ICU-Admission Hyperphosphataemia Is Related to Shock and Tissue Damage, Indicating Injury Severity and Mortality in Polytrauma Patients
Abstract
1. Introduction
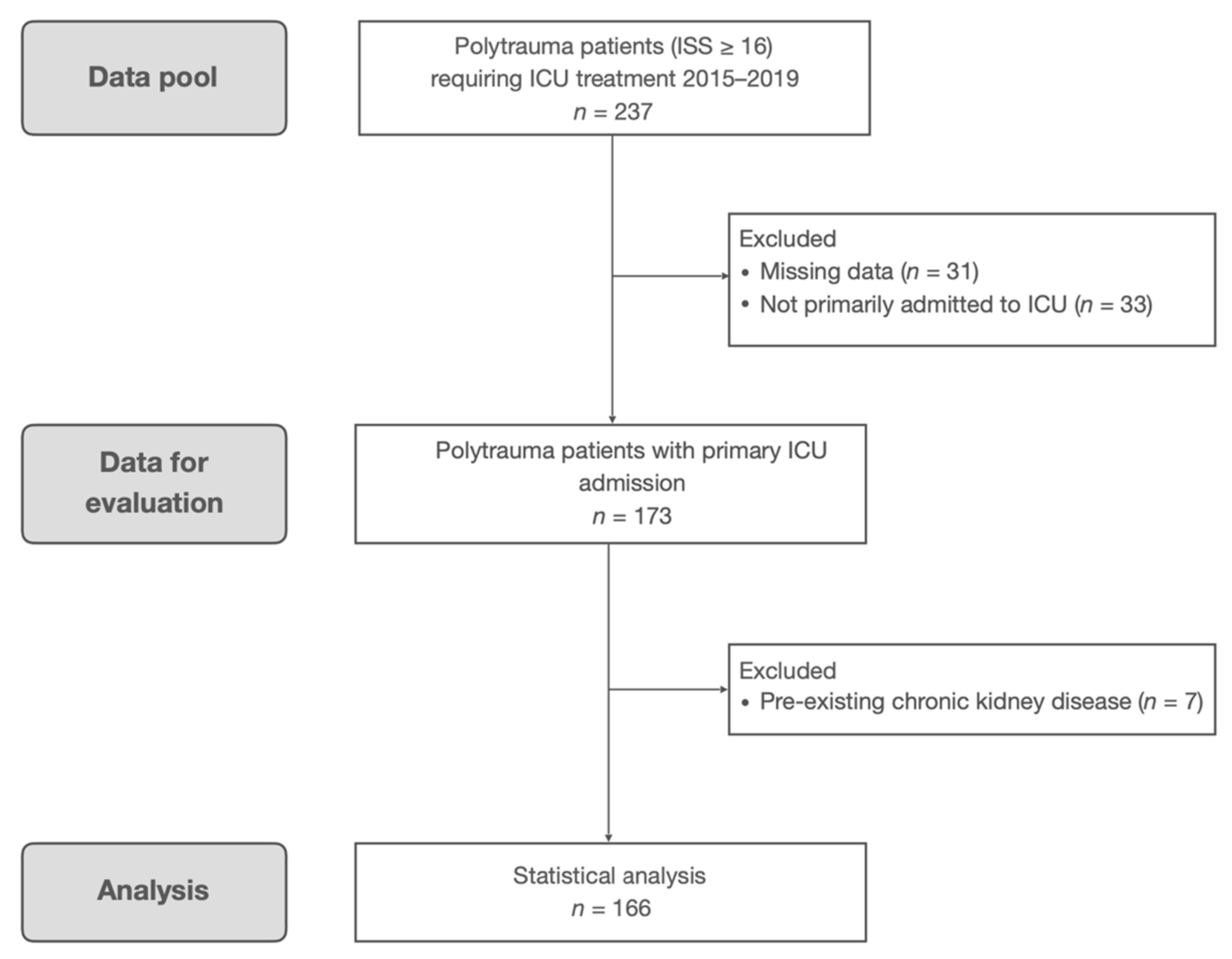
2. Materials and Methods
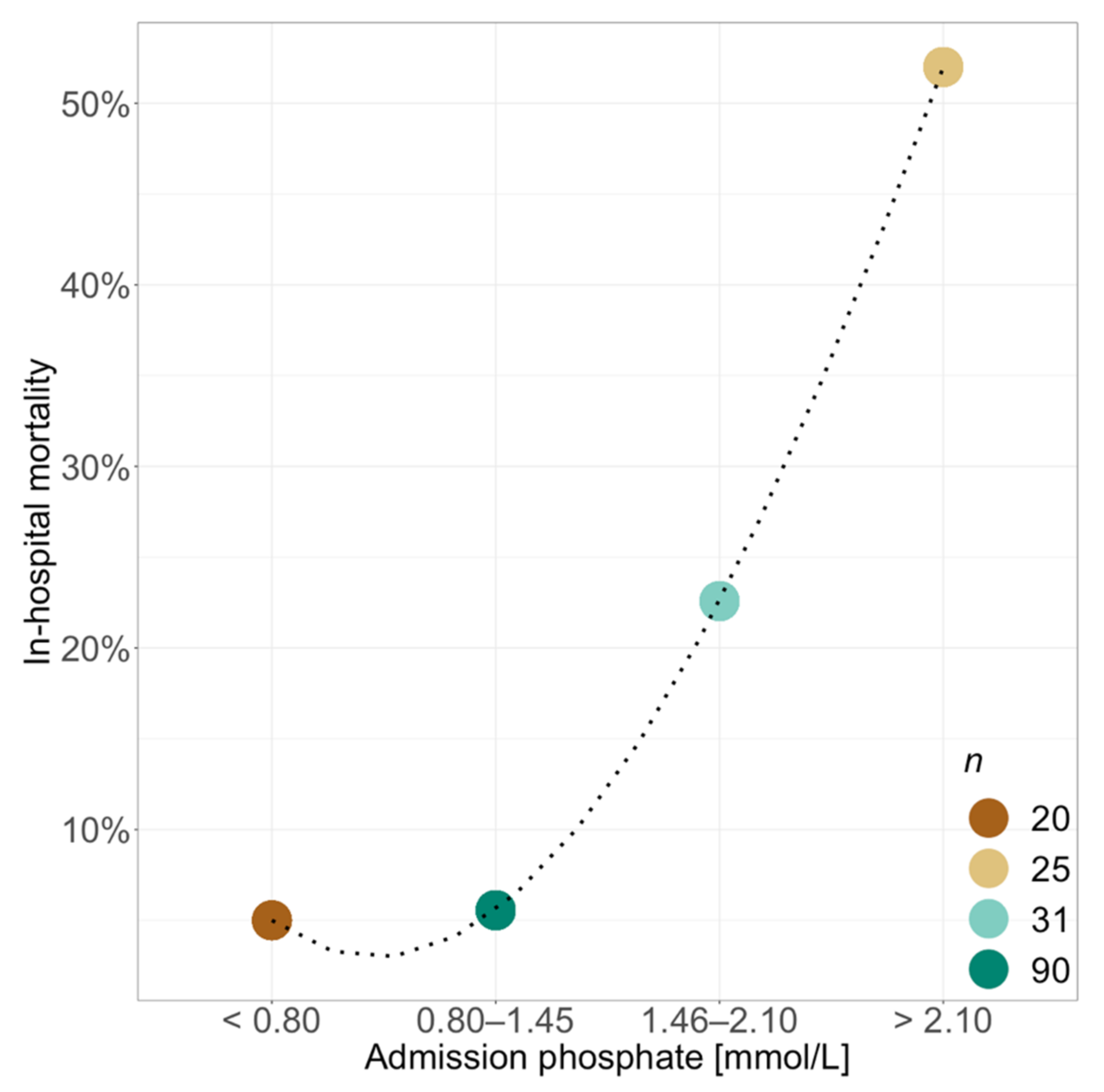
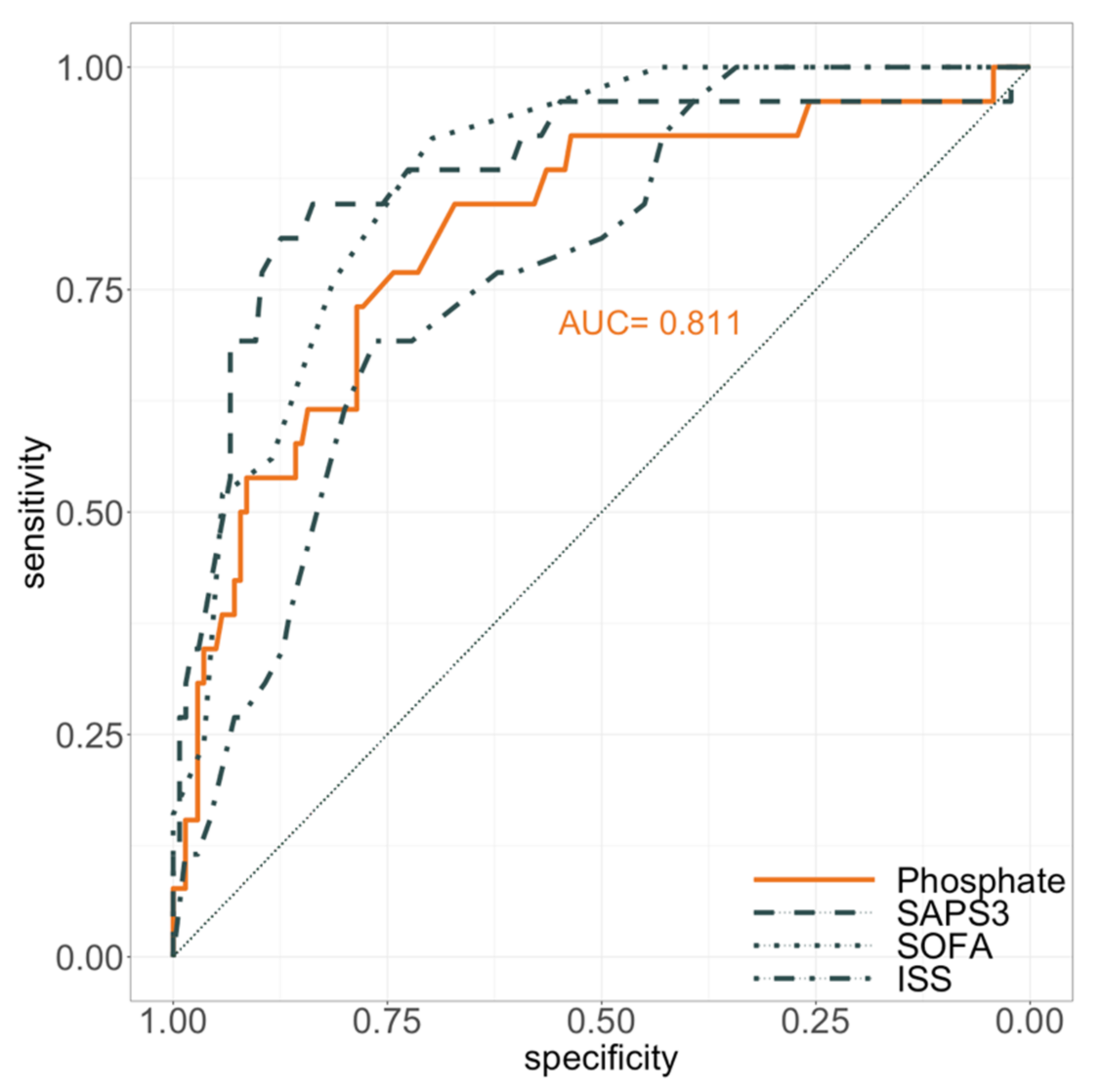
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Penido, M.G.M.G.; Alon, U.S. Phosphate Homeostasis and Its Role in Bone Health. Pediatr. Nephrol. 2012, 27, 2039–2048. [Google Scholar] [CrossRef] [PubMed]
- Bowen, J.W.; Levinson, C. Phosphate Concentration and Transport in Ehrlich Ascites Tumor Cells: Effect of Sodium. J. Cell. Physiol. 1982, 110, 149–154. [Google Scholar] [CrossRef]
- Knochel, J.P.; Haller, R.; Ferguson, E. Phosphate and Minerals in Health and Disease. Adv. Exp. Med. Biol. 1980, 323–334. [Google Scholar] [CrossRef]
- Noorwali, A.; Preston, C.J.; Challa, A.; Russell, R.G.G. Regulation of Phosphate and Mineral Metabolism. Adv. Exp. Med. Biol. 1982, 137–146. [Google Scholar] [CrossRef]
- Saks, V.A.; Strumia, E. Phosphocreatine: Molecular and Cellular Aspects of the Mechanism of Cardioprotective Action. Curr. Ther. Res. 1993, 53, 565–598. [Google Scholar] [CrossRef]
- Lederer, E. Regulation of Serum Phosphate. J. Physiol. 2014, 592, 3985–3995. [Google Scholar] [CrossRef]
- Komaba, H.; Fukagawa, M. Phosphate—A Poison for Humans? Kidney Int. 2016, 90, 753–763. [Google Scholar] [CrossRef]
- Weisinger, J.R.; Bellorín-Font, E. Magnesium and Phosphorus. Lancet 1998, 352, 391–396. [Google Scholar] [CrossRef]
- Hems, D.A.; Brosnan, J.T. Effects of Ischaemia on Content of Metabolites in Rat Liver and Kidney in Vivo. Biochem. J. 1970, 120, 105–111. [Google Scholar] [CrossRef][Green Version]
- O’Connor, L.R.; Klein, K.L.; Bethune, J.E. Hyperphosphatemia in Lactic Acidosis. N. Engl. J. Med. 1977, 297, 707–709. [Google Scholar] [CrossRef] [PubMed]
