Clinical Assessment
Subjects were required to complete a structured questionnaire involving a survey of demographics, medical history, comorbid conditions, current alcohol consumption, and drug use. Subjects underwent complete clinical examination with laboratory tests. BMI was calculated as weight (kg) divided by height (m2). Waist circumference was measured at the midpoint between the lowest rib and top of the iliac crest. Blood pressure (BP) was measured by trained nurses using an automatic tonometer after 5 min of rest. Hypertension was defined as systolic BP ≥ 140 mmHg, diastolic BP ≥ 90 mmHg, or current use of antihypertensive medication. Diabetes was defined as fasting plasma glucose level ≥ 126 mg/dL, glycated hemoglobin (HbA1c) level ≥ 6.5%, and/or current use of antihyperglycemic medications. Impaired fasting glucose was defined as a fasting serum glucose level of 100–125 mg/dL [12]. Metabolic syndrome was diagnosed if three or more of the following risks were present [13]: (a) waist circumference ≥ 90 cm in males or ≥ 80 cm in females (in accordance with the International Obesity Task Force criteria for the Asia-Pacific population); (b) triglyceride level ≥ 150 mg/dL; (c) high-density lipoprotein (HDL) cholesterol level < 40 mg/dL in males and < 50 mg/dL in females; (d) blood pressure level ≥ 130/85 mm Hg or current use of antihypertensive medications; (e) fasting blood glucose level ≥ 100 mg/dL or current use of antidiabetic medications. Participants were considered to have NAFLD when they fulfilled all inclusion criteria and showed fatty liver on USG.
2.5. Abdominal USG
Abdominal USG was performed by experienced radiologists. Semi-quantitative grading of fatty liver was performed based on standard criteria [14]. Fatty liver status was defined as presence versus absence.
2.6. Target Populations
The subjects were divided into the following five groups: (1) USG-diagnosed NAFLD; (2) elevated liver enzyme (aminotransferases), defined as ≥1.5-fold the upper limit of normal; (3) metabolic syndrome; (4) impaired fasting glucose; and (5) type 2 diabetes regardless of fatty liver.
2.7. Indices of Hepatic Fibrosis
The European Association for the Study of the Liver (EASL) and Italian guidelines suggest the use of NFS and FIB-4 index as non-invasive methods to identify patients with different risks of advanced fibrosis [4,15]. Furthermore, American Association for the Study of Liver Disease (AASLD) guidelines report a recent study in which both NAFLD fibrosis score (NFS) and Fibrosis-4 (FIB-4) index showed the best predictive value for advanced fibrosis among histologically proven NAFLD patients compared with other tests [16]. Therefore, single use of these two non-invasive tests was evaluated. FIB-4 index score ≥ 0.92 and NFS ≥ −3.16 based on the following formulas were used as the cut-off values for excluding significant hepatic fibrosis [17,18]:
FIB-4 [19]:
NFS [20]:
−1.675 + 0.037 × age [years] + 0.094 × BMI [kg/m2] + 1.13 × impaired fasting glycemia or diabetes [yes = 1, no = 0] + 0.99 × AST/ALT ratio − 0.013 × platelet [×109/L] − 0.66 × albumin [g/dL]
2.8. Decision Curve Analysis and Net Benefit
Test performance has been reported in the literature as area under the receiver operating characteristic curve (AUROC), sensitivity, and specificity. However, these measures do not determine whether a test is fit for clinical use.
In the present study, decision curve analysis was used to compare and describe the clinical effects between subgroups. Decision curve analysis involves calculating the net benefit, which places benefits and harms on the same scale and is presented in unit of true positives [21]. The cost component is not included in the analysis. Net benefit can be calculated using the following formula:
(TP: true positive, FP: false positive, N: total sample size, Pt: probability threshold)
The Pt is defined by considering the number of patients a clinician would need to perform a test on to obtain one correct diagnosis. For example, if a clinician found it acceptable to subject 10 patients to liver biopsy to obtain one correct diagnosis of cirrhosis, then Pt is 10%. A strategy is considered to have clinical value if it has the highest net benefit across a range of Pts.
