Absence of Stress Hyperglycemia Indicates the Most Severe Form of Blunt Liver Trauma
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Study Population
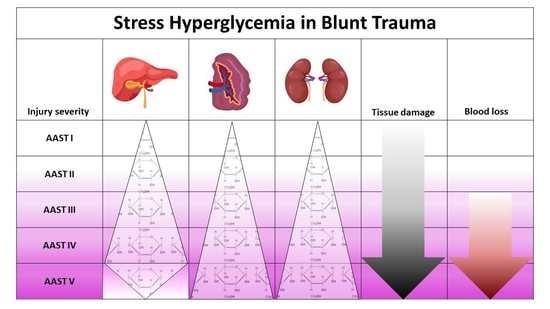
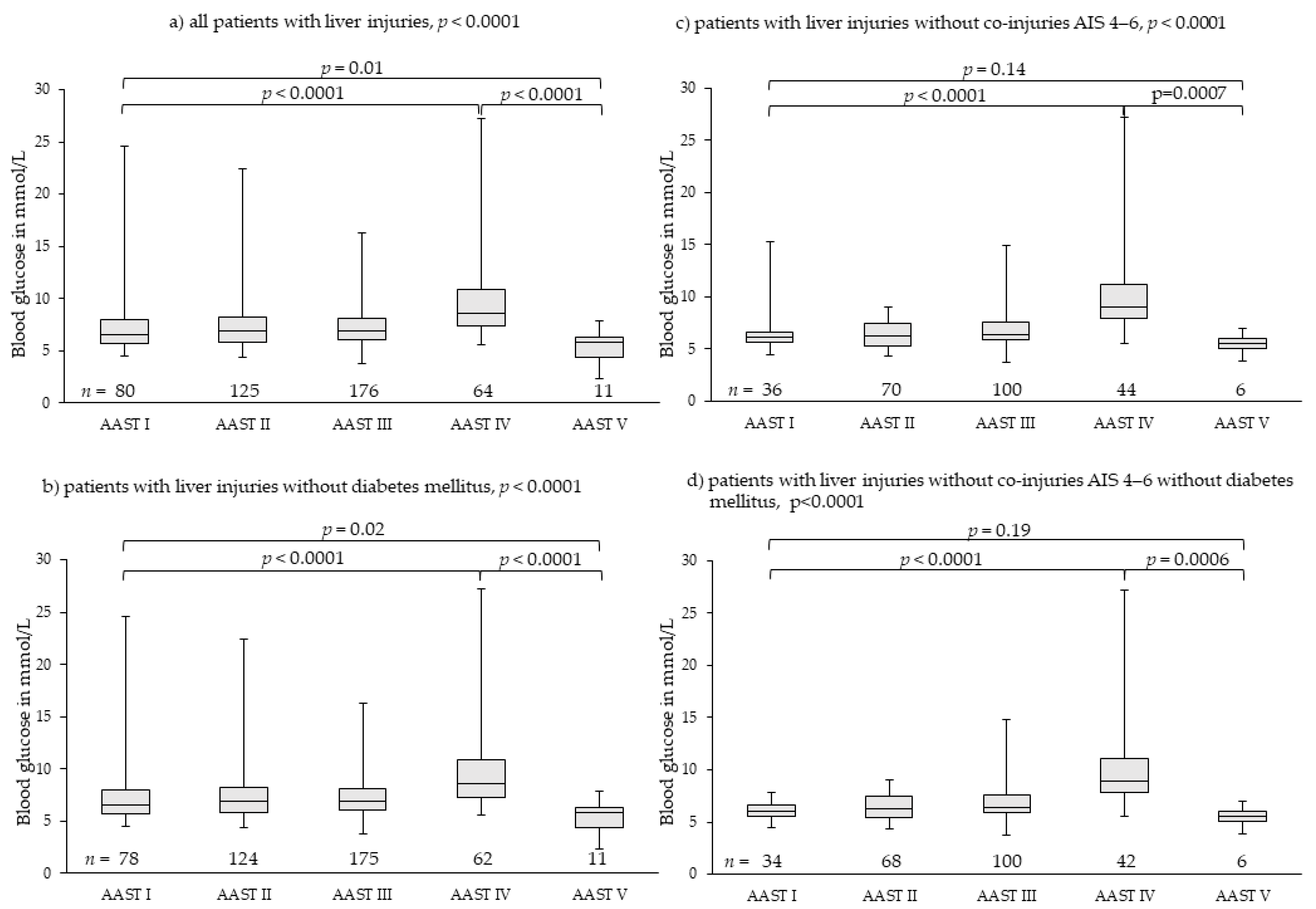
3.2. Liver Injuries
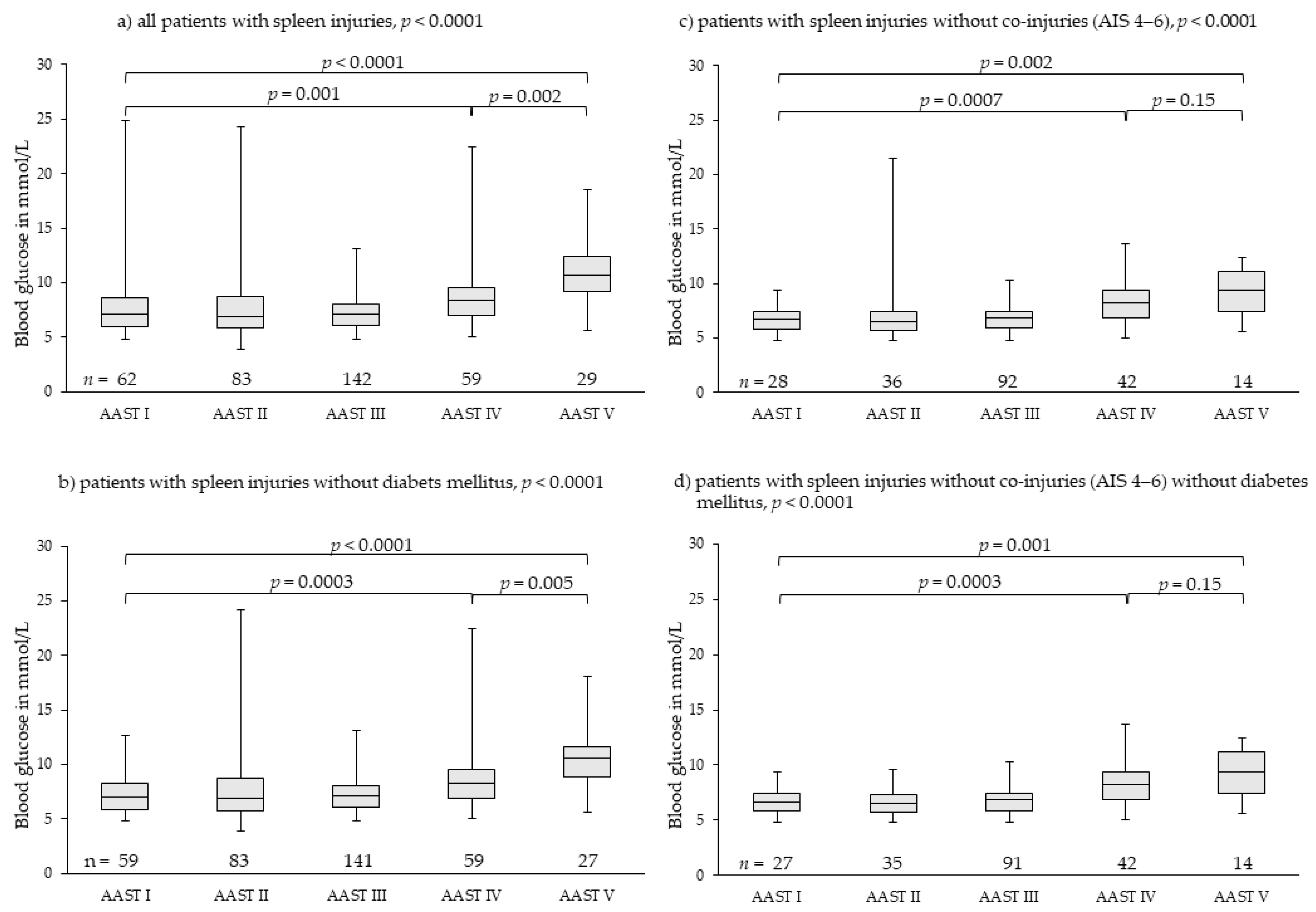
3.3. Spleen Injuries
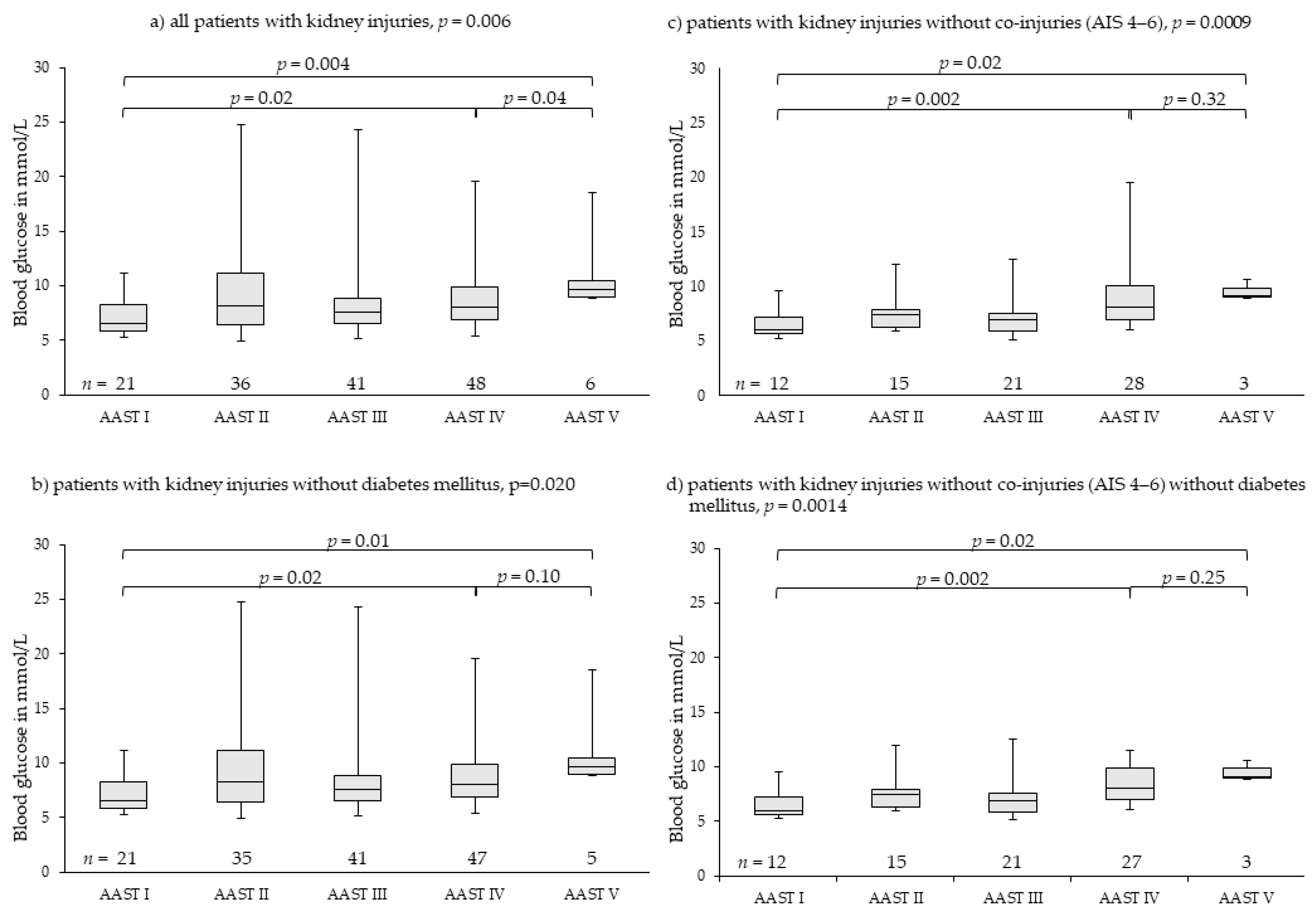
3.4. Kidney Injuries
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mamtani, M.; Kulkarni, H.; Bihari, S.; Prakash, S.; Chavan, S.; Huckson, S.; Pilcher, D. Degree of Hyperglycemia Independently Associates With Hospital Mortality and Length of Stay in Critically Ill, Nondiabetic Patients: Results From the ANZICS CORE Binational Registry. J. Crit. Care 2020, 55, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Kreutziger, J.; Schmid, S.; Umlauf, N.; Ulmer, H.; Nijsten, M.W.; Werner, D. Association between Blood Glucose and cardiac Rhythms during pre-hospital care of Trauma Patients—A retrospective Analysis. Scand. J. Trauma Resusc. Emerg. Med. 2018, 26, 58. [Google Scholar] [CrossRef] [PubMed]
- Kreutziger, J.; Lederer, W.; Schmid, S.; Ulmer, H.; Wenzel, V.; Nijsten, M.W.; Werner, D.; Schlechtriemen, T. Blood glucose concentrations in prehospital trauma patients with traumatic shock. A retrospective analysis. Eur. J. Anaesthesiol. 2018, 35, 33–42. [Google Scholar] [CrossRef]
- Vogelzang, M.; Nijboer, J.M.; van der Horst, I.C.; Zijlstra, F.; ten Duis, H.J.; Nijsten, M.W. Hyperglycemia Has a Stronger Relation with Outcome in Trauma Patients Than in Other Critically Ill Patients. J. Trauma 2006, 60, 873–877. [Google Scholar] [CrossRef] [PubMed]