- Kebler, R.; McDonald, F.D.; Cadnapaphornchai, P. Dynamic Changes in Serum Phosphorus Levels in Diabetic Ketoacidosis. Am. J. Med. 1985, 79, 571–576. [Google Scholar] [CrossRef]
- Tranquada, R.E.; Grant, W.J.; Peterson, C.R. Lactic Acidosis. Arch. Intern. Med. 1966, 117, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Sternbach, G.L.; Varon, J. Severe Hyperphosphatemia Associated with Hemorrhagic Shock. Am. J. Emerg. Med. 1992, 10, 331–332. [Google Scholar] [CrossRef]
- Heine, G.H.; Nangaku, M.; Fliser, D. Calcium and Phosphate Impact Cardiovascular Risk. Eur. Heart J. 2013, 34, 1112–1121. [Google Scholar] [CrossRef]
- Lau, W.L.; Festing, M.; Giachelli, C. Phosphate and Vascular Calcification: Emerging Role of the Sodium-Dependent Phosphate Co-Transporter PiT-1. Thromb. Haemost. 2010, 104, 464–470. [Google Scholar] [CrossRef]
- Cozzolino, M.; Ciceri, P.; Galassi, A. Hyperphosphatemia: A Novel Risk Factor for Mortality in Chronic Kidney Disease. Ann. Transl. Med. 2019, 7, 55. [Google Scholar] [CrossRef]
- Askar, A.M. Hyperphosphatemia. Saudi Med. J. 2015, 36, 13–19. [Google Scholar] [CrossRef]
- Block, G.; Hulbert-Shearon, T.; Levin, N.; Port, F. Association of Serum Phosphorus and Calcium x Phosphate Product with Mortality Risk in Chronic Hemodialysis Patients: A National Study. Am. J. Kidney Dis. 1998, 31, 607–617. [Google Scholar] [CrossRef]
- Thongprayoon, C.; Cheungpasitporn, W.; Mao, M.A.; Sakhuja, A.; Erickson, S.B. Admission Hyperphosphatemia Increases the Risk of Acute Kidney Injury in Hospitalized Patients. J. Nephrol. 2018, 31, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Thongprayoon, C.; Cheungpasitporn, W.; Chewcharat, A.; Mao, M.A.; Thirunavukkarasu, S.; Kashani, K.B. Admission Serum Phosphate Levels and the Risk of Respiratory Failure. Int. J. Clin. Pract. 2020, 74, e13461. [Google Scholar] [CrossRef]