2.9. Statistical Analysis
For descriptive statistics, results were expressed as mean ± standard deviation (SD) or median with interquartile range (IQR) for continuous variables with normal or asymmetric distribution, respectively. Categorical variables were presented as numbers and percentages. To compare variables based on gender, Chi-square test was used for categorical variables, and Student’s t-test was used for continuous data. To assess the performance of the FIB-4 index based on the screening algorithm to detect significant fibrosis, AUROCs were determined. IBM SPSS Statistics version 23.0 (IBM Co., Armonk, NY, USA) was used, and p < 0.05 was considered statistically significant.
3. Results
3.1. Baseline Characteristics
The clinical data of subjects are summarized in Table 1. The mean age of the subjects was approximately 50 years, and there were more males (77.2%) than females. The mean body mass index (BMI) was 24.58 ± 3.08. Among the 1288 subjects, 169 (13.12%) had diabetes, 418 (32.45%) had hypertension, and 317 (24.61%) had metabolic syndrome. The proportion of significant hepatic fibrosis with 3.0 kPa or higher based on MRE criteria was 3.88%, and the rate of advanced hepatic fibrosis with 3.6 kPa or higher was 0.47%.

Table 1.
Baseline characteristics.
3.2. Prevalence of Significant Fibrosis Based on Various Conditions
The prevalence of significant hepatic fibrosis was evaluated based on five conditions (USG-diagnosed NAFLD, elevated liver enzyme, metabolic syndrome, impaired fasting glucose, and diabetes); the prevalence of significant hepatic fibrosis (≥3.0 kPa) was 5.9%, 6.7%, 7.6%, 3.5%, and 14.2%, respectively. The prevalence of significant hepatic fibrosis in diabetic patients with USG-diagnosed fatty liver was significantly higher than in diabetic patients without fatty liver (6.67% vs. 20.2%, p = 0.027). A difference was not observed in the prevalence of significant hepatic fibrosis in patients with elevated liver enzyme, metabolic syndrome, or impaired fasting glucose based on the presence of fatty liver on USG (elevated liver enzyme; 3.91% vs. 8.66%, p = 0.115, metabolic syndrome; 2.68% vs. 4.84%, p = 0.158, and impaired fasting glucose; 2.46% vs. 4.69%, p = 0.20).
3.3. Performance of FIB-4 Index and NFS in Screening for Significant Hepatic Fibrosis in Various Conditions
Table 2 summarizes sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) for the FIB-4 index and NFS based on various conditions. All groups had high NPV (>90%) for the exclusion of significant fibrosis. Notably, the diabetes group showed the best PPV for both the FIB-4 index and NFS among the groups (17.7% and 14.7%, respectively). For the FIB-4 index and NFS, the PPV was 6.9% and 6.2% in the USG-diagnosed group and 6.2% and 6.5% in the elevated liver enzyme group, respectively. The sensitivity for identifying significant fibrosis in patients with diabetes was low and similar for the FIB-4 index and NFS (40.0% and 46.0%, respectively).

Table 2.
Performance of FIB-4 index and NFS based on various conditions.
The use of the FIB-4 index resulted in a 6.9% true positive rate and 93.1% false positive rate in the USG-diagnosed NAFLD group (Figure 2). In the diabetes group, the FIB-4 index showed a true positive rate of 17.7% and false positive rate of 82.3% (Figure 2).
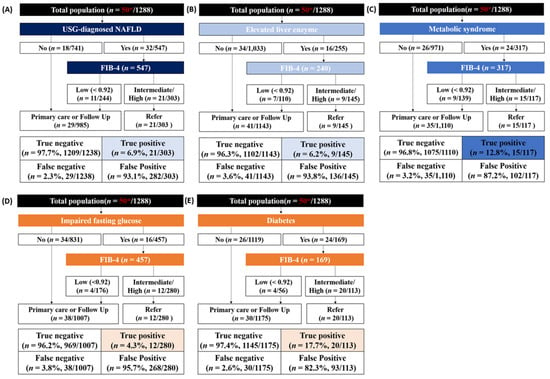
Figure 2.
Test outcomes and referral rates of FIB-4 index-based screening in five conditions. (A) USG-diagnosed NAFLD; (B) Elevated liver enzyme; (C) Metabolic syndrome; (D) Impaired fasting glucose; (E) Diabetes. * The number of patients with significant fibrosis with 3.0 kPa or higher based on MRE criteria.
