Diagnostics and Management of Male Infertility in Primary Ciliary Dyskinesia
Abstract
:1. Introduction
2. Role of PCD Genes in Male Fertility
2.1. Sperm Specific Dynein Heavy Chain Genes
2.2. Male Specific Motile Cilia
3. Diagnosing Male Infertility in PCD Patients
3.1. Semen Quality Analysis
3.2. Sperm Viability
4. Treating Male Infertility in PCD
5. Success of ART in PCD Patients
6. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Olbrich, H.; Schmidts, M.; Werner, C.; Onoufriadis, A.; Loges, N.T.; Raidt, J.; Banki, N.F.; Shoemark, A.; Burgoyne, T.; Al Turki, S.; et al. Recessive HYDIN mutations cause primary ciliary dyskinesia without randomization of left-right body asymmetry. Am. J. Hum. Genet. 2012, 91, 672–684. [Google Scholar] [CrossRef] [Green Version]
- Heuser, T.; Dymek, E.E.; Lin, J.; Smith, E.F.; Nicastro, D. The CSC connects three major axonemal complexes involved in dynein regulation. Mol. Biol. Cell 2012, 23, 3143–3155. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.; Ostrowski, L.E. Motile cilia genetics and cell biology: Big results from little mice. Cell. Mol. Life Sci. 2021, 78, 769–797. [Google Scholar] [CrossRef]
- Fabczak, H.; Osinka, A. Role of the Novel Hsp90 Co-Chaperones in Dynein Arms’ Preassembly. Int. J. Mol. Sci. 2019, 20, 6174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osinka, A.; Poprzeczko, M.; Zielinska, M.M.; Fabczak, H.; Joachimiak, E.; Wloga, D. Ciliary proteins: Filling the gaps. Recent advances in deciphering the protein composition of motile ciliary complexes. Cells 2019, 8, 730. [Google Scholar] [CrossRef] [Green Version]
- Lehti, M.S.; Sironen, A. Formation and function of sperm tail structures in association with sperm motility defects. Biol. Reprod. 2017, 97, 522–536. [Google Scholar] [CrossRef] [PubMed]
- Martinez, G.; Kherraf, Z.E.; Zouari, R.; Fourati Ben Mustapha, S.; Saut, A.; Pernet-Gallay, K.; Bertrand, A.; Bidart, M.; Hograindleur, J.P.; Amiri-Yekta, A.; et al. Whole-Exome sequencing identifies mutations in FSIP2 as a recurrent cause of multiple morphological abnormalities of the sperm flagella. Hum. Reprod. 2018, 33, 1973–1984. [Google Scholar] [CrossRef]
- Elkina, Y.L.; Kuravsky, M.L.; Bragina, E.E.; Kurilo, L.F.; Khayat, S.S.; Sukhomlinova, M.Y.; Schmalhausen, E.V. Detection of a mutation in the intron of sperm-specific glyceraldehyde-3-phosphate dehydrogenase gene in patients with fibrous sheath dysplasia of the sperm flagellum. Andrologia 2017, 49, e12606. [Google Scholar] [CrossRef]
- Liu, M.; Sun, Y.; Li, Y.; Sun, J.; Yang, Y.; Shen, Y. Novel mutations in FSIP2 lead to multiple morphological abnormalities of the sperm flagella and poor ICSI prognosis. Gene 2021, 781, 145536. [Google Scholar] [CrossRef]
- Fishman, E.L.; Jo, K.; Nguyen, Q.P.H.; Kong, D.; Royfman, R.; Cekic, A.R.; Khanal, S.; Miller, A.L.; Simerly, C.; Schatten, G.; et al. A novel atypical sperm centriole is functional during human fertilization. Nat. Commun. 2018, 9, 2210. [Google Scholar] [CrossRef]
- Wu, B.; Gao, H.; Liu, C.; Li, W. The coupling apparatus of the sperm head and tail. Biol. Reprod. 2020, 102, 988–998. [Google Scholar] [CrossRef] [PubMed]
- Zur Lage, P.; Newton, F.G.; Jarman, A.P. Survey of the ciliary motility machinery of drosophila sperm and ciliated mechanosensory neurons reveals unexpected cell-type specific variations: A model for motile ciliopathies. Front. Genet. 2019, 10, 24. [Google Scholar] [CrossRef]
- Whitfield, M.; Thomas, L.; Bequignon, E.; Schmitt, A.; Stouvenel, L.; Montantin, G.; Tissier, S.; Duquesnoy, P.; Copin, B.; Chantot, S.; et al. Mutations in DNAH17, encoding a sperm-specific axonemal outer dynein arm heavy chain, cause isolated male infertility due to asthenozoospermia. Am. J. Hum. Genet. 2019, 105, 98–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishimoto, K.; Bloomfield-Gadêlha, H.; Gaffney, E.; Smith, D.; Kirkman-Brown, J.C. Coarse-Graining the Fluid Flow around a Human Sperm. Phys. Rev. Lett. 2017, 118, 124501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, D.; Gaffney, E.; Blake, J. Modelling mucociliary clearance. Respir. Physiol. Neurobiol. 2008, 163, 178–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Legendre, M.; Zaragosi, L.E.; Mitchison, H.M. Motile cilia and airway disease. Semin. Cell Dev. Biol. 2021, 110, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Backman, K.; Mears, W.E.; Waheeb, A.; Beaulieu Bergeron, M.; McClintock, J.; de Nanassy, J.; Reisman, J.; Osmond, M.; Hartley, T.; Mears, A.J.; et al. A splice site and copy number variant responsible for TTC25-related primary ciliary dyskinesia. Eur. J. Med. Genet. 2021, 64, 104193. [Google Scholar] [CrossRef] [PubMed]
