The Non-Cancer Specific Elevation of the Serum Squamous Cell Carcinoma Antigen during the Post-Radiotherapy Follow-Up of Cervical Cancer Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Cohort
2.2. Data Collection
2.3. Literature Review on Non-Cancer Specific SCC Elevation
2.4. Statistical Analysis
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of Incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Marth, C.; Landoni, F.; Mahner, S.; McCormack, M.; Gonzalez-Martin, A.; Colombo, N.; ESMO Guidelines Committee. Cervical cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2017, 28, iv72–iv83. [Google Scholar] [CrossRef]
- Gadducci, A.; Tana, R.; Cosio, S.; Genazzani, A.R. The serum assay of tumour markers in the prognostic evaluation, treatment monitoring and follow-up of patients with cervical cancer: A review of the literature. Crit. Rev. Oncol. Hematol. 2008, 66, 10–20. [Google Scholar] [CrossRef]
- Ngan, H.Y.; Cheng, G.T.; Yeung, W.S.; Wong, L.C.; Ma, H.K. The prognostic value of TPA and SCC in squamous cell carcinoma of the cervix. Gynecol. Oncol. 1994, 52, 63–68. [Google Scholar] [CrossRef]
- Holloway, R.W.; To, A.; Moradi, M.; Boots, L.; Watson, N.; Shingleton, H. Monitoring the course of cervical carcinoma with the squamous cell carcinoma serum radioimmunoassay. Obstet. Gynecol. 1989, 74, 944–949. [Google Scholar] [CrossRef]
- Scambia, G.; Panici, P.B.; Baiocchi, G.; Amoroso, M.; Foti, E.; Greggi, S.; Mancuso, S. The value of squamous cell carcinoma antigen in patients with locally advanced cervical cancer undergoing neoadjuvant chemotherapy. Am. J. Obstet. Gynecol. 1991, 164, 631–636. [Google Scholar] [CrossRef]
- Ohno, T.; Nakayama, Y.; Nakamoto, S.; Kato, S.; Imai, R.; Nonaka, T.; Ishikawa, H.; Harashima, K.; Suzuki, Y. Measurement of serum squamous cell carcinoma antigen levels as a predictor of radiation response in patients with carcinoma of the uterine cervix. Cancer 2003, 97, 3114–3120. [Google Scholar] [CrossRef]
- Hong, J.H.; Tsai, C.S.; Chang, J.T.; Wang, C.C.; Lai, C.H.; Lee, S.P.; Tseng, C.J.; Chang, T.C.; Tang, S.G. The prognostic significance of pre- and posttreatment SCC levels in patients with squamous cell carcinoma of the cervix treated by radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 1998, 41, 823–830. [Google Scholar] [CrossRef]