- Cheungpasitporn, W.; Thongprayoon, C.; Mao, M.A.; Kittanamongkolchai, W.; Sakhuja, A.; Erickson, S.B. Admission Serum Phosphate Levels Predict Hospital Mortality. Hosp. Pract. 2018, 46, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Cheungpasitporn, W.; Thongprayoon, C.; Hansrivijit, P.; Medaura, J.; Chewcharat, A.; Bathini, T.; Mao, M.; Erickson, S. Impact of Admission Calcium-Phosphate Product on 1-Year Mortality among Hospitalized Patients. Adv. Biomed. Res. 2020, 9, 14. [Google Scholar] [CrossRef] [PubMed]
- Naffaa, M.E.; Mustafa, M.; Azzam, M.; Nasser, R.; Andria, N.; Azzam, Z.S.; Braun, E. Serum Inorganic Phosphorus Levels Predict 30-Day Mortality in Patients with Community Acquired Pneumonia. BMC Infect. Dis. 2015, 15, 332. [Google Scholar] [CrossRef]
- Kuo, G.; Lee, C.-C.; Yang, S.-Y.; Hsiao, Y.-C.; Chuang, S.-S.; Chang, S.-W.; Tu, K.-H.; Fan, P.-C.; Tian, Y.-C.; Chen, Y.-C.; et al. Hyperphosphatemia Is Associated with High Mortality in Severe Burns. PLoS ONE 2018, 13, e0190978. [Google Scholar] [CrossRef]
- Haider, D.G.; Lindner, G.; Wolzt, M.; Ahmad, S.S.; Sauter, T.; Leichtle, A.B.; Fiedler, G.-M.; Fuhrmann, V.; Exadaktylos, A.K. Hyperphosphatemia Is an Independent Risk Factor for Mortality in Critically Ill Patients: Results from a Cross-Sectional Study. PLoS ONE 2015, 10, e0133426. [Google Scholar] [CrossRef]
- Metnitz, P.G.; Moreno, R.P.; Almeida, E.; Jordan, B.; Bauer, P.; Campos, R.; Iapichino, G.; Edbrooke, D.; Capuzzo, M.; Gall, J.-R. SAPS 3—From Evaluation of the Patient to Evaluation of the Intensive Care Unit. Part 1: Objectives, Methods and Cohort Description. Intensive Care Med. 2005, 31, 1336–1344. [Google Scholar] [CrossRef] [PubMed]
- Moreno, R.P.; Metnitz, P.G.H.; Almeida, E.; Jordan, B.; Bauer, P.; Campos, R.A.; Iapichino, G.; Edbrooke, D.; Capuzzo, M.; Gall, J.-R.L. SAPS 3—From Evaluation of the Patient to Evaluation of the Intensive Care Unit. Part 2: Development of a Prognostic Model for Hospital Mortality at ICU Admission. Intensive Care Med. 2005, 31, 1345–1355. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.-L.; Moreno, R.; Takala, J.; Willatts, S.; Mendonça, A.D.; Bruining, H.; Reinhart, C.K.; Suter, P.M.; Thijs, L.G. The SOFA (Sepsis-Related Organ Failure Assessment) Score to Describe Organ Dysfunction/Failure. Intensive Care Med. 1996, 22, 707–710. [Google Scholar] [CrossRef]
- Vincent, J.-L.; de Mendonca, A.; Cantraine, F.; Moreno, R.; Takala, J.; Suter, P.M.; Sprung, C.L.; Colardyn, F.; Blecher, S. Use of the SOFA Score to Assess the Incidence of Organ Dysfunction/Failure in Intensive Care Units. Crit. Care Med. 1998, 26, 1793–1800. [Google Scholar] [CrossRef]
- Hruska, K.A.; Mathew, S.; Lund, R.; Qiu, P.; Pratt, R. Hyperphosphatemia of Chronic Kidney Disease. Kidney Int. 2008, 74, 148–157. [Google Scholar] [CrossRef]
- Foley, R.N.; Collins, A.J.; Ishani, A.; Kalra, P.A. Calcium-Phosphate Levels and Cardiovascular Disease in Community-Dwelling Adults: The Atherosclerosis Risk in Communities (ARIC) Study. Am. Heart J. 2008, 156, 556–563. [Google Scholar] [CrossRef] [PubMed]
- Foley, R.N.; Collins, A.J.; Herzog, C.A.; Ishani, A.; Kalra, P.A. Serum Phosphorus Levels Associate with Coronary Atherosclerosis in Young Adults. J. Am. Soc. Nephrol. 2009, 20, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Ellam, T.J.; Chico, T.J.A. Phosphate: The New Cholesterol? The Role of the Phosphate Axis in Non-Uremic Vascular Disease. Atherosclerosis 2012, 220, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Opie, L.H.; Thomas, M.; Owen, P.; Shulman, G. Increased Coronary Venous Inorganic Phosphate Concentrations during Experimental Myocardial Ischemia. Am. J. Cardiol. 1972, 30, 503–513. [Google Scholar] [CrossRef]
- Kreutziger, J.; Wenzel, V.; Kurz, A.; Constantinescu, M.A. Admission Blood Glucose Is an Independent Predictive Factor for Hospital Mortality in Polytraumatised Patients. Intensive Care Med. 2009, 35, 1234–1239. [Google Scholar] [CrossRef]