- Fagerberg, L.; Hallström, B.M.; Oksvold, P.; Kampf, C.; Djureinovic, D.; Odeberg, J.; Habuka, M.; Tahmasebpoor, S.; Danielsson, A.; Edlund, K.; et al. Analysis of the Human tissue-specific expression by genome-wide integration of transcriptomics and antibody-based proteomics. Mol. Cell. Proteom. 2014, 13, 397–406. [Google Scholar] [CrossRef] [Green Version]
- Shoemark, A.; Harman, K. Primary Ciliary Dyskinesia. Semin. Respir. Crit. Care Med. 2021, 42, 537–548. [Google Scholar] [CrossRef] [PubMed]
- Sironen, A.; Shoemark, A.; Patel, M.; Loebinger, M.R.; Mitchison, H.M. Sperm defects in primary ciliary dyskinesia and related causes of male infertility. Cell. Mol. Life Sci. 2019, 77, 2029–2048. [Google Scholar] [CrossRef] [Green Version]
- Tu, C.; Nie, H.; Meng, L.; Wang, W.; Li, H.; Yuan, S.; Cheng, D.; He, W.; Liu, G.; Du, J.; et al. Novel mutations in SPEF2 causing different defects between flagella and cilia bridge: The phenotypic link between MMAF and PCD. Qual. Life Res. 2020, 139, 257–271. [Google Scholar] [CrossRef]
- Liu, C.; Lv, M.; He, X.; Zhu, Y.; Amiri-Yekta, A.; Li, W.; Wu, H.; Kherraf, Z.E.; Liu, W.; Zhang, J.; et al. Homozygous mutations in SPEF2 induce multiple morphological abnormalities of the sperm flagella and male infertility. J. Med. Genet. 2019, 57, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Sha, Y.; Li, Y.; Mei, L.; Lin, S.; Huang, X.; Lu, J.; Ding, L.; Kong, S.; Lu, Z. Loss-of-function mutations in SPEF2 cause multiple morphological abnormalities of the sperm flagella (MMAF). J. Med. Genet. 2019, 56, 678–684. [Google Scholar] [CrossRef] [PubMed]
- Coutton, C.; Martinez, G.; Kherraf, Z.E.; Amiri-Yekta, A.; Boguenet, M.; Saut, A.; He, X.; Zhang, F.; Cristou-Kent, M.; Escoffier, J.; et al. Bi-allelic Mutations in ARMC2 lead to severe astheno-teratozoospermia due to sperm flagellum malformations in humans and mice. Am. J. Hum. Genet. 2019, 104, 331–340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, F.N.; Amiri-Yekta, A.; Martinez, G.; Saut, A.; Tek, J.; Stouvenel, L.; Lores, P.; Karaouzene, T.; Thierry-Mieg, N.; Satre, V.; et al. Absence of CFAP69 causes male infertility due to multiple morphological abnormalities of the flagella in human and mouse. Am. J. Hum. Genet. 2018, 102, 636–648. [Google Scholar] [CrossRef] [Green Version]
- He, X.; Li, W.; Wu, H.; Lv, M.; Liu, W.; Liu, C.; Zhu, F.; Li, C.; Fang, Y.; Yang, C.; et al. Novel homozygous CFAP69 mutations in humans and mice cause severe asthenoteratospermia with multiple morphological abnormalities of the sperm flagella. J. Med. Genet. 2018, 56, 96–103. [Google Scholar] [CrossRef]
- Lorès, P.; Coutton, C.; El Khouri, E.; Stouvenel, L.; Givelet, M.; Thomas, L.; Rode, B.; Schmitt, A.; Louis, B.; Sakheli, Z.; et al. Homozygous missense mutation L673P in adenylate kinase 7 (AK7) leads to primary male infertility and multiple morphological anomalies of the flagella but not to primary ciliary dyskinesia. Hum. Mol. Genet. 2018, 27, 1196–1211. [Google Scholar] [CrossRef]
- Mata, M.; Lluch-Estellés, J.; Armengot, M.; Sarrión, I.; Carda, C.; Cortijo, J. New Adenylate Kinase 7 (AK7) Mutation in Primary Ciliary Dyskinesia. Am. J. Rhinol. Allergy 2012, 26, 260–264. [Google Scholar] [CrossRef]
- Milara, J.; Armengot, M.; Mata, M.; Morcillo, E.; Cortijo, J. Role of Adenylate kinase Type 7 expression on cilia motility: Possible link in primary ciliary dyskinesia. Am. J. Rhinol. Allergy 2010, 24, 181–185. [Google Scholar] [CrossRef]
- Jeanson, L.; Copin, B.; Papon, J.F.; Moal, F.D.-L.; Duquesnoy, P.; Montantin, G.; Cadranel, J.; Corvol, H.; Coste, A.; Désir, J.; et al. RSPH3 Mutations cause primary ciliary dyskinesia with central-complex defects and a near absence of radial spokes. Am. J. Hum. Genet. 2015, 97, 153–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vanaken, G.J.; Bassinet, L.; Boon, M.; Mani, R.; Honore, I.; Papon, J.F.; Cuppens, H.; Jaspers, M.; Lorent, N.; Coste, A.; et al. Infertility in an adult cohort with primary ciliary dyskinesia: Pheno-type-gene association. Eur. Respir. J. 2017, 50, 1700314. [Google Scholar] [CrossRef] [PubMed]