- Bilotta, F.; Caramia, R.; Paoloni, F.P.; Delfini, R.; Rosa, G. Safety and Efficacy of Intensive Insulin Therapy in Critical Neurosurgical Patients. Anesthesiology 2009, 110, 611–619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Capes, S.E.; Hunt, D.; Mamberg, K.; Pathak, P.; Gerstein, H.C. Stress Hyperglycemia and Prognosis of Stroke in Nondiabetic and Diabetic Patients: A Systematic Overview. Stroke 2001, 32, 2426–2432. [Google Scholar] [CrossRef] [Green Version]
- Capes, S.E.; Hunt, D.; Malmberg, K.; Gerstein, H.C. Stress Hyperglycaemia and Increased Risk of Death After Myocardial Infarction in Patients with and without Diabetes: A Systematic Overview. Lancet 2000, 355, 773–778. [Google Scholar] [CrossRef]
- Di Battista, A.P.; Rhind, S.G.; Hutchison, M.G.; Hassan, S.; Shiu, M.Y.; Inaba, K.; Topolovec-Vranic, J.; Capone Neto, A.; Rizoli, S.B.; Baker, A.J. Inflammatory cytokine and chemokine profils are associated with patient outcome and the hyperadrenergic state following acute brain injury. J. Neuroinflamm. 2016, 13, 40. [Google Scholar] [CrossRef] [Green Version]
- Woiciechowsky, C.; Asadullah, K.; Nestler, D.; Eberhardt, B.; Platzer, C.; Schöning, B.; Glöckner, F.; Lanksch, W.R.; Volk, H.D.; Döcke, W.D. Sympathetic activation triggers systemic interleukin-10 release in immunodepression induced by brain injury. Nat. Med. 1998, 4, 808–813. [Google Scholar] [CrossRef]
- Kreutziger, J.; Rafetseder, A.; Mathis, S.; Wenzel, V.; El Attal, R.; Schmid, S. Admission blood glucose predicted haemorrhagic shock in multiple trauma patients. Injury 2015, 46, 15–20. [Google Scholar] [CrossRef]