- Rose, P.G.; Baker, S.; Fournier, L.; Nelson, B.E.; Hunter, R.E. Serum squamous cell carcinoma antigen levels in invasive cervical cancer: Prediction of response and recurrence. Am. J. Obstet. Gynecol. 1993, 168, 942–946. [Google Scholar] [CrossRef]
- Kato, H.; Torigoe, T. Radioimmunoassay for tumor antigen of human cervical squamous cell carcinoma. Cancer 1977, 40, 1621–1628. [Google Scholar] [CrossRef]
- Uemura, Y.; Pak, S.C.; Luke, C.; Cataltepe, S.; Tsu, C.; Schick, C.; Kamachi, Y.; Pomeroy, S.L.; Perlmutter, D.H.; Silverman, G.A. Circulating serpin tumor markers SCCA1 and SCCA2 are not actively secreted but reside in the cytosol of squamous carcinoma cells. Int. J. Cancer 2000, 89, 368–377. [Google Scholar] [CrossRef]
- Schmidt-Rhode, P.; Schulz, K.D.; Sturm, G.; Häfner, H.; Prinz, H.; Künzig, H.J. Squamous cell carcinoma antigen for monitoring cervical cancer. Int. J. Biol. Markers 1988, 3, 87–94. [Google Scholar] [CrossRef]
- De Bruijn, H.W.; Duk, J.M.; Van der Zee, A.G.; Pras, E.; Willemse, P.H.; Boonstra, H.; Hollema, H.; Mourits, M.J.; de Vries, E.G.; Aalders, J.G. The clinical value of squamous cell carcinoma antigen in cancer of the uterine cervix. Tumour Biol. 1998, 19, 505–516. [Google Scholar] [CrossRef]
- Maruo, T.; Shibata, K.; Kimura, A.; Hoshina, M.; Mochizuki, M. Tumor-associated antigen, TA-4, in the monitoring of the effects of therapy for squamous cell carcinoma of the uterine cervix. Serial determinations and tissue localization. Cancer 1985, 56, 302–308. [Google Scholar] [CrossRef]
- Ohno, T.; Noda, S.E.; Okonogi, N.; Murata, K.; Shibuya, K.; Kiyohara, H.; Tamaki, T.; Ando, K.; Oike, T.; Ohkubo, Y.; et al. In-room computed tomography-based brachytherapy for uterine cervical cancer: Results of a 5-year retrospective study. J. Radiat. Res. 2017, 58, 543–551. [Google Scholar] [CrossRef]
- Ashitani, J.; Mukae, H.; Ihiboshi, H.; Taniguchi, H.; Mashimoto, H.; Matsukura, S. Adult respiratory distress syndrome with increased serum and bronchoalveolar lavage fluid levels of squamous cell carcinoma-related antigen. Intern. Med. 1996, 35, 497–501. [Google Scholar] [CrossRef][Green Version]
- Wei, X.; Su, J.; Yang, K.; Wei, J.; Wan, H.; Cao, X.; Tan, W.; Wang, H. Elevations of serum cancer biomarkers correlate with severity of COVID-19. J. Med. Virol. 2020, 92, 2036–2041. [Google Scholar] [CrossRef]
- Sano, A. Transient elevation of squamous cell carcinoma antigen levels with influenza virus infection. Respirol. Case Rep. 2018, 6, e00362. [Google Scholar] [CrossRef]