- Castleman, V.H.; Romio, L.; Chodhari, R.; Hirst, R.A.; de Castro, S.C.; Parker, K.A.; Ybot-Gonzalez, P.; Emes, R.D.; Wilson, S.; Wallis, C.; et al. Mutations in Radial Spoke Head Protein Genes RSPH9 and RSPH4A Cause Primary Ciliary Dyskinesia with Central-Microtubular-Pair Abnormalities. Am. J. Hum. Genet. 2009, 84, 197–209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abbasi, F.; Miyata, H.; Shimada, K.; Morohoshi, A.; Nozawa, K.; Matsumura, T.; Xu, Z.; Pratiwi Pikawa, M. RSPH6A is required for sperm flagellum formation and male fertility in mice. J. Cell Sci. 2018, 131, jcs221648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aprea, I.; Raidt, J.; Höben, I.M.; Loges, N.T.; Nöthe-Menchen, T.; Pennekamp, P.; Olbrich, H.; Kaiser, T.; Biebach, L.; Tüttelmann, F.; et al. Defects in the cytoplasmic assembly of axonemal dynein arms cause morphological abnormalities and dysmotility in sperm cells leading to male infertility. PLoS Genet. 2021, 17, e1009306. [Google Scholar] [CrossRef]
- Thomas, L.; Bouhouche, K.; Whitfield, M.; Thouvenin, G.; Coste, A.; Louis, B.; Szymanski, C.; Bequignon, E.; Papon, J.F.; Castelli, M.; et al. TTC12 Loss-of-Function mutations cause primary ciliary dyskinesia and unveil distinct dynein assembly mechanisms in motile cilia versus flagella. Am. J. Hum. Genet. 2020, 106, 153–169. [Google Scholar] [CrossRef]
- Fliegauf, M.; Olbrich, H.; Horvath, J.; Wildhaber, J.H.; Zariwala, M.A.; Kennedy, M.; Knowles Mromran, H. Mislocalization of DNAH5 and DNAH9 in respiratory cells from patients with primary ciliary dyskinesia. Am. J. Respir. Crit. Care Med. 2005, 171, 1343–1349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwabe, G.C.; Hoffmann, K.; Loges, N.T.; Birker, D.; Rossier, C.; de Santi, M.M.; Olbrich, H.; Fliegauf, M.; Failly, M.; Liebers, U.; et al. Primary ciliary dyskinesia associated with normal axoneme ultrastructure is caused by DNAH11 mutations. Hum. Mutat. 2008, 29, 289–298. [Google Scholar] [CrossRef]
- Wang, X.; Jin, H.; Han, F.; Cui, Y.; Chen, J.; Yang, C.; Zhu, P.; Jiao, G.; Wang, W.; Hao, C.; et al. Homozygous DNAH1 frameshift mutation causes multiple morphological anomalies of the sperm flagella in Chinese. Clin. Genet. 2016, 91, 313–321. [Google Scholar] [CrossRef] [Green Version]
- Amiri-Yekta, A.; Coutton, C.; Kherraf, Z.E.; Karaouzene, T.; Le Tanno, P.; Sanati, M.H.; Sabbaghian, M.; Almadani, N.; Sadighi Gilani, M.A.; Hosseini, S.H.; et al. Whole-exome sequencing of familial cases of multiple morphological abnormalities of the sperm flagella (MMAF) reveals new DNAH1 mutations. Hum. Reprod. 2016, 31, 2872–2880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imtiaz, F.; Allam, R.; Ramzan, K.; Al-Sayed, M. Variation in DNAH1 may contribute to primary ciliary dyskinesia. BMC Med. Genet. 2015, 16, 14. [Google Scholar] [CrossRef] [Green Version]
- Ben Khelifa, M.; Coutton, C.; Zouari, R.; Karaouzène, T.; Rendu, J.; Bidart, M.; Yassine, S.; Pierre, V.; Delaroche, J.; Hennebicq, S.; et al. Mutations in DNAH1, which encodes an inner arm heavy chain dynein, lead to male infertility from multiple morphological abnormalities of the sperm flagella. Am. J. Hum. Genet. 2014, 94, 95–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Sha, Y.; Wang, X.; Ding, L.; Liu, W.; Ji, Z.; Mei, L.; Huang, X.; Lin, S.; Kong, S.; et al. DNAH2 is a novel candidate gene associated with multiple morphological abnormalities of the sperm flagella. Clin. Genet. 2019, 95, 590–600. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Miyata, H.; Gao, Y.; Sha, Y.; Tang, S.; Xu, Z.; Whitfield, M.; Patrat, C.; Wu, H.; Dulioust, E.; et al. Bi-Allelic DNAH8 variants lead to multiple morphological abnormalities of the sperm flagella and primary male infertility. Am. J. Hum. Genet. 2020, 107, 330–341. [Google Scholar] [CrossRef]