- Xu, J.; Kim, H.T.; Ma, Y.; Zhao, L.; Zhai, L.; Kokorina, N.; Wang, P.; Messina, J.L. Trauma and Hemorrhage-Induced Acute Hepatic Insulin Resistance: Dominant Role of Tumor Necrosis Factor (TNF)-alpha. Endocrinology 2008, 149, 2369–2382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burk, A.M.; Martin, M.; Flierl, M.A.; Rittirsch, D.; Helm, M.; Lampl, L.; Bruckner, U.; Stahl, G.L.; Blom, A.M.; Perl, M.; et al. Early complementopathy after multile injuries in humans. Shock 2012, 37, 348–354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huber-Lang, M.; Lambris, J.D.; Ward, P.A. Innate immune responses to trauma. Nat. Immunol. 2018, 19, 327–341. [Google Scholar] [CrossRef] [PubMed]
- Roberts, D.J.; Jenne, C.N.; Léger, C.; Kramer, A.H.; Gallagher, C.N.; Todd, S.; Parney, I.A.; Doig, C.J.; Yong, V.W.; Kubes, P.; et al. Association between the cerebral inflammatory and matrix metalloproteinase responses after severe traumatic brain injury in humans. J. Neurtrauma. 2013, 30, 1727–1736. [Google Scholar] [CrossRef]
- Gentile, L.F.; Cuenca, A.G.; Vanzant, E.L.; Efron, P.A.; McKinley, B.; Moore, F.; Moldawer, L.L. Is there value in plasma cytokine measurements in patients with severe trauma and sepsis? Methods 2013, 61, 3–9. [Google Scholar] [CrossRef] [Green Version]
- Meade, P.; Shoemaker, W.C.; Donnelly, T.J.; Abraham, E.; Jagels, M.A.; Cryer, H.G.; Hugli, T.E.; Bishop, M.H.; Wo, C.C. Temporal patterns of hemodynamics, oxygen transport, cytokine activity, and complement activity in the development of adult respiratory distress syndrome after severe injury. J. Trauma 1994, 36, 651–657. [Google Scholar] [CrossRef]
- McDonald, B.; Poittman, K.; Menezes, G.B.; Hirota, S.A.; Slaba, I.; Waherhouse, C.C.M.; Beck, P.L.; Muruve, D.A.; Kubes, P. Intravascular danger signals guide neutrophils to sites of sterile inflammation. Science 2010, 330, 362–366. [Google Scholar] [CrossRef]