- Sakito, O.; Kadota, J.; Kohno, S.; Itoh, N.; Takahara, O.; Hara, K. Pulmonary infiltration with eosinophilia and increased serum levels of squamous cell carcinoma-related antigen and neuron specific enolase. Intern. Med. 1994, 33, 550–553. [Google Scholar] [CrossRef]
- Kagohashi, K.; Satoh, H.; Kurishima, K.; Kadono, K.; Ishikawa, H.; Ohtsuka, M.; Sekizawa, K. Squamous cell carcinoma antigen in lung cancer and nonmalignant respiratory diseases. Lung 2008, 186, 323–326. [Google Scholar] [CrossRef]
- Body, J.J.; Sculier, J.P.; Raymakers, N.; Paesmans, M.; Ravez, P.; Libert, P.; Richez, M.; Dabouis, G.; Lacroix, H.; Bureau, G.; et al. Evaluation of squamous cell carcinoma antigen as a new marker for lung cancer. Cancer 1990, 65, 1552–1556. [Google Scholar] [CrossRef]
- Mitsuishi, K.; Nakamura, T.; Sakata, Y.; Yuyama, N.; Arima, K.; Sugita, Y.; Suto, H.; Izuhara, K.; Ogawa, H. The squamous cell carcinoma antigens as relevant biomarkers of atopic dermatitis. Clin. Exp. Allergy 2005, 35, 1327–1333. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.C.; Zheng, N.N.; Zhong, L.S. Different expression of squamous cell carcinoma antigens in psoriasis vulgaris and other papulosquamous dermatoses. Australas. J. Dermatol. 2020, 61, e261–e262. [Google Scholar] [CrossRef]
- Sun, A.; Chiang, C.P. Levamisole and/or Chinese medicinal herbs can modulate the serum level of squamous cell carcinoma associated antigen in patients with erosive oral lichen planus. J. Oral. Pathol. Med. 2001, 30, 542–548. [Google Scholar] [CrossRef]
- Hayashi, A.; Shiono, H.; Okumura, M. Thymoma accompanied by lichen planus. Interact. Cardiovasc. Thorac. Surg. 2008, 7, 347–348. [Google Scholar] [CrossRef] [PubMed]
- Iriki, H.; Tanese, K.; Furuichi, Y.; Takeshita, K.; Saito, M. Marked decrease in serum squamous cell carcinoma antigen level after antitumor necrosis factor alpha therapy in six cases of severe psoriasis. Int. J. Dermatol. 2016, 55, e364–e366. [Google Scholar] [CrossRef] [PubMed]
- Campbell, B.; De’Ambrosis, B. Squamous cell carcinoma antigen in patients with cutaneous disorders. J. Am. Acad. Dermatol. 1990, 22, 639–642. [Google Scholar] [CrossRef]
- Zheng, N.N.; Zhang, R.C.; Yang, X.X.; Tao, Y.K.; Zhong, L.S. Squamous cell carcinoma antigen is useful in the differential diagnosis of erythroderma. Int. J. Dermatol. 2019, 58, e158–e159. [Google Scholar] [CrossRef] [PubMed]