- Kreutziger, J.; Lederer, W.; Schmid, S.; Ulmer, H.; Wenzel, V.; Nijsten, M.W.; Werner, D.; Schlechtriemen, T. Blood Glucose Concentrations in Prehospital Trauma Patients with Traumatic Shock. Eur. J. Anaesthesiol. 2018, 35, 33–42. [Google Scholar] [CrossRef]
- Kreutziger, J.; Rafetseder, A.; Mathis, S.; Wenzel, V.; Attal, R.E.; Schmid, S. Admission Blood Glucose Predicted Haemorrhagic Shock in Multiple Trauma Patients. Injury 2015, 46, 15–20. [Google Scholar] [CrossRef]
- Innerhofer, P.; Fries, D.; Mittermayr, M.; Innerhofer, N.; von Langen, D.; Hell, T.; Gruber, G.; Schmid, S.; Friesenecker, B.; Lorenz, I.H.; et al. Reversal of Trauma-Induced Coagulopathy Using First-Line Coagulation Factor Concentrates or Fresh Frozen Plasma (RETIC): A Single-Centre, Parallel-Group, Open-Label, Randomised Trial. Lancet Haematol. 2017, 4, e258–e271. [Google Scholar] [CrossRef]
- Spahn, D.R.; Bouillon, B.; Cerny, V.; Duranteau, J.; Filipescu, D.; Hunt, B.J.; Komadina, R.; Maegele, M.; Nardi, G.; Riddez, L.; et al. The European Guideline on Management of Major Bleeding and Coagulopathy Following Trauma: Fifth Edition. Crit. Care 2019, 23, 98. [Google Scholar] [CrossRef]
- Higaki, M.; Tanemoto, M.; Shiraishi, T.; Taniguchi, K.; Fujigaki, Y.; Uchida, S. Acute Kidney Injury Facilitates Hypocalcemia by Exacerbating the Hyperphosphatemic Effect of Muscle Damage in Rhabdomyolysis. Nephron 2015, 131, 11–16. [Google Scholar] [CrossRef]
- Lochy, S.; Jacobs, R.; Honoré, P.; Waele, E.D.; Joannes-Boyau, O.; Regt, J.D.; Gorp, V.V.; Spapen, H. Phosphate Induced Crystal Acute Kidney Injury—An under-Recognized Cause of Acute Kidney Injury Potentially Leading to Chronic Kidney Disease: Case Report and Review of the Literature. Int. J. Nephrol. Renov. Dis. 2013, 6, 61–64. [Google Scholar] [CrossRef]
| All Patients | Admission Phosphate | Admission Phosphate | p | ||