- Chen, X.-L.; Sun, L.; Guo, F.; Wang, F.; Liu, S.; Liang, X.; Wang, R.-S.; Wang, Y.-J.; Sun, Y.-X. High-mobility group box-1 induces proinflammatory cytokines production of Kupffer cells through TLRs-dependent signaling pathway after burn injury. PLoS ONE 2012, 7, e50668. [Google Scholar] [CrossRef]
- Thobe, B.M.; Frink, M.; Hildebrand, F.; Schwacha, M.G.; Hubbard, W.J.; Choudhry, M.A.; Chaudry, I.H. The role of MAPK in Kupffer cell toll-like receptor (TLR) 2-, TLR4-, and TLR9-mediated signaling following trauma-hemorrhage. J. Cell Physiol. 2007, 210, 667–675. [Google Scholar] [CrossRef]
- Hodis, J.; Kutinová-Canovà, N.; Potmĕšsil, P.; Kameníková, L.; Kmoníčková, E.; Zídek, Z.; Farghali, H. The Role of Adrenergic Agonists on Glycogenolysis in Rat Hepatocyte Cultures and Possible Involvement of NO. Physiol. Res. 2007, 56, 419–425. [Google Scholar]
- Li, L.; Thompson, L.H.; Zhao, L.; Messina, J.L. Tissue Specific Difference in the Molecular Mechanisms for the Development of Acute Insulin Resistance Following Injury. Endocrinology 2009, 150, 24–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCowen, K.C.; Ling, P.R.; Ciccarone, A.; Mao, Y.; Chow, J.C.; Bistrian, B.R.; Smith, R.J. Sustained endotoxemia leads to marked down-regulation of early steps in the insulin-signaling cascade. Crit. Care Med. 2001, 29, 839–846. [Google Scholar] [CrossRef]
- Leclercq, I.A.; Da Silva Morais, A.; Schroyen, B.; Van Hul, N.; Geerts, A. Insulin resistance in hepatocytes and sinusoidal liver cells: Mechanisms and consequences. J. Hepatol. 2007, 47, 142–156. [Google Scholar] [CrossRef] [PubMed]
- Penn, A.H.; Schmid-Schönbein, G.W. The intestine as source of cytotoxic mediators in shock. Free fatty acis and degradation of lipid-binding proteins. Am. J. Physiol. Heart Circ. Physiol. 2008, 294, H1779–H1792. [Google Scholar] [CrossRef] [Green Version]