- Duk, J.M.; van Voorst Vader, P.C.; ten Hoor, K.A.; Hollema, H.; Doeglas, H.M.; de Bruijn, H.W. Elevated levels of squamous cell carcinoma antigen in patients with a benign disease of the skin. Cancer 1989, 64, 1652–1656. [Google Scholar] [CrossRef]
- Chen, J.; Tao, F.; Zhang, B.; Chen, Q.; Qiu, Y.; Luo, Q.; Gen, Y.; Meng, J.; Zhang, J.; Lu, H. Elevated Squamous Cell Carcinoma Antigen, Cytokeratin 19 Fragment, and Carcinoembryonic Antigen Levels in Diabetic Nephropathy. Int. J. Endocrinol. 2017, 2017, 5304391. [Google Scholar] [CrossRef] [PubMed]
- Kashiwabara, K.; Nakamura, H.; Yagyu, H.; Kishi, K.; Matsuoka, T.; Esaki, T. Changes in squamous cell carcinoma-related antigen levels before and after hemodialysis in relation to the model of dialyzer employed. Intern. Med. 2000, 39, 291–295. [Google Scholar] [CrossRef][Green Version]
- Tong, H.L.; Dong, Z.N.; Wen, X.Y.; Gao, J.; Wang, B.; Tian, Y.P. Impact of chronic kidney disease on serum tumor markers concentrations. Chin. Med. J. 2013, 126, 274–279. [Google Scholar]
- Koike, N.; Oi, H.; Naruse, K.; Kanayama, N.; Kobayashi, H. Squamous cell carcinoma antigen as a novel candidate marker for amniotic fluid embolism. J. Obstet. Gynaecol. Res. 2017, 43, 1815–1820. [Google Scholar] [CrossRef]
- Phocas, I.; Sarandakou, A.; Rizos, D.; Dimitriadou, F.; Mantzavinos, T.; Zourlas, P.A. Tumour-associated antigens, CEA, CA 125 and SCC in serum and follicular fluid of stimulated and unstimulated cycles. Eur. J. Obstet. Gynecol. Reprod. Biol. 1994, 54, 131–136. [Google Scholar] [CrossRef]
- Sarandakou, A.; Kontoravdis, A.; Kontogeorgi, Z.; Rizos, D.; Phocas, I. Expression of CEA, CA-125 and SCC antigen by biological fluids associated with pregnancy. Eur. J. Obstet. Gynecol. Reprod. Biol. 1992, 44, 215–220. [Google Scholar] [CrossRef]
- Schlageter, M.H.; Larghero, J.; Cassinat, B.; Toubert, M.E.; Borschneck, C.; Rain, J.D. Serum carcinoembryonic antigen, cancer antigen 125, cancer antigen 15-3, squamous cell carcinoma, and tumor-associated trypsin inhibitor concentrations during healthy pregnancy. Clin. Chem. 1998, 44, 1995–1998. [Google Scholar] [CrossRef]
- Yuyama, N.; Davies, D.E.; Akaiwa, M.; Matsui, K.; Hamasaki, Y.; Suminami, Y.; Yoshida, N.L.; Maeda, M.; Pandit, A.; Lordan, J.L.; et al. Analysis of novel disease-related genes in bronchial asthma. Cytokine 2002, 19, 287–296. [Google Scholar] [CrossRef]