- Yang, Y.; Jiang, C.; Zhang, X.; Liu, X.; Li, J.; Qiao, X.; Liu, H.; Shen, Y. Loss-of-function mutation in DNAH8 induces asthenoteratospermia associated with multiple morphological abnormalities of the sperm flagella. Clin. Genet. 2020, 98, 396–401. [Google Scholar] [CrossRef]
- Zhou, Z.; Mao, X.; Chen, B.; Mu, J.; Wang, W.; Li, B.; Yan, Z.; Dong, J.; Li, Q.; Kuang, Y.; et al. A novel splicing variant in DNAH8 causes asthenozoospermia. J. Assist. Reprod. Genet. 2021, 1545–1550. [Google Scholar] [CrossRef] [PubMed]
- Weng, M.; Sha, Y.; Zeng, Y.U.; Huang, N.; Liu, W.; Zhang, X.; Zhou, H. Mutations in DNAH8 contribute to multiple morphological abnormalities of sperm flagella and male infertility. Acta Biochim. Biophys. Sin. 2021, 53, 472–480. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Ma, H.; Khan, T.; Ma, A.; Li, T.; Zhang, H.; Gao, J.; Zhou, J.; Li, Y.; Yu, C.; et al. A DNAH17 missense variant causes flagella destabilization and asthenozoospermia. J. Exp. Med. 2020, 217, e20182365. [Google Scholar] [CrossRef] [Green Version]
- Sha, Y.; Wei, X.; Ding, L.; Mei, L.; Huang, X.; Lin, S.; Su, Z.; Kong, L.; Zhang, Y.; Ji, Z. DNAH17 is associated with asthenozoospermia and multiple morphological abnormalities of sperm flagella. Ann. Hum. Genet. 2019, 84, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Khan, I.; Liu, C.; Ma, A.; Khan, A.; Zhang, Y.; Zhang, H.; Kakakhel, M.B.S.; Zhou, J.; Zhang, W.; et al. Novel loss-of-function variants in DNAH17 cause multiple morphological abnormalities of the sperm flagella in humans and mice. Clin. Genet. 2020, 99, 176–186. [Google Scholar] [CrossRef]
- Tang, D.; Sha, Y.; Gao, Y.; Zhang, J.; Cheng, H.; Zhang, J.; Ni, X.; Wang, C.; Xu, C.; Geng, H.; et al. Novel variants in DNAH9 lead to nonsyndromic severe asthenozoospermia. Reprod. Biol. Endocrinol. 2021, 19, 27. [Google Scholar] [CrossRef]
- Fassad, M.R.; Shoemark, A.; Legendre, M.; Hirst, R.A.; Koll, F.; le Borgne, P.; Louis, B.; Daudvohra, F.; Patel, M.P.; Thomas, L.; et al. Mutations in outer dynein arm heavy chain DNAH9 cause motile cilia defects and situs inversus. Am. J. Hum. Genet. 2018, 103, 984–994. [Google Scholar] [CrossRef] [Green Version]
- Loges, N.T.; Antony, D.; Maver, A.; Deardorff, M.A.; Gulec, E.Y.; Gezdirici, A.; Nothe-Menchen, T.; Hoben, I.M.; Jelten, L.; Frank, D.; et al. Recessive DNAH9 Loss-of-Function mutations cause laterality defects and subtle respiratory ciliary-beating defects. Am. J. Hum. Genet. 2018, 103, 995–1008. [Google Scholar] [CrossRef] [Green Version]
- Terré, B.; Lewis, M.; Gil-Gómez, G.; Han, Z.; Hao, L.; Aguilera, M.; Prats, N.; Roy, S.; Zhao, H.; Stracker, T.H. Defects in efferent duct multiciliogenesis underlie male infertility in GEMC1, MCIDAS or CCNO deficient mice. Development 2019, 146, 162628. [Google Scholar] [CrossRef] [Green Version]
- Yuan, S.; Liu, Y.; Peng, H.; Tang, C.; Hennig, G.W.; Wang, Z.; Wang, L.; Yu, T.; Klukovich, R.; Zhang, Y.; et al. Motile cilia of the male reproductive system require miR-34/miR-449 for development and function to generate luminal turbulence. Proc. Natl. Acad. Sci. USA 2019, 116, 3584–3593. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Chen, L.; Li, J.; Li, H.; Hong, Z.; Xie, M.; Chen, S.; Yao, B. The Semen pH Affects Sperm Motility and Capacitation. PLoS ONE 2015, 10, e0132974. [Google Scholar] [CrossRef] [Green Version]
- Shibahara, H.; Hamada, Y.; Hasegawa, A.; Wakimoto, E.; Toji, H.; Shigeta, M.; Koyama, K. Relationship between the sperm motility index assessed by the sperm quality analyzer and the outcome of intracytoplasmic sperm injection. J. Assist. Reprod. Genet. 1999, 16, 540–545. [Google Scholar] [CrossRef]
- Westlander, G.; Barry, M.; Petrucco, O.; Norman, R. Different fertilization rates between immotile testicular spermatozoa and immotile ejaculated spermatozoa for ICSI in men with Kartagener’s syndrome: Case reports. Hum. Reprod. 2003, 18, 1286–1288. [Google Scholar] [CrossRef]
- Niu, Z.-H.; Huang, X.-F.; Jia, X.-F.; Zheng, J.; Yuan, Y.; Shi, T.-Y.; Diao, H.; Yu, H.-G.; Sun, F.; Zhang, H.-Q.; et al. A sperm viability test using SYBR-14/propidium iodide flow cytometry as a tool for rapid screening of primary ciliary dyskinesia patients and for choosing sperm sources for intracytoplasmic sperm injection. Fertil. Steril. 2011, 95, 389–392. [Google Scholar] [CrossRef]