- Chu, C.A.; Galassetti, P.; Igawa, K.; Sindelar, A.K.; Neal, D.W.; Burish, M.; Cherrington, A.D. Interaction of free fatty acids and epinephrine in regulating hepatic glucose production in conscious dogs. Am. J. Physiol. Endocrinol. Metab. 2003, 284, 291–301. [Google Scholar] [CrossRef] [Green Version]
- Chu, C.A.; Sindelar, D.K.; Neal, D.W.; Cherrington, A.D. Portal adrenergic blockade does not inhibit the gluconeogenetic effects of circulating chatecholamines on the liver. Metabolism 1997, 46, 458–465. [Google Scholar] [CrossRef]
- Stumvoll, M.; Chintalapudi, U.; Perriello, G.; Welle, S.; Gutierrez, O.; Gerich, J. Uptake and release of glucose by the human kidney. Postabsorptive rates and responses to epinephrine. J. Clin. Investig. 1995, 96, 2528–2533. [Google Scholar] [CrossRef]
- Li, L.; Messina, J.L. Acute insulin resistance following injury. Trends Endocrinol. Metab. 2009, 20, 429–435. [Google Scholar] [CrossRef] [Green Version]
- Berthet, J.; Rall, T.W.; Sutherland, E.W. The relationship of epinephrine and glucagon to liver phosphorylase. IV. Effect of epinephrine and glucagon on the reactivation of phosphorylase in liver homogenates. J. Biol. Chem. 1957, 224, 463–475. [Google Scholar] [PubMed]
- Walsh, D.A.; Perkins, J.P.; Krebs, E.G. An adenosine 3‘,5‘-monophosphate-dependant protein kinase from rabbit skeletal muscle. J. Biol. Chem. 1968, 243, 3763–3765. [Google Scholar] [CrossRef]
- Studer, R.K.; Borle, A.B. Differences between male and female rats in the regulation of hepatic glycogenolysis. The relative role of calcium and cAMP in phosphorylase activation by catecholamines. J. Biol. Chem. 1982, 257, 7987–7993. [Google Scholar] [CrossRef]