- Takeuchi, S.; Furusyo, N.; Ono, J.; Azuma, Y.; Takemura, M.; Esaki, H.; Yamamura, K.; Mitamura, Y.; Tsuji, G.; Kiyomatsu-Oda, M.; et al. Serum squamous cell carcinoma antigen (SCCA)-2 correlates with clinical severity of pediatric atopic dermatitis in Ishigaki cohort. J. Dermatol. Sci. 2019, 95, 70–75. [Google Scholar] [CrossRef]
- Kawashima, H.; Nishimata, S.; Kashiwagi, Y.; Numabe, H.; Sasamoto, M.; Iwatsubo, H.; Takekuma, K.; Hoshika, A. Squamous cell carcinoma-related antigen in children with atopic dermatitis. Pediatr. Int. 2000, 42, 448–450. [Google Scholar] [CrossRef]
- Kahn, N.; Riedlinger, J.; Roeßler, M.; Rabe, C.; Lindner, M.; Koch, I.; Schott-Hildebrand, S.; Herth, F.J.; Schneider, M.A.; Meister, M.; et al. Blood-sampling collection prior to surgery may have a significant influence upon biomarker concentrations measured. Clin. Proteom. 2015, 12, 19. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, T.C.; Song, Y.S.; Yu, S.F.; Zhang, J.; Wang, H.; Gu, Y.E.; Chen, T.; Jia, G. Association of folate deficiency and selected tumor marker concentrations in long-term hexavalent chromium exposed population. Int. J. Hyg. Environ. Health 2014, 217, 88–94. [Google Scholar] [CrossRef]
- Li, T.H.; Kao, Y.F.; Lui, C.C.; Chen, W.H. An increase of blood squamous cell carcinoma antigen in Pseudomonas aeruginosa spondylitis. Clin. Biochem. 2006, 39, 662–665. [Google Scholar] [CrossRef] [PubMed]
- Promsopa, C.; Suwansri, S.; Khuntikij, P. The serum squamous cell carcinoma antigen level in inverted sinonasal papilloma and nasal polyps patients. World J. Otorhinolaryngol. Head Neck Surg. 2020, 7, 23–27. [Google Scholar] [CrossRef]
- Gao, M.L.; Chen, L.; Li, Y.F.; Xue, X.C.; Chen, L.; Wang, L.N.; Shah, W.; Kong, Y. Synergistic increase of oxidative stress and tumor markers in PAH-exposed workers. Asian Pac. J. Cancer Prev. 2014, 15, 7105–7112. [Google Scholar] [CrossRef]
- Minato, T.; Toyoshima, M.; Imai, N.; Kasai, A.; Yaegashi, N. Mature ovarian cystic teratoma with disseminated nodular lesions in the pleural and peritoneal cavities: A case report. Radiol. Case Rep. 2018, 13, 671–675. [Google Scholar] [CrossRef]
- Kasiske, B.L.; Wheeler, D.C. Kidney Disease: Improving Global Outcomes CKD Work Group. KDIGO 2012 Clinical practice guideline for the evaluation and Management of Chronic Kidney Disease. Kidney Int. Suppl. 2013, 3, 1–150. [Google Scholar]