In the diabetes group, the FIB-4 index performed better than the NFS (AUROC: 0.69 vs. 0.58, p = 0.05) (Figure 3). However, the performance of these two methods was not significantly different in the other subgroups.
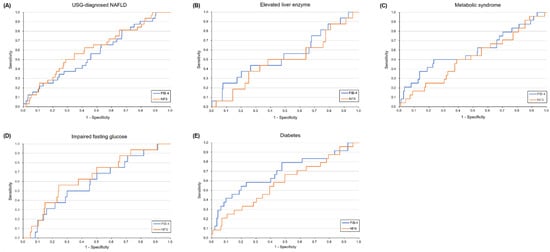
Figure 3.
Diagnostic performance of FIB-4 index and NFS for diagnosis of significant fibrosis in five conditions. (A) USG-diagnosed NAFLD; (B) Elevated liver enzyme; (C) Metabolic syndrome; (D) Impaired fasting glucose; (E) Diabetes.
3.4. Decision Curve Analysis and Net Benefit
The diabetes group showed the highest net benefit compared with the USG-diagnosed NAFLD and elevated liver enzyme groups for the detection of significant fibrosis across a wide range of Pts (Figure 4, Supplementary Table S1). The net benefit for each group was lower than for the strategy of “refer all” for Pt below approximately 0.03. At Pt 0.07, where an FIB-4 index-based strategy provides greater net benefit than liver biopsy [22], the diabetes group showed the greatest net benefit, followed by metabolic syndrome. Notably, net benefit was not apparent when using a Pt of 0.07 for patients with USG-diagnosed NAFLD. At the traditional Pt of 0.2 (five or fewer referrals for each correct diagnosis of fibrosis), a positive net benefit was not observed in all groups.
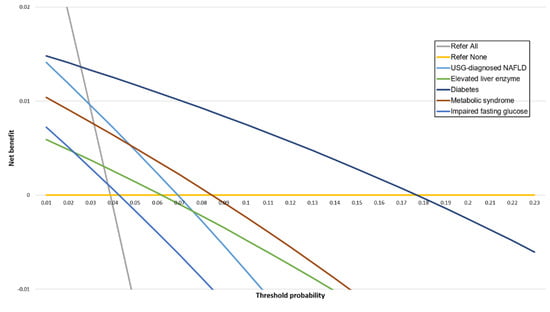
Figure 4.
Decision curves for FIB-4 index in five groups.
4. Discussion
In the majority of previous studies, hepatic fibrosis screening was assessed to determine the optimal diagnostic tests in the referral process. To the best of our knowledge, no prior studies investigated who should undergo screening for hepatic fibrosis in the community setting. In the present study, which target population in a low-risk cohort might most benefit from screening for hepatic fibrosis was determined. The patients with diabetes showed better performance (AUROC: 0.69) with the best combination of PPV and NPV (17.7% and 97.5%, respectively) compared with the USG-diagnosed NAFLD (AUROC: 0.57) and elevated liver enzyme (AUROC: 0.55) patients using the FIB-4 index-based algorithm. The diabetes group showed a 2.5-fold increase in the detection of significant fibrosis (7% to 18%) and simultaneously reduced the percentage of unnecessary referrals (93.1% to 82.3%) compared with the USG-diagnosed NAFLD group (Figure 5). In decision curve analysis, the diabetes group showed the highest net benefit compared with the USG-diagnosed NAFLD and elevated liver enzyme groups for the detection of significant fibrosis across a wide range of Pts. Taken together, these results indicate a possible rationale for the targeted screening of patients with diabetes.
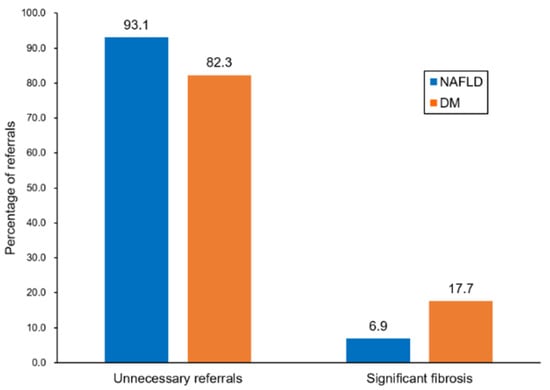
Figure 5.
Evaluation of patients referred to secondary care.