- Studer, R.K.; Snowdowne, K.W.; Borle, A.B. Regulation of hepatic glycogenoylsis by glucagon in male and female rats. Role of cAMP and Ca2+ and interactions between epinephrine and glucagon. J. Biol. Chem. 1984, 259, 3596–3604. [Google Scholar] [CrossRef]
- El-Maghrabi, M.R.; Claus, T.; Pilkis, J.; Pilkis, S.J. Regulation of 6-phosphfructo-2-kinase activity by cyclic AMP-dependent phosphorylation. Proc. Natl. Acad. Sci. USA 1982, 79, 315–319. [Google Scholar] [CrossRef] [Green Version]
- Richards, C.S.; Yokoyama, M.; Furuya, E.; Uyeda, K. Reciprocal changes in fructose-2,6-bisphosphate, 2-kinase and fructose-2,6-bisphosphatase activity in response to glucagon and epinephrine. Biochem. Biophys. Res. Commun. 1982, 104, 1073–1079. [Google Scholar] [CrossRef]
- Thompson, L.H.; Kim, H.T.; Kokorina, N.A.; Mesina, J.L. Acute muscle-type specific insulin resistance following injury. Mol. Med. 2008, 14, 715–723. [Google Scholar] [CrossRef]
- Hotamisligil, G.S.; Erbay, E. Nutrient sensing and inflammation in metabolic diseases. Nat. Rev. Immunol. 2008, 8, 923–934. [Google Scholar] [CrossRef] [Green Version]
- Wouters, M.; Posma, R.A.; van der Weerd, L.; van Putten, T.S.; Wendt, K.W. Incidence, Causes and Consequences of Early Hypoglycaemia in Severe Trauma Patients. Diploma Thesis, ESICM Paris, Paris, France, 2013. [Google Scholar]
- Skjelstad, T.; Sørensen, M.A.; Nielsen, E.W. Alpine cross-country skier with energy depletion and reduced consciousness. Tidsskr. Nor. Legeforen. 2017, 137, 289–292. [Google Scholar] [CrossRef]
- Moore, E.E.; Shackford, S.R.; Pachter, H.L.; McAninch, J.W.; Browner, B.D.; Champion, H.R.; Flint, L.M.; Gennarelli, T.A.; Malangoni, M.A.; Ramenofsky, M