- Sallam, H.N.; Farrag, A.; Agameya, A.F.; El-Garem, Y.; Ezzeldin, F. The use of the modified hypo-osmotic swelling test for the selection of immotile testicular spermatozoa in patients treated with ICSI: A randomized controlled study. Hum. Reprod. 2005, 20, 3435–3440. [Google Scholar] [CrossRef] [Green Version]
- Nordhoff, V. How to select immotile but viable spermatozoa on the day of intracytoplasmic sperm injection? An embryologist’s view. Andrology 2014, 3, 156–162. [Google Scholar] [CrossRef] [Green Version]
- Kordus, R.J.; Price, R.L.; Davis, J.M.; Whitman-Elia, G.F. Successful twin birth following blastocyst culture of embryos derived from the immotile ejaculated spermatozoa from a patient with primary ciliary dyskinesia: A case report. J. Assist. Reprod. Genet. 2008, 25, 437–443. [Google Scholar] [CrossRef] [Green Version]
- Geber, S.; Lemgruber, M.; Taitson, P.F.; Valle, M.; Sampaio, M. Birth of healthy twins after intracytoplasmic sperm injection using ejaculated immotile spermatozoa from a patient with Kartagener’s syndrome. Andrologia 2011, 44, 842–844. [Google Scholar] [CrossRef]
- Mangoli, V.; Mangoli, R.; Dandekar, S.; Suri, K.; Desai, S. Selection of viable spermatozoa from testicular biopsies: A comparative study between pentoxifylline and hypoosmotic swelling test. Fertil. Steril. 2011, 95, 631–634. [Google Scholar] [CrossRef]
- Yildirim, G.; Ficicioglu, C.; Akçin, O.; Attar, R.; Tecellioglu, N.; Yencilek, F. Can pentoxifylline improve the sperm motion and ICSI success in the primary ciliary dyskinesia? Arch. Gynecol. Obstet. 2008, 279, 213–215. [Google Scholar] [CrossRef]
- De Oliveira, N.M.; Sánchez, R.V.; Fiesta, S.R.; Salgado, T.L.; Rodríguez, R.; Bethencourt, J.C.; Zamora, R.B. Pregnancy with frozen-thawed and fresh testicular biopsy after motile and immotile sperm microinjection, using the mechanical touch technique to assess viability. Hum. Reprod. 2004, 19, 262–265. [Google Scholar] [CrossRef] [Green Version]
- Wu, H.; Wang, J.; Cheng, H.; Gao, Y.; Liu, W.; Zhang, Z.; Jiang, H.; Li, W.; Zhu, F.; Lv, M.; et al. Patients with severe asthenoteratospermia carrying SPAG6 or RSPH3 mutations have a positive pregnancy outcome following intracytoplasmic sperm injection. J. Assist. Reprod. Genet. 2020, 37, 829–840. [Google Scholar] [CrossRef]
- Chen, H.; Feng, G.; Zhang, B.; Zhou, H.; Shu, J.; Gan, X. A successful pregnancy using completely immotile but viable frozen-thawed spermatozoa selected by laser. Clin. Exp. Reprod. Med. 2017, 44, 52–55. [Google Scholar] [CrossRef] [Green Version]
- Gerber, P.A.; Kruse, R.; Hirchenhain, J.; Krussel, J.S.; Neumann, N.J. Pregnancy after laser-assisted selection of viable sper-matozoa before intracytoplasmatic sperm injection in a couple with male primary cilia dyskinesia. Fertil. Steril. 2008, 89, 1826.e9–1826.e12. [Google Scholar] [CrossRef]
- Ozkavukcu, S.; Celik-Ozenci, C.; Konuk, E.; Atabekoğlu, C.S. Live birth after Laser Assisted Viability Assessment (LAVA) to detect pentoxifylline resistant ejaculated immotile spermatozoa during ICSI in a couple with male Kartagener’s syndrome. Reprod. Biol. Endocrinol. 2018, 16, 10. [Google Scholar] [CrossRef] [Green Version]
- Samplaski, M.K.; Dimitromanolakis, A.; Lo, K.C.; Grober, E.D.; Mullen, B.; Garbens, A.; Jarvi, K.A. The relationship between sperm viability and DNA fragmentation rates. Reprod. Biol. Endocrinol. 2015, 13, 42. [Google Scholar] [CrossRef] [Green Version]
- Aydos, O.S.; Yukselten, Y.; Kaplan, F.; Sunguroglu, A.; Aydos, K. Analysis of the correlation between sperm DNA integrity and conventional semen parameters in infertile men. Turk. J. Urol. 2015, 41, 191–197. [Google Scholar] [CrossRef]
- Belloc, S.; Benkhalifa, M.; Cohen-Bacrie, M.; Dalleac, A.; Amar, E.; Zini, A. Sperm deoxyribonucleic acid damage in normo-zoospermic men is related to age and sperm progressive motility. Fertil. Steril. 2014, 101, 1588–1593. [Google Scholar] [CrossRef]
- Derijck, A.A.; van der Heijden, G.W.; Ramos, L.; Giele, M.; Kremer, J.; Ade Boer, P. Motile human normozoospermic and oligozoospermic semen samples show a difference in double-strand DNA break incidence. Hum. Reprod. 2007, 22, 2368–2376. [Google Scholar] [CrossRef] [Green Version]