- Tomizawa, K.; Kaminuma, T.; Murata, K.; Noda, S.E.; Irie, D.; Kumazawa, T.; Oike, T.; Ohno, T. FIGO 2018 Staging for Cervical Cancer: Influence on Stage Distribution and Outcomes in the 3D-Image-Guided Brachytherapy Era. Cancers 2020, 12, 1770. [Google Scholar] [CrossRef]
- Okazaki, S.; Murata, K.; Noda, S.E.; Kumazaki, Y.; Hirai, R.; Igari, M.; Abe, T.; Komatsu, S.; Nakano, T.; Kato, S. Dose-volume parameters and local tumor control in cervical cancer treated with central-shielding external-beam radiotherapy and CT-based image-guided brachytherapy. J. Radiat. Res. 2019, 60, 490–500. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Offord, C.P.; Kimura, G.; Kuribayashi, S.; Takeda, H.; Tsuchiya, S.; Shimojo, H.; Kanno, H.; Bozic, I.; Nowak, M.A.; et al. Tumour and immune cell dynamics explain the PSA bounce after prostate cancer brachytherapy. Br. J. Cancer 2016, 115, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Bogani, G.; Sopracordevole, F.; Di Donato, V.; Ciavattini, A.; Ghelardi, A.; Lopez, S.; Simoncini, T.; Plotti, F.; Casarin, J.; Serati, M.; et al. High-risk HPV-positive and -negative high-grade cervical dysplasia: Analysis of 5-year outcomes. Gynecol. Oncol. 2021, 161, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Casarin, J.; Bogani, G.; Papadia, A.; Ditto, A.; Pinelli, C.; Garzon, S.; Donadello, N.; Laganà, A.S.; Cromi, A.; Mueller, M.; et al. Preoperative conization and risk of recurrence in patients undergoing laparoscopic radical hysterectomy for early stage cervical cancer: A multicenter study. J. Minim. Invasive Gynecol. 2021, 28, 117–123. [Google Scholar] [CrossRef] [PubMed]
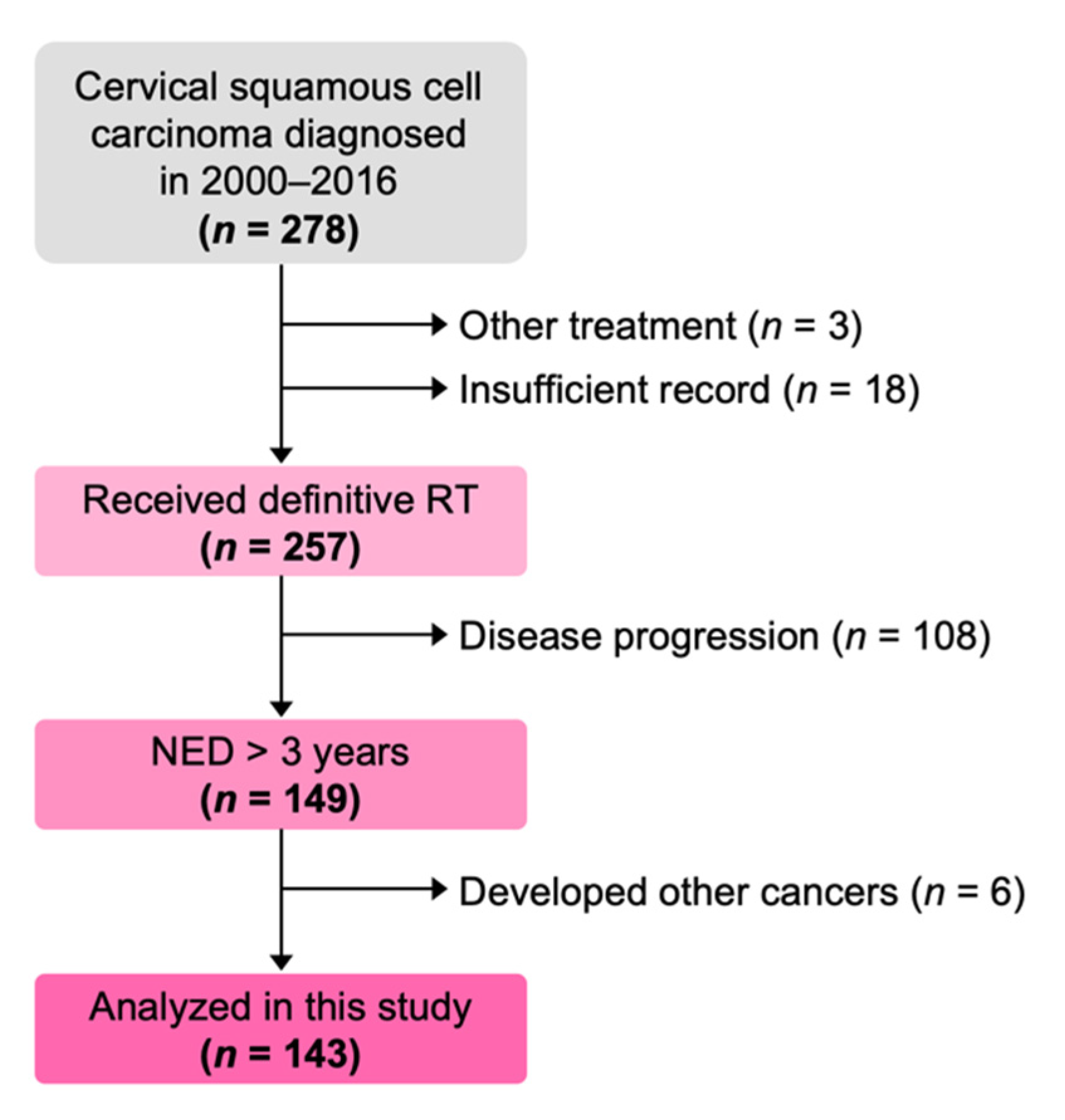
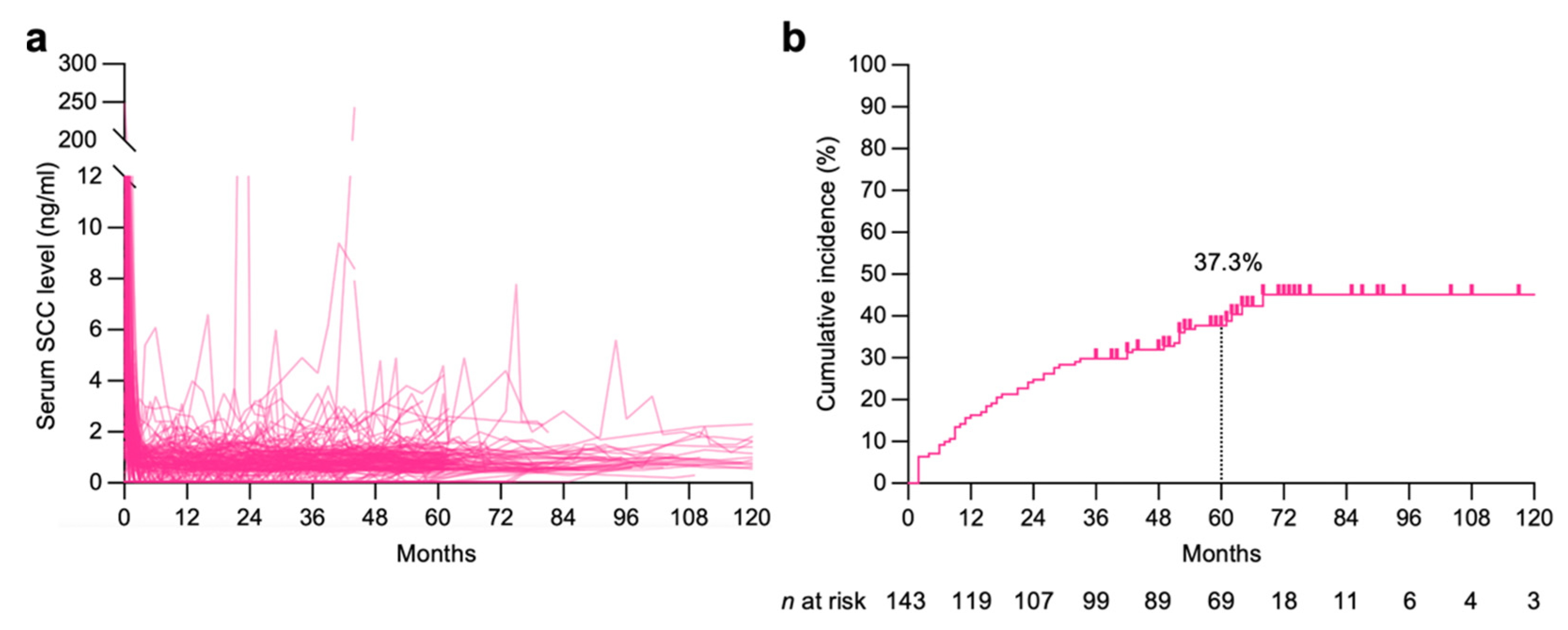
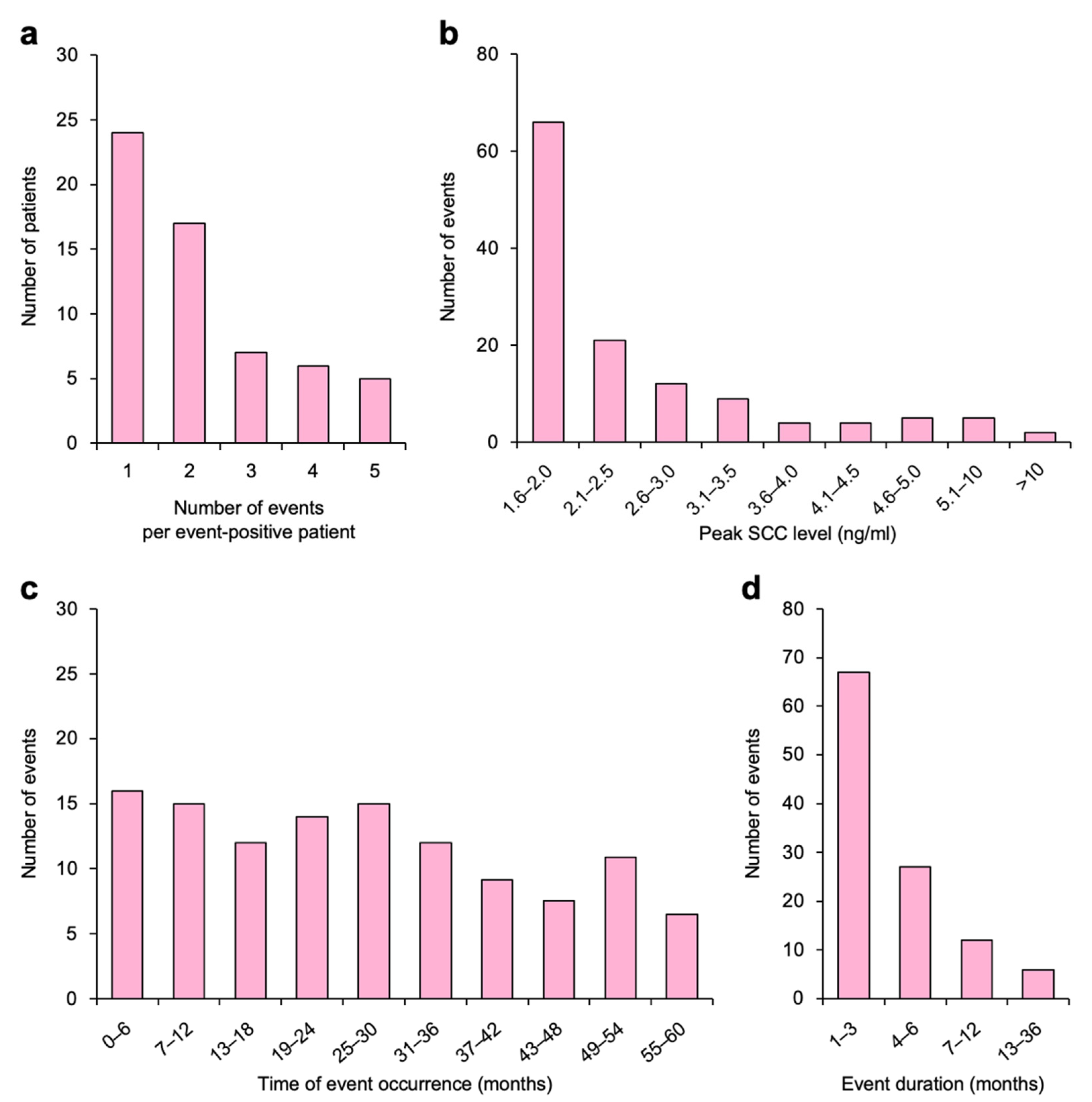
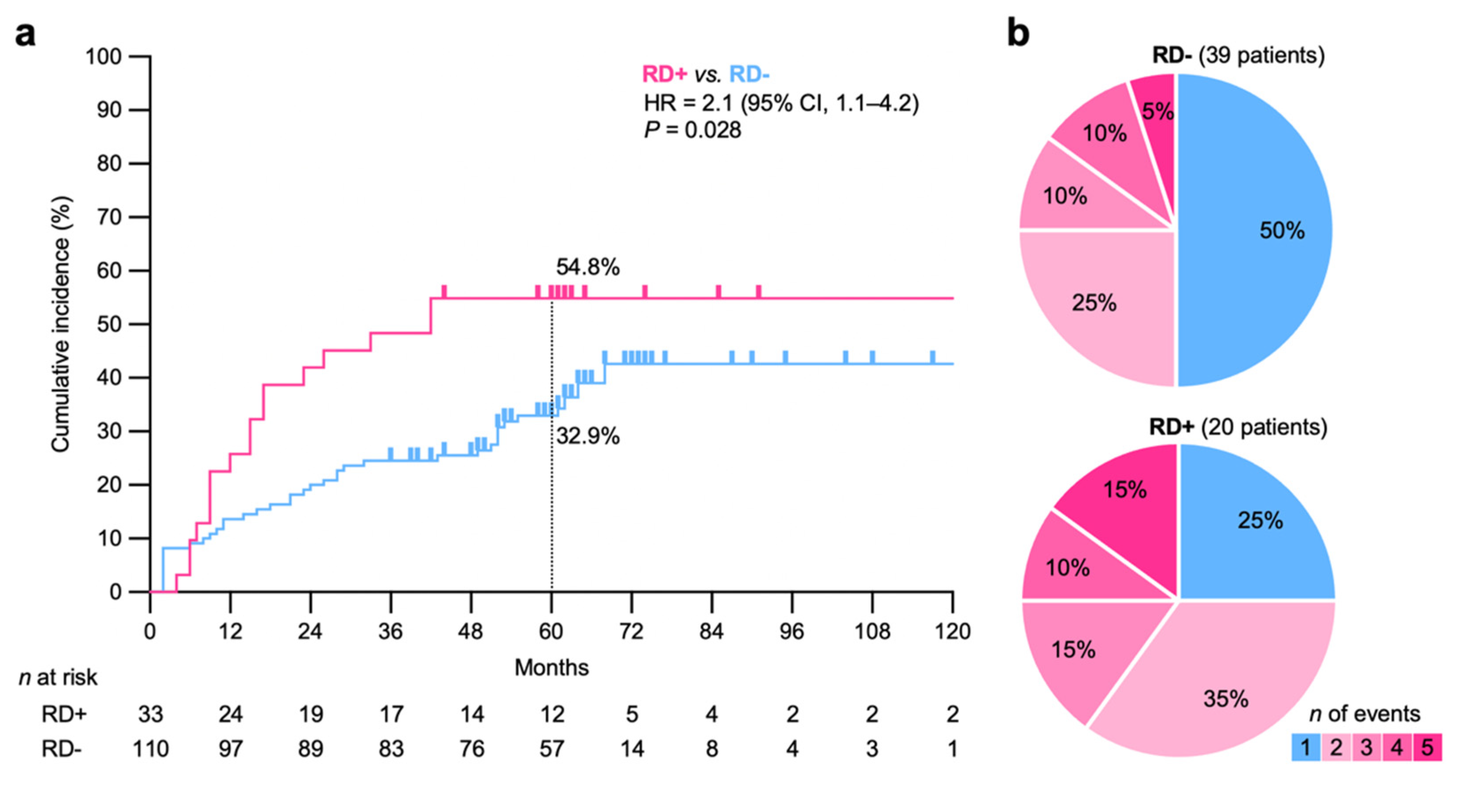
| Characteristics | N |
|---|---|
| Age | 57 (27–88) |
| FIGO stage | |
| IB | 28 (19%) |
| II | 71 (50%) |
| III | 37 (26%) |
| IVA | 7 (5%) |
| Tumor volume | |
| <30 cm3 | 61 (43%) |
| 30–100 cm3 | 57 (40%) |
| >100 cm3 | 22 (15%) |
| NA | 3 (2%) |
| Pelvic LN | |
| Positive | 61 (43%) |
| Negative | 82 (57%) |
| PALN | |
| Positive | 17 (12%) |
| Negative | 126 (88%) |
| Treatment | |
| CCRT | 88 (62%) |
| RT alone | 55 (38%) |
| Clinical Factors | Study Design | N | Serum SCC (ng/mL) | Refs. |
|---|---|---|---|---|
| Respiratory disease | ||||
| ARDS | Case report | 1 | 29.5 | [16] |
| COVID-19 | Retro MI | 252 | NA | [17] |
| Influenza | Case report | 1 | 17.1 | [18] |