It is very well known that screening for hepatic fibrosis is necessary in diabetes or NAFLD cohorts. In this study, we tried to compare the size of net benefits for hepatic fibrosis screening among known various high-risk groups in community cohorts. So far, a few social economic analyses were performed in selected high-risk populations (NAFLD cohort or diabetes cohort) with conflicting results [7,8], but a comprehensive net benefit analysis according to various high-risk groups in primary care settings has not been reported. We believe that the results of this study can be helpful in understanding the target population and priority of screening algorithms in various high-risk groups in primary care centers.
Because awareness regarding the health risks associated with hepatic fibrosis in patients with diabetes is increasing, there is renewed interest in screening for hepatic fibrosis in diabetic patients. In this context, the feasibility of non-invasive tests for hepatic fibrosis screening in patients with diabetes was evaluated in two recent studies [23,24]. The results showed intermediate performance (AUROC of FIB-4 index: 0.70 and 0.78), which is similar to slightly higher compared with our results (AUROC: 0.69). However, these validation cohorts included diabetic patients with NAFLD and did not properly validate a diabetic cohort regardless of fatty liver. As the effects of diabetes and fatty liver might be additive in the same individual, diagnostic performance derived from cohorts with high fibrosis prevalence might have been overestimated in the previous study. The presence of diabetes is associated independently with liver fibrosis [3,25]. Therefore, to accurately estimate the performance of non-invasive tests in patients with diabetes, subjects without fatty liver need to be included in the validation group.
In the present study, the FIB-4 index had better performance for screening significant hepatic fibrosis in the diabetes group than did the NFS (Figure 3). Consistent results were found in two previous studies [23,24]. This finding is clinically relevant because the NFS, different from the FIB-4 index, includes the presence of diabetes in the formula. This indicates that the diagnostic performance of the NFS could be significantly influenced in a cohort of diabetic patients. Although several international guidelines recommended the FIB-4 index or NFS as the first-line examination for detecting fibrosis in NAFLD patients, the FIB-4 index is preferable in diabetic clinics.
In the present study, decision curve analysis was used to quantify the harms and benefits of using two simple non-invasive fibrosis tests based on various conditions rather than only presenting the diagnostic test accuracy. Fortunately, the diabetes group provided the greatest diagnostic utility in a low-risk population.
The present study had several limitations. First, for diagnosing liver fibrosis, biopsy remains the gold standard. However, performing a biopsy in asymptomatic participants in this low-risk cohort would have been unethical. Therefore, MRE was used, which is currently considered the most accurate imaging tool for the detection of liver fibrosis. Second, only the FIB-4 index and NFS were used, without considering other available non-invasive tests (e.g., AST/ALT ratio, AST to platelet ratio index, vibration-controlled transient elastography) or a combination of tests. However, the FIB-4 index and NFS are the most widely used and best validated tests. In addition, the main objective was to compare the diagnostic performance between groups rather than providing AUROC. Third, modified thresholds of the FIB-4 index and NFS used to predict significant fibrosis are not properly validated yet. The FIB-4 index and NFS were originally proposed to exclude advanced fibrosis using the cut-off values of 1.3 and −1.455. It would be inappropriate to use 1.3 and −1.455 as a lower cut-off in the primary care setting as it would include significant fibrosis. These modified cut-off values need to be validated in a large cohort in a low-risk population. Additionally, a reasonable range of Pts is determined by the preference of clinicians or patients and is not objective. However, rank order between groups irrespective of Pt was provided and demonstrated the highest net benefit in the diabetes group. The cohort in this study comprised relatively young and healthy individuals, and whether these results can be generalized to the elderly population is unclear. Finally, financial costs were not evaluated, which is necessary to apply research results in clinical practice. However, model-based economic evaluation is afflicted with certain limitations because an unrealistic simplification of assumptions is required.
5. Conclusions
In conclusion, patients with diabetes, even in the absence of fatty liver, would be preferable for hepatic fibrosis screening in a primary care setting. However, the cost effectiveness remains unclear and should be addressed in future studies.
Supplementary Materials
The following is available online at https://www.mdpi.com/article/10.3390/diagnostics11091605/s1, Table S1: Net benefit of FIB-4 at different Pts.