- Nuñez, R.; López-Fernández, C.; Arroyo, F.; Caballero, P.; Gosalvez, J. Characterization of sperm DNA damage in Kartagener’s syndrome with recurrent fertilization failure: Case revisited. Sex. Reprod. Healthc. 2010, 1, 73–75. [Google Scholar] [CrossRef]
- Da Costa, R.; Redmann, K.; Schlatt, S. Simultaneous detection of sperm membrane integrity and DNA fragmentation by flow cytometry: A novel and rapid tool for sperm analysis. Andrology 2021, 9, 1254–1263. [Google Scholar] [CrossRef] [PubMed]
- Campagne, D.M. Can Male Fertility Be Improved Prior to Assisted Reproduction through The Control of Uncommonly Considered Factors? Int. J. Fertil. Steril. 2013, 6, 214–223. [Google Scholar] [PubMed]
- Loutradi, K.E.; Tarlatzis, B.C.; Goulis, D.G.; Zepiridis, L.; Pagou, T.; Chatziioannou, E.; Grimbizis, G.F.; Papadimas IBontis, I. The effects of sperm quality on embryo development after intracytoplasmic sperm injection. J. Assist. Reprod. Genet. 2006, 23, 69–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borges, E.; Setti, A.S.; Braga, D.P.A.F.; Figueira, R.C.S.; Iaconelli, A. Total motile sperm count has a superior predictive value over the WHO 2010 cut-off values for the outcomes of intracytoplasmic sperm injection cycles. Andrology 2016, 4, 880–886. [Google Scholar] [CrossRef]
- Hotaling, J.M.; Smith, J.F.; Rosen, M.; Muller, C.H.; Walsh, T.J. The relationship between isolated teratozoospermia and clinical pregnancy after in vitro fertilization with or without intracytoplasmic sperm injection: A systematic review and meta-analysis. Fertil. Steril. 2011, 95, 1141–1145. [Google Scholar] [CrossRef] [PubMed]
- De Vos, A.; van De Velde, H.; Joris, H.; Verheyen, G.; Devroey, P.; van Steirteghem, A. Influence of individual sperm mor-phology on fertilization, embryo morphology, and pregnancy outcome of intracytoplasmic sperm injection. Fertil. Steril. 2003, 79, 42–48. [Google Scholar] [CrossRef]
- Magli, M.C.; Gianaroli, L.; Ferraretti, A.P.; Gordts, S.; Fredericks, V.; Crippa, A. Paternal contribution to aneuploidy in pre-implantation embryos. Reprod. Biomed. Online 2009, 18, 536–542. [Google Scholar] [CrossRef]
- Munro, N.C.; Currie, D.C.; Lindsay, K.S.; Ryder, T.A.; Rutman, A.; Dewar, A.; Greenstone, M.A.; Hendry, W.F.; Cole, P.J. Fertility in men with primary ciliary dyskinesia presenting with respiratory infection. Thorax 1994, 49, 684–687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cayan, S.; Conaghan, J.; Schriock, E.D.; Ryan, I.P.; Black, L.D.; Turek, P.J. Birth after intracytoplasmic sperm injection with use of testicular sperm from men with Kartagener/immotile cilia syndrome. Fertil. Steril. 2001, 76, 612–614. [Google Scholar] [CrossRef]
- Vicdan, K.; Akarsu, C.; Vicdan, A.; Sozen, E.; Buluc, B.; Biberoĝlu, K.; Ozogul, C. Birth of a healthy boy using fresh testicular sperm in a patient with Klinefelter syndrome combined with Kartagener syndrome. Fertil. Steril. 2011, 96, 577–579. [Google Scholar] [CrossRef]
- Hattori, H.; Nakajo, Y.; Ito, C.; Toyama, Y.; Toshimori, K.; Kyono, K. Birth of a healthy infant after intracytoplasmic sperm injection using pentoxifylline-activated sperm from a patient with Kartagener’s syndrome. Fertil. Steril. 2011, 95, 2431.e9–2431.e11. [Google Scholar] [CrossRef]
- Ebner, T.; Maurer, M.; Oppelt, P.; Mayer, R.B.; Duba, H.C.; Costamoling, W.; Shebl, O. Healthy twin live-birth after ionophore treatment in a case of theophylline-resistant Kartagener syndrome. J. Assist. Reprod. Genet. 2015, 32, 873–877. [Google Scholar] [CrossRef] [Green Version]
- Schlegel, P.N. Testicular sperm extraction: Microdissection improves sperm yield with minimal tissue excision. Hum. Reprod. 1999, 14, 131–135. [Google Scholar] [CrossRef]
- Deruyver, Y.; Vanderschueren, D.; van Der Aa, F. Outcome of microdissection TESE compared with conventional TESE in non-obstructive azoospermia: A systematic review. Andrology 2013, 2, 20–24. [Google Scholar] [CrossRef]
- Bernie, A.M.; Mata, D.A.; Ramasamy, R.; Schlegel, P.N. Comparison of microdissection testicular sperm extraction, conven-tional testicular sperm extraction, and testicular sperm aspiration for nonobstructive azoospermia: A systematic review and meta-analysis. Fertil. Steril. 2015, 104, 1099–1103.e3. [Google Scholar] [CrossRef] [PubMed]