| PIE syndrome | Case report | 1 | 8.1 | [19] |
| Various | Retro MI | 299 | mean, 1.3; range, 0.5–18.3 | [20] |
| Various | Retro MI | 89 | median, 4.6 | [21] |
| Skin disease | ||||
| Atopic dermatitis | Retro MI | 30 | NA | [22] |
| Generalized eczema | Retro MI | 51 | 4.1 ± 4.3 | [23] |
| Lichen planus | Retro MI | 109 | 1.3 ± 1.2 | [24] |
| Lichen planus | Case report | 1 | 9.0 | [25] |
| Psoriasis | Retro MI | 68 | 6.5 ± 5.2 | [23] |
| Psoriasis | Retro MI | 6 | 12.7 (1.7–33.3) | [26] |
| Various | Retro MI | 83 | NA | [27] |
| Various | Retro MI | 72 | range, 3.5–117.7 | [28] |
| Various | Retro MI | 66 | range, 1.4–73.2 | [29] |
| Renal dysfunction | ||||
| Diabetic nephropathy | Retro MI | 77 | 1.2 (0.5–1.8) | [30] |
| Various, with dialysis | Retro MI | 94 | NA | [31] |
| Various, without dialysis | Retro MI | 539 | median, 0.8 | [32] |
| Pregnancy-related events | ||||
| Amniotic embolism | Retro MI | 4 | 112.0 ± 169.4 | [33] |
| Follicle stimulation | Retro MI | 42 | 2.0 (1.1–17.8) | [34] |
| Normal pregnancy | Retro MI | 56 | 1.7 (0.8–17.0) | [35] |
| Normal pregnancy | Prospective | 12 | range, 0.1–4.3 | [36] |
| Pediatric diseases | ||||
| Asthma | Retro MI | 32 | 2.1 (1.4–3.3) | [37] |
| Atopic dermatitis | Prospective | 96 | NA | [38] |
| Atopic dermatitis | Retro MI | 95 | NA | [39] |
| Others | ||||
| Arterial puncture | Retro MI | 13 | NA | [40] |
| Chromium hexavalent | Retro MI | 115 | 1.2 ± 1.2 | [41] |
| Infectious spondylitis | Case report | 1 | 2.2 | [42] |
| Inverted papilloma | Prospective | 30 | 3.9 (IQR, 2.3–7.9) | [43] |
| PAH | Retro MI | 136 | range, 0.2–2.4 | [44] |
| Teratoma | Case report | 1 | 6.4 | [45] |
| Clinical Factor | SCC Elevation | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|---|
| + | − | Odds Ratio (95% CI) | p | Odds Ratio (95% CI) | p | ||
| Age | NA | ||||||
| <39 | 8 | 7 | Ref | ||||
| 40–49 | 12 | 14 | 0.75 (0.21–2.68) | 0.65 | |||
| 50–59 | 14 | 25 | 0.49 (0.14–1.63) | 0.24 | |||
| 60–69 | 15 | 22 | 0.59 (0.17–1.99) | 0.40 | |||
| ≥70 | 10 | 16 | 0.54 (0.15–1.97) | 0.35 | |||
| FIGO stage | NA | ||||||
| IB | 12 | 16 | Ref | ||||
| II | 29 | 42 | 0.92 (0.38–2.23) | 0.85 | |||
| III | 14 | 23 | 0.81 (0.29–2.20) | 0.68 | |||
| IVA | 4 | 3 | 1.77 (0.33–9.47) | 0.50 | |||
| Tumor volume | |||||||
| <34.3 cm3 | 25 | 46 | Ref | ||||
| ≥34.3 cm3 | 32 | 37 | 1.70 (0.86–3.37) | 0.12 | 1.38 (0.65–2.93) | 0.39 | |