Author Contributions
Conceptualization, D.W.J. and K.A.K.; Data curation, S.H.P.; Formal analysis, J.H.L. and H.P.H.; Investigation, S.H.P.; Methodology, S.H.P.; Project administration, K.A.K.; Resources, K.A.K.; Software, J.H.L.; Supervision, D.W.J.; Validation, H.J.P. and J.N.K.; Visualization, J.H.L.; Writing—review and editing, S.H.P., D.W.J. and K.A.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board (or Ethics Committee) of the Kangbuk Samsung Hospital Institutional Review Board (IRB No. KBSMC 2020-11-029, approved on 26 November 2020).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hagstrom, H.; Nasr, P.; Ekstedt, M.; Hammar, U.; Stal, P.; Hultcrantz, R.; Kechagias, S. Fibrosis stage but not NASH predicts mortality and time to development of severe liver disease in biopsy-proven NAFLD. J. Hepatol. 2017, 67, 1265–1273. [Google Scholar] [CrossRef]
- Gines, P.; Graupera, I.; Lammert, F.; Angeli, P.; Caballeria, L.; Krag, A.; Guha, I.N.; Murad, S.D.; Castera, L. Screening for liver fibrosis in the general population: A call for action. Lancet Gastroenterol. Hepatol. 2016, 1, 256–260. [Google Scholar] [CrossRef]
- Kang, K.A.; Jun, D.W.; Kim, M.S.; Kwon, H.J.; Nguyen, M.H. Prevalence of significant hepatic fibrosis using magnetic resonance elastography in a health check-up clinic population. Aliment. Pharmacol. Ther. 2020, 51, 388–396. [Google Scholar] [CrossRef]
- EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J. Hepatol. 2016, 64, 1388–1402. [CrossRef]
- Bril, F.; Cusi, K. Management of Nonalcoholic Fatty Liver Disease in Patients With Type 2 Diabetes: A Call to Action. Diabetes Care 2017, 40, 419–430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castera, L. Non--invasive tests for liver fibrosis in NAFLD: Creating pathways between primary healthcare and liver clinics. Liver Int. 2020, 40, 77–81. [Google Scholar] [CrossRef] [Green Version]
- Corey, K.E.; Klebanoff, M.J.; Tramontano, A.C.; Chung, R.T.; Hur, C. Screening for Nonalcoholic Steatohepatitis in Individuals with Type 2 Diabetes: A Cost-Effectiveness Analysis. Dig. Dis. Sci. 2016, 61, 2108–2117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noureddin, M.; Jones, C.; Alkhouri, N.; Gomez, E.V.; Dieterich, D.T.; Rinella, M.E. Screening for Non-Alcoholic Fatty Liver Disease in Persons with Type 2 Diabetes in the U.S. is Cost Effective: A Comprehensive Cost-Utility Analysis. Gastroenterology 2020, 159, 1985–1987. [Google Scholar] [CrossRef]
- Kotronen, A.; Peltonen, M.; Hakkarainen, A.; Sevastianova, K.; Bergholm, R.; Johansson, L.M.; Lundbom, N.; Rissanen, A.; Ridderstråle, M.; Groop, L. Prediction of non-alcoholic fatty liver disease and liver fat using metabolic and genetic factors. Gastroenterology 2009, 137, 865–872. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Kim, D.; Kim, H.J.; Lee, C.-H.; Yang, J.I.; Kim, W.; Kim, Y.J.; Yoon, J.-H.; Cho, S.-H.; Sung, M.-W. Hepatic steatosis index: A simple screening tool reflecting nonalcoholic fatty liver disease. Dig. Liver Dis. 2010, 42, 503–508. [Google Scholar] [CrossRef]
- Hsu, C.; Caussy, C.; Imajo, K.; Chen, J.; Singh, S.; Kaulback, K.; Le, M.-D.; Hooker, J.; Tu, X.; Bettencourt, R. Magnetic resonance vs transient elastography analysis of patients with nonalcoholic fatty liver disease: A systematic review and pooled analysis of individual participants. Clin. Gastroenterol. Hepatol. 2019, 17, 630–637.e638. [Google Scholar] [CrossRef] [Green Version]