- Coutton, C.; Escoffier, J.; Martinez, G.; Arnoult, C.; Ray, P.F. Teratozoospermia: Spotlight on the main genetic actors in the human. Hum. Reprod. Update 2015, 21, 455–485. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, V.; Rives, N.; Albert, M.; Peers, M.C.; Selva, J.; Clavier, B.; Escudier, E.; Escalier, D. Outcome of ICSI with ejaculated spermatozoa in a series of men with distinct ultrastructural flagellar abnormalities. Hum. Reprod. 2006, 21, 2065–2074. [Google Scholar] [CrossRef] [Green Version]
- Fauque, P.; Albert, M.; Serres, C.; Viallon, V.; Davy, C.; Epelboin, S.; Chalas, C.; Jouannet, P.; Patrat, C. From ultrastructural flagellar sperm defects to the health of babies conceived by ICSI. Reprod. Biomed. Online 2009, 19, 326–336. [Google Scholar] [CrossRef]
- Terada, Y.; Schatten, G.; Hasegawa, H.; Yaegashi, N. Essential roles of the sperm centrosome in human fertilization: Developing the therapy for fertilization failure due to sperm centrosomal dysfunction. Tohoku J. Exp. Med. 2010, 220, 247–258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaushal, M.; Baxi, A. Birth after intracytoplasmic sperm injection with use of testicular sperm from men with Kartagener or immotile cilia syndrome. Fertil. Steril. 2007, 88, 497.e9–497.e11. [Google Scholar] [CrossRef] [PubMed]
- Aktan, T.M.; Montag, M.; Duman, S.; Gorkemli, H.; Rink, K.; Yurdakul, T. Use of a laser to detect viable but immotile sper-matozoa. Andrologia 2004, 36, 366–369. [Google Scholar] [CrossRef]
- Garza, S.D.; Patrizio, P. Reproductive outcomes in patients with male infertility because of Klinefelter’s syndrome, Kartagener’s syndrome, round-head sperm, dysplasia fibrous sheath, and ‘stump’ tail sperm. Curr. Opin. Obstet. Gynecol. 2013, 25, 229–246. [Google Scholar] [CrossRef]
- Vodistincn Zumbusch, A.; Fiedler, K.; Mayerhofer, A.; Jessberger, B.; Ring, J.; Vogt, H.J. Birth of healthy children after intracytoplasmic sperm injection in two couples with male Kartagener’s syndrome. Fertil. Steril. 1998, 70, 643–646. [Google Scholar] [CrossRef]
- Kay, V.; Irvine, D. Successful in-vitro fertilization pregnancy with spermatozoa from a patient with Kartagener’s syndrome: Case Report. Hum. Reprod. 2000, 15, 135–138. [Google Scholar] [CrossRef]
- Matsumoto, Y.; Goto, S.; Hashimoto, H.; Kokeguchi, S.; Shiotani, M.; Okada, H. A healthy birth after intracytoplasmic sperm injection using ejaculated spermatozoa from a patient with Kartagener’s syndrome. Fertil. Steril. 2010, 93, 2074.e17–2074.e19. [Google Scholar] [CrossRef]
- McLachlan, R.I.; Ishikawa, T.; Osianlis, T.; Robinson, P.; Merriner, D.J.; Healy, D.; de Kretser, D.; O’Bryan, M.K. Normal live birth after testicular sperm extraction and intracytoplasmic sperm injection in variant primary ciliary dyskinesia with completely immotile sperm and structurally abnormal sperm tails. Fertil. Steril. 2012, 97, 313–318. [Google Scholar] [CrossRef]
- Montjean, D.; Courageot, J.; Altié, A.; Amar-Hoffet, A.; Rossin, B.; Geoffroy-Siraudin, C.; Tourame, P.; Boyer, P. Normal live birth after vitrified/warmed oocytes intracytoplasmic sperm injection with immotile spermatozoa in a patient with Kartagener’s syndrome. Andrologia 2014, 47, 839–845. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, C.; Zhang, X.; Liu, M.; Sun, Y.; Yang, Y.; Shen, Y. The effect of a novel LRRC6 mutation on the flagellar ultrastructure in a primary ciliary dyskinesia patient. J. Assist. Reprod. Genet. 2021, 38, 689–696. [Google Scholar] [CrossRef] [PubMed]
- Sha, Y.; Wei, X.; Ding, L.; Ji, Z.; Mei, L.; Huang, X.; Su, Z.; Wang, W.; Zhang, X.; Lin, S. Biallelic mutations of CFAP74 may cause human primary ciliary dyskinesia and MMAF phenotype. J. Hum. Genet. 2020, 65, 961–969. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tu, C.; Nie, H.; Meng, L.; Li, D.; Wang, W.; Zhang, H.; Lu, G.; Lin, G.; Tan, Y.-Q.; et al. Novel DNAAF6 variants identified by whole-exome sequencing cause male infertility and primary ciliary dyskinesia. J. Assist. Reprod. Genet. 2020, 37, 811–820. [Google Scholar] [CrossRef] [PubMed]
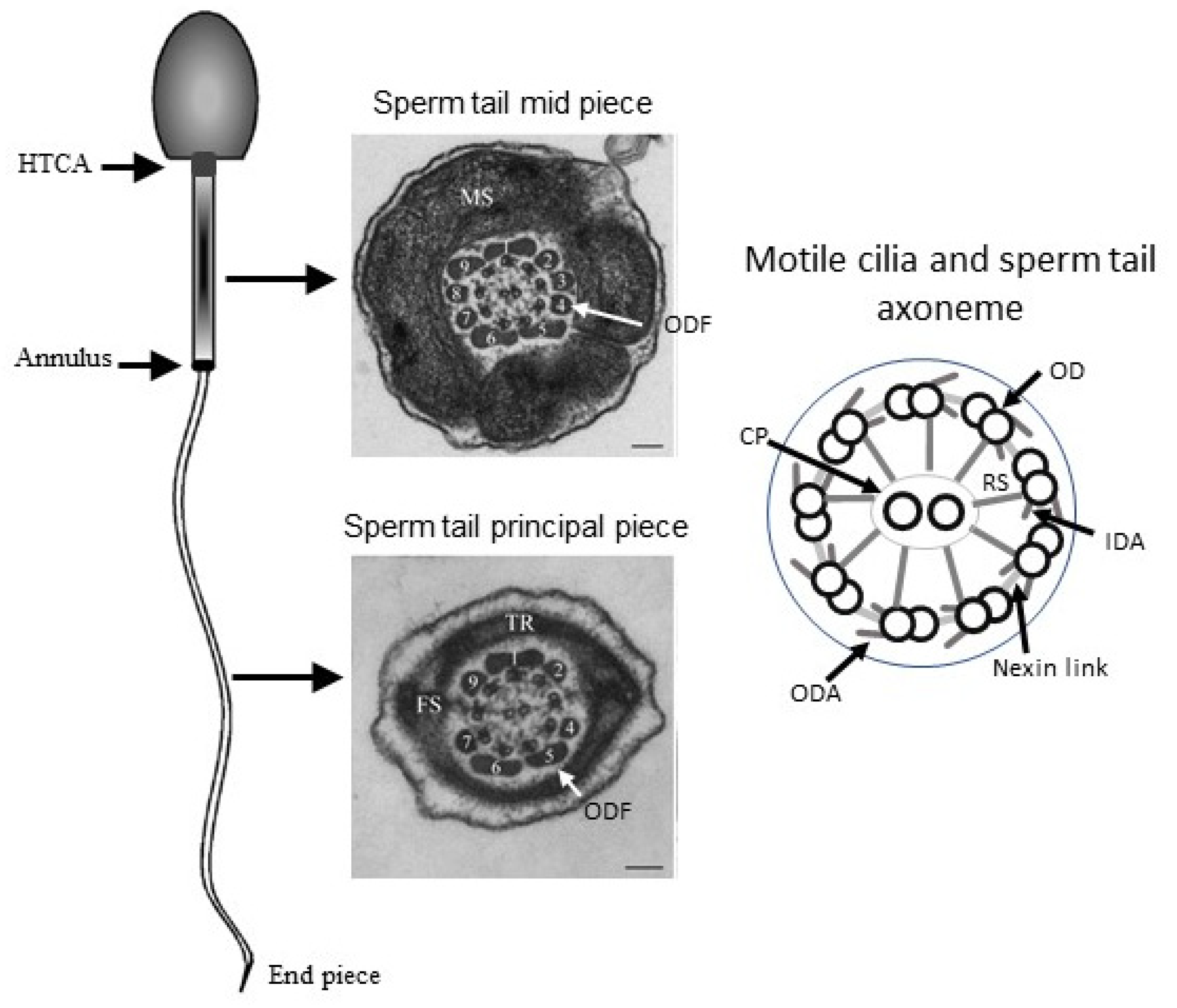
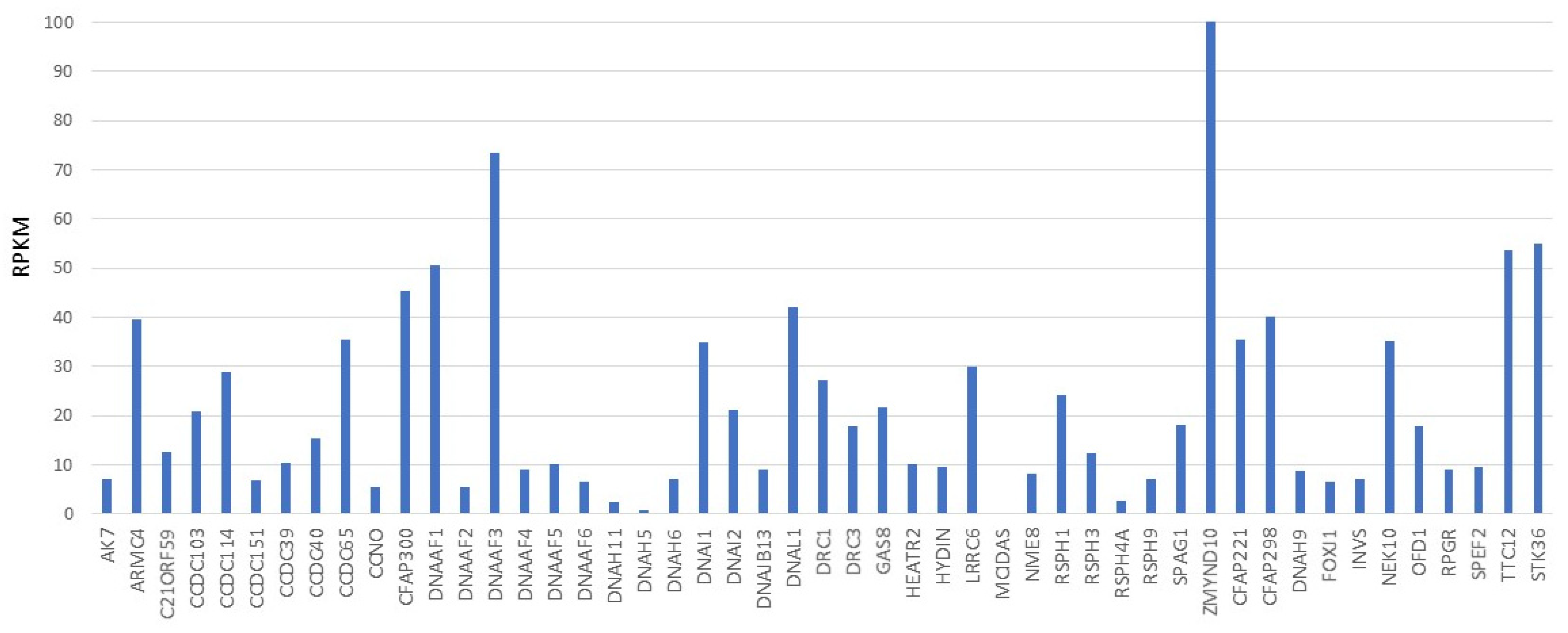
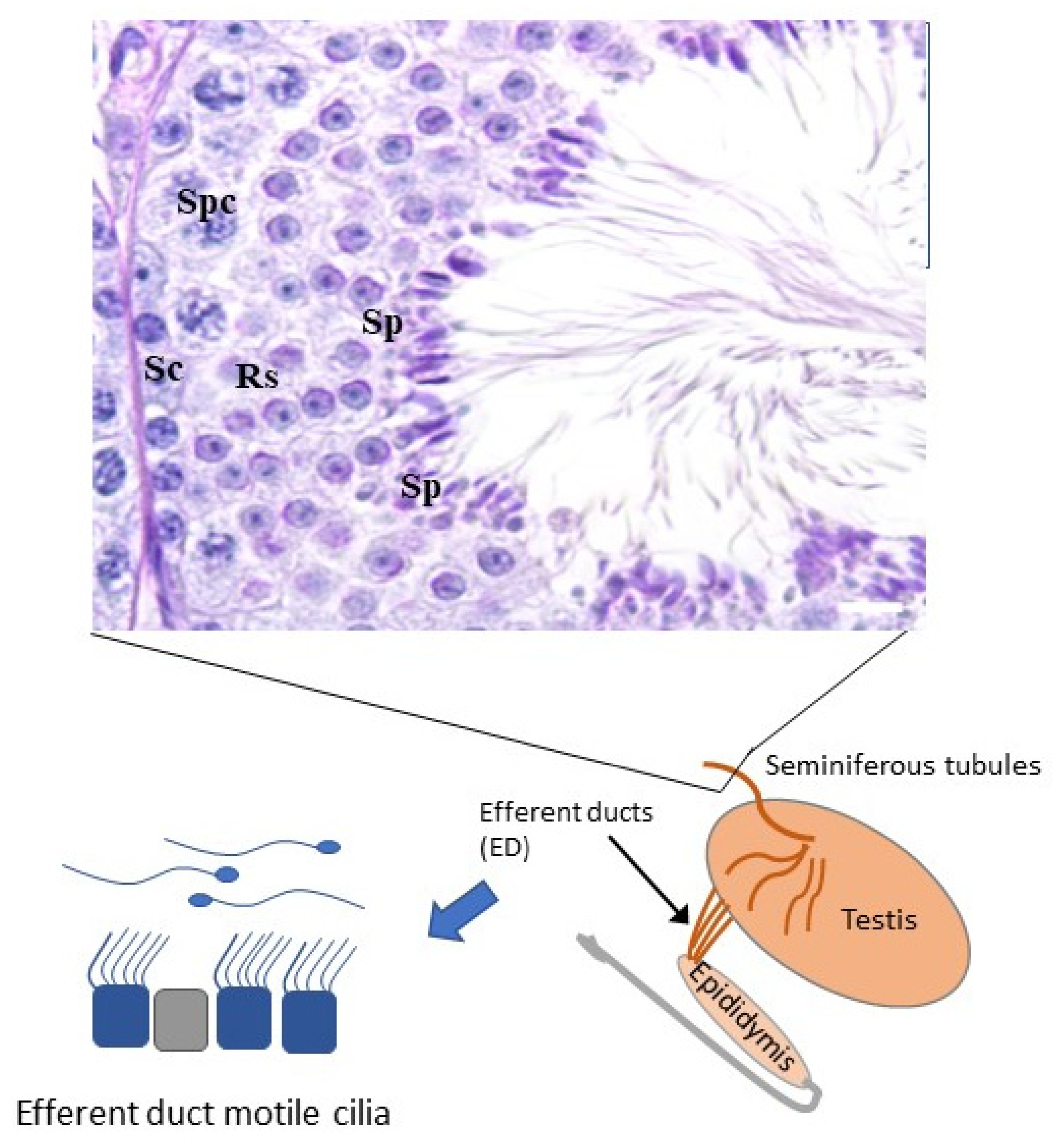