|---|---|---|---|---|---|
| ≤1.45 mmol/L | >1.45 mmol/L | p | |||
| (n = 166) | (n = 110) | (n = 56) | (Bonferroni Corrected) | ||
| n (%) or Median (IQR) | n (%) or Median (IQR) | n (%) or Median (IQR) | |||
| ICU-admission phosphate [mmol/L] | 1.20 (0.92–1.57) | 1.00 (0.86–1.20) | 1.94 (1.57–2.68) | <0.001 | <0.001 |
| Age [yrs] | 47 (31–57) | 47 (33–57) | 46 (28–58) | 0.743 | 1 |
| Sex | |||||
| Male | 129 (77.7) | 90 (81.8) | 39 (69.6) | 0.113 | 1 |
| Female | 37 (22.3) | 20 (18.2) | 17 (30.4) | ||
| BMI [kg/m2] | 24.7 (23.1–26.3) | 24.7 (23.4–26.3) | 25.4 (22.5–26.3) | 0.289 | 1 |
| Comorbidities | |||||
| Any | 61 (36.7) | 41 (37.3) | 20 (35.7) | 0.979 | 1 |
| Arterial hypertension | 25 (15.1) | 17 (15.5) | 8 (14.3) | 1.000 | 1 |
| Cerebrovascular disease | 5 (3.0) | 5 (4.5) | 0 (0) | 0.254 | 1 |
| COPD | 3 (1.8) | 2 (1.8) | 1 (1.8) | 1.000 | 1 |
| Coronary artery disease | 11 (6.6) | 8 (7.3) | 3 (5.4) | 0.889 | 1 |
| Diabetes mellitus type 2 | 5 (3.0) | 4 (3.6) | 1 (1.8) | 0.858 | 1 |
| Heart failure | 7 (4.2) | 4 (3.6) | 3 (5.4) | 0.910 | 1 |
| Chronic kidney disease | 0 (0) | 0 (0) | 0 (0) | 1.000 | 1 |
| Peripheral artery disease | 0 (0) | 0 (0) | 0 (0) | 1.000 | 1 |
| Other | 50 (30.1) | 35 (31.8) | 15 (26.8) | 0.625 | 1 |
| Injury severity score | 29 (22–41) | 26 (22–34) | 38 (30–44) | <0.001 | <0.001 |
| Abbreviated injury scale | |||||
| Head or neck | 2 (0–4) | 2 (0–4) | 2 (0–4) | 0.576 | 1 |
| Face | 0 (0–1) | 0 (0–1) | 0 (0–0) | 0.141 | 1 |
| Chest | 3 (3–4) | 3 (2–4) | 3 (3–4) | 0.081 | 1 |
| Abdomen | 2 (0–3) | 1 (0–2) | 3 (0–4) | <0.001 | 0.002 |
| Extremities/pelvic girdle | 2 (0–3) | 2 (0–3) | 2 (0–3) | 0.796 | 1 |
| External | 0 (0–1) | 0 (0–1) | 0 (0–1) | 0.447 | 1 |
| Body temp. at hospital arrival [°C] | 35.4 (34.8–36.4) | 36.0 (35.3–36.5) | 35.0 (33.4–35.5) | 0.013 | 0.521 |
| SAPS 3 (ICU admission) | 50 (38–63) | 44 (35–56) | 63 (52–71) | <0.001 | <0.001 |