- Marathe, P.H.; Gao, H.X.; Close, K.L. American D iabetes A ssociation S tandards of M edical C are in D iabetes 2017. J. Diabetes 2017, 9, 320–324. [Google Scholar] [CrossRef] [PubMed]
- Who, E.C. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004, 363, 157. [Google Scholar]
- Saadeh, S.; Younossi, Z.M.; Remer, E.M.; Gramlich, T.; Ong, J.P.; Hurley, M.; Mullen, K.D.; Cooper, J.N.; Sheridan, M.J. The utility of radiological imaging in nonalcoholic fatty liver disease. Gastroenterology 2002, 123, 745–750. [Google Scholar] [CrossRef] [PubMed]
- Lonardo, A.; Nascimbeni, F.; Targher, G.; Bernardi, M.; Bonino, F.; Bugianesi, E.; Casini, A.; Gastaldelli, A.; Marchesini, G.; Marra, F. AISF position paper on nonalcoholic fatty liver disease (NAFLD): Updates and future directions. Dig. Liver Dis. 2017, 49, 471–483. [Google Scholar] [CrossRef]
- Kaswala, D.H.; Lai, M.; Afdhal, N.H. Fibrosis assessment in nonalcoholic fatty liver disease (NAFLD) in 2016. Dig. Dis. Sci. 2016, 61, 1356–1364. [Google Scholar] [CrossRef]
- Meneses, D.; Olveira, A.; Corripio, R.; Del Carmen Mendez, M.; Romero, M.; Calvo-Vinuelas, I.; Herranz, L.; Vicent, D.; de-Cos-Blanco, A.I. Performance of Noninvasive Liver Fibrosis Scores in the Morbid Obese Patient, Same Scores but Different Thresholds. Obes. Surg. 2020, 30, 2538–2546. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.-J.; Ye, F.-Z.; Li, Y.-Y.; Pan, X.-Y.; Chen, Y.-X.; Wu, X.-X.; Xiong, J.-J.; Liu, W.-Y.; Xu, S.-H.; Chen, Y.-P. Individualized risk prediction of significant fibrosis in non-alcoholic fatty liver disease using a novel nomogram. United Eur. Gastroenterol. J. 2019, 7, 1124–1134. [Google Scholar] [CrossRef]
- Sterling, R.K.; Lissen, E.; Clumeck, N.; Sola, R.; Correa, M.C.; Montaner, J.; Mark, S.S.; Torriani, F.J.; Dieterich, D.T.; Thomas, D.L.; et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006, 43, 1317–1325. [Google Scholar] [CrossRef]
- Angulo, P.; Hui, J.M.; Marchesini, G.; Bugianesi, E.; George, J.; Farrell, G.C.; Enders, F.; Saksena, S.; Burt, A.D.; Bida, J.P.; et al. The NAFLD fibrosis score: A noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology 2007, 45, 846–854. [Google Scholar] [CrossRef]
- Vickers, A.J.; Van Calster, B.; Steyerberg, E.W. Net benefit approaches to the evaluation of prediction models, molecular markers, and diagnostic tests. Bmj 2016, 352, i6. [Google Scholar] [CrossRef] [Green Version]
- Majumdar, A.; Campos, S.; Gurusamy, K.; Pinzani, M.; Tsochatzis, E.A. Defining the minimum acceptable diagnostic accuracy of noninvasive fibrosis testing in cirrhosis: A decision analytic modeling study. Hepatology 2020, 71, 627–642. [Google Scholar] [CrossRef]
- Alkayyali, T.; Qutranji, L.; Kaya, E.; Bakir, A.; Yilmaz, Y. Clinical utility of noninvasive scores in assessing advanced hepatic fibrosis in patients with type 2 diabetes mellitus: A study in biopsy-proven non-alcoholic fatty liver disease. Acta Diabetol. 2020, 57, 613–618. [Google Scholar] [CrossRef] [PubMed]
- Bril, F.; McPhaul, M.J.; Caulfield, M.P.; Clark, V.C.; Soldevilla-Pico, C.; Firpi-Morell, R.J.; Lai, J.; Shiffman, D.; Rowland, C.M.; Cusi, K. Performance of Plasma Biomarkers and Diagnostic Panels for Nonalcoholic Steatohepatitis and Advanced Fibrosis in Patients With Type 2 Diabetes. Diabetes Care 2020, 43, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Angulo, P.; Keach, J.C.; Batts, K.P.; Lindor, K.D. Independent predictors of liver fibrosis in patients with nonalcoholic steatohepatitis. Hepatology 1999, 30, 1356–1362. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).