| Pelvic LN | NA | ||||||
| Negative | 34 | 48 | Ref | ||||
| Positive | 25 | 36 | 0.98 (0.50–1.92) | 0.95 | |||
| PALN | NA | ||||||
| Negative | 52 | 74 | Ref | ||||
| Positive | 7 | 10 | 0.99 (0.35–2.78) | 0.99 | |||
| Treatment | NA | ||||||
| CCRT | 24 | 31 | Ref | ||||
| RT | 35 | 53 | 0.85 (0.43–1.68) | 0.64 | |||
| AE, skin | |||||||
| Grade 0 | 51 | 64 | Ref | ||||
| Grade ≥1 | 8 | 20 | 0.50 (0.20–1.23) | 0.13 | 0.48 (0.18–1.29) | 0.14 | |
| AE, GI | NA | ||||||
| Grade 0–1 | 37 | 47 | Ref | ||||
| Grade ≥2 | 22 | 37 | 0.75 (0.38–1.49) | 0.41 | |||
| AE, GU | NA | ||||||
| Grade 0 | 38 | 58 | Ref | ||||
| Grade ≥1 | 21 | 26 | 1.23 (0.60–2.49) | 0.56 | |||
| AE, hematology | |||||||
| Grade 0–1 | 17 | 35 | Ref | ||||
| Grade ≥2 | 42 | 49 | 1.76 (0.86–3.59) | 0.11 | 1.53 (0.69–3.38) | 0.28 | |
| Respiratory disease | NA | ||||||
| Negative | 49 | 72 | Ref | ||||
| Positive | 10 | 12 | 1.22 (0.49–3.05) | 0.66 | |||
| Skin disease | |||||||
| Negative | 47 | 78 | Ref | ||||
| Positive | 12 | 6 | 3.31 (1.16–9.43) | 0.024 | 2.63 (0.88–7.84) | 0.083 | |
| Renal dysfunction | |||||||
| Negative | 39 | 71 | Ref | ||||
| Positive | 20 | 13 | 2.80 (1.25–6.23) | 0.012 | 2.38 (1.01–5.61) | 0.046 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oike, T.; Oike, T.; Ando, K.; Iwase, A.; Ohno, T. The Non-Cancer Specific Elevation of the Serum Squamous Cell Carcinoma Antigen during the Post-Radiotherapy Follow-Up of Cervical Cancer Patients. Diagnostics 2021, 11, 1585. https://doi.org/10.3390/diagnostics11091585
Oike T, Oike T, Ando K, Iwase A, Ohno T. The Non-Cancer Specific Elevation of the Serum Squamous Cell Carcinoma Antigen during the Post-Radiotherapy Follow-Up of Cervical Cancer Patients. Diagnostics. 2021; 11(9):1585. https://doi.org/10.3390/diagnostics11091585
Chicago/Turabian StyleOike, Tae, Takahiro Oike, Ken Ando, Akira Iwase, and Tatsuya Ohno. 2021. "The Non-Cancer Specific Elevation of the Serum Squamous Cell Carcinoma Antigen during the Post-Radiotherapy Follow-Up of Cervical Cancer Patients" Diagnostics 11, no. 9: 1585. https://doi.org/10.3390/diagnostics11091585
APA StyleOike, T., Oike, T., Ando, K., Iwase, A., & Ohno, T. (2021). The Non-Cancer Specific Elevation of the Serum Squamous Cell Carcinoma Antigen during the Post-Radiotherapy Follow-Up of Cervical Cancer Patients. Diagnostics, 11(9), 1585. https://doi.org/10.3390/diagnostics11091585