| SOFA Score (ICU admission) | 10 (7–12) | 9 (6–10) | 12 (10–14) | <0.001 | <0.001 |
| Intensive care unit | |||||
| Length of stay [d] | 7 (3–14) | 8 (2–14) | 7 (3–12) | 0.828 | 1 |
| Mortality | 23 (13.9) | 5 (4.5) | 18 (32.1) | <0.001 | <0.001 |
| In-hospital | |||||
| Length of stay [d] | 17 (9–34) | 21 (11–34) | 12 (4–27) | 0.001 | 0.043 |
| Mortality | 26 (15.7) | 6 (5.5) | 20 (35.7) | <0.001 | <0.001 |
| Admission Phosphate | Admission Phosphate | p | Correlation with Admission Phosphate Levels Spearman’s Rho (p) | ||
|---|---|---|---|---|---|
| ≤1.45 mmol/L | >1.45 mmol/L | p | |||
| (n = 110) | (n = 56) | (Bonferroni Corrected) | |||
| n (%) or Median (IQR) | n (%) or Median (IQR) | ||||
| Markers of tissue damage on admission | |||||
| ASAT [U/L] | 72 (46–154) | 170 (90–431) | 0.001 | 0.043 | 0.44 (<0.001) |
| ALAT [U/L] | 53 (33–121) | 120 (62–319) | 0.001 | 0.043 | 0.39 (<0.001) |
| LDH [U/L] | 341 (253–425) | 485 (317–666) | 0.005 | 0.187 | 0.48 (<0.001) |
| Myoglobin [µg/L] | 1840 (518–2094) | 3037 (1454–6362) | 0.002 | 0.078 | 0.44 (<0.001) |
| Creatinine [mg/dL] | 0.96 (0.86–1.10) | 1.25 (1.08–1.47) | <0.001 | <0.001 | 0.49 (<0.001) |
| Markers of shock on admission | |||||
| Lactate [mg/dL] | 16 (11–23) | 46 (25–78) | <0.001 | <0.001 | 0.65 (<0.001) |
| Glucose [mg/dL] | 138 (116–165) | 176 (137–255) | <0.001 | 0.029 | 0.39 (<0.001) |
| Admission day resuscitation # | |||||
| Packed red blood cells [mL] | 0 (0–560) | 500 (0–1585) | 0.029 | 1 | |
| Fresh frozen plasma [mL] | 0 (0–0) | 0 (0–288) | <0.001 | 0.024 | |
| Colloids [mL] | 3002 (2000–4500) | 4002 (2500–5500) | 0.014 | 0.575 | |
| Crystalloids [mL] | 2449 (1601–3497) | 2188 (1569–3516) | 0.326 | 1 | |
| Norepinephrine [µg/kg/min] * | 0.11 (0.04–0.21) | 0.28 (0.16–0.39) | <0.001 | 0.001 |
| Variable | Crude Odds Ratio | Adjusted Odds Ratio | p |
|---|---|---|---|
| (95% CI) | (95% CI) | ||
| Age | 1.03 (1.00–1.05) | 1.03 (1.00–1.07) | 0.048 |