| Defect | Sperm Phenotype |
|---|---|
| Normozoospermia | Normal ejaculate and semen parameters |
| Azoospermia | No sperm in the ejaculate |
| Oligozoospermia | Sperm concentration <15 million/mL |
| Asthenozoospermia | Sperm motility <40% |
| Teratozoospermia | Normal morphology <4% |
| Oligoasthenozoospermia | Sperm concentration <15 million/mL |
| Sperm motility <40% | |
| Oligoteratozoospermia | Sperm concentration <15 million/mL |
| Normal morphology <4% | |
| Asthenoteratozoospermia | Sperm motility <40% |
| Normal morphology <4% | |
| Oligoasthenoteratozoospermia | Sperm concentration <15 million/mL |
| Sperm motility <40% | |
| Normal morphology <4% |
| WHO Reference Range | |
|---|---|
| Total sperm count in ejaculate | 39–928 million |
| Ejaculate volume | 1.5–7.6 mL |
| Sperm concentration | 15–259 million per mL |
| Total motility (progressive and non-progressive) | 40–81 percent |
| Progressive motility | 32–75 percent |
| Sperm morphology | 4–48 percent |
| Gene | ICSI Result | Sperm Origin | Reported Sperm Phenotype | Viability % | Sperm Count | Reference |
|---|---|---|---|---|---|---|
| - | live birth | ejaculated sperm | immotile | 90 | normal | [97] |
| - | live birth | ejaculated sperm, swim-up | 25% motility | nd | normal | [98] |
| - | live birth | testicular sperm | azoospermia | nd | 0 | [83] |
| - | live birth | testicular sperm | immotile | <5% | low (4.8) | [83] |
| - | live birth | testicular sperm | immotile, severe oligospermia | nd | extremely low | [57] |
| - | pregnancy | testicular sperm | immotile | >65 | normal | [57] |
| - | live birth | testicular sperm | immotile, malformed | nd | normal | [94] |
| - | live birth | viable ejaculated sperm | immotile, dynein arm defect | 40 | normal | [61] |
| - | live birth | ejaculated sperm, swim-up | 0.3% motile, lack of dynein arms | 30 | normal | [99] |
| - | no pregnancy | viable ejaculated and testicular | 0.3% motile, 76.4% DNA fragmentation | 30 | low (1.2) | [74] |
| - | live birth | viable ejaculated sperm | immotile, dynein arm defect | 54 | low (0.9) | [85] |
| - | live birth | testicular sperm | azoospermia | nd | 0 | [84] |
| - | live birth | viable ejaculated sperm | immotile | nd | normal | [62] |
| - | live birth | testicular sperm | immotile, malformed | 20 | low (10.1) | [100] |
| - | live birth | viable ejaculated sperm, | immotile, abnormal morphology | 32 | low (1.8) | [86] |
| - | live birth | viable ejaculated sperm | immotile | 60–74 | normal | [101] |
| - | no pregnancy | viable ejaculated sperm | immotile | 35–65 | normal | [101] |
| ZMYND10 | healthy baby | viable ejaculated sperm | immotile, oligoasthenoteratozoospermia, lack of dynein arms | 54 | low (2–9) | [69] |
| CFAP74 | healthy baby | ejaculated sperm | low motility (2%), malformed | nd | normal | [103] |
| DNAAF6 | healthy baby | viable ejaculated sperm | immotile | nd | low (5.2–11.2) | [104] |
| DNAAF7 | healthy baby | viable ejaculated sperm | low motility (8.6%) | nd | low (3.5–8.7) | [104] |
| SPAG6 | healthy baby | viable ejaculated sperm | immotile, malformed | nd | normal | [66] |
| RSPH3 | healthy baby | viable ejaculated sperm | immotile, malformed | nd | normal | [66] |
| DNAH9 | clinical pregnancy | motile ejaculated sperm | very low motility (0.3–0.8% motile) | 75–82 | normal | [50] |
| LRRC6 | clinical pregnancy | testicular sperm | immotile, axoneme defects | 75 | normal | [102] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jayasena, C.N.; Sironen, A. Diagnostics and Management of Male Infertility in Primary Ciliary Dyskinesia. Diagnostics 2021, 11, 1550. https://doi.org/10.3390/diagnostics11091550
Jayasena CN, Sironen A. Diagnostics and Management of Male Infertility in Primary Ciliary Dyskinesia. Diagnostics. 2021; 11(9):1550. https://doi.org/10.3390/diagnostics11091550
Chicago/Turabian StyleJayasena, Channa N., and Anu Sironen. 2021. "Diagnostics and Management of Male Infertility in Primary Ciliary Dyskinesia" Diagnostics 11, no. 9: 1550. https://doi.org/10.3390/diagnostics11091550
APA StyleJayasena, C. N., & Sironen, A. (2021). Diagnostics and Management of Male Infertility in Primary Ciliary Dyskinesia. Diagnostics, 11(9), 1550. https://doi.org/10.3390/diagnostics11091550