| Sex (female vs. male) | 1.70 (0.67–4.30) | 1.35 (0.41–4.53) | 0.622 |
| Pre-existing comorbidity | 1.09 (0.46–2.58) | 0.93 (0.30–2.88) | 0.896 |
| Injury severity score | 1.07 (1.03–1.10) | 1.03 (1.00–1.07) | 0.089 |
| Admission phosphate levels | |||
| 0.80–1.45 mmol/L | reference | ||
| <0.80 mmol/L | 0.89 (0.10–8.11) | 0.95 (0.10–8.99) | 0.966 |
| 1.46–2.10 mmol/L | 4.96 (1.44–17.03) | 3.96 (1.03–15.16) | 0.045 |
| >2.10 mmol/L | 18.42 (5.57–60.87) | 12.81 (3.45–47.48) | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rugg, C.; Bachler, M.; Kammerlander, R.; Niederbrunner, D.; Bösch, J.; Schmid, S.; Kreutziger, J.; Ströhle, M. ICU-Admission Hyperphosphataemia Is Related to Shock and Tissue Damage, Indicating Injury Severity and Mortality in Polytrauma Patients. Diagnostics 2021, 11, 1548. https://doi.org/10.3390/diagnostics11091548
Rugg C, Bachler M, Kammerlander R, Niederbrunner D, Bösch J, Schmid S, Kreutziger J, Ströhle M. ICU-Admission Hyperphosphataemia Is Related to Shock and Tissue Damage, Indicating Injury Severity and Mortality in Polytrauma Patients. Diagnostics. 2021; 11(9):1548. https://doi.org/10.3390/diagnostics11091548
Chicago/Turabian StyleRugg, Christopher, Mirjam Bachler, Robert Kammerlander, Daniel Niederbrunner, Johannes Bösch, Stefan Schmid, Janett Kreutziger, and Mathias Ströhle. 2021. "ICU-Admission Hyperphosphataemia Is Related to Shock and Tissue Damage, Indicating Injury Severity and Mortality in Polytrauma Patients" Diagnostics 11, no. 9: 1548. https://doi.org/10.3390/diagnostics11091548
APA StyleRugg, C., Bachler, M., Kammerlander, R., Niederbrunner, D., Bösch, J., Schmid, S., Kreutziger, J., & Ströhle, M. (2021). ICU-Admission Hyperphosphataemia Is Related to Shock and Tissue Damage, Indicating Injury Severity and Mortality in Polytrauma Patients. Diagnostics, 11(9), 1548. https://doi.org/10.3390/diagnostics11091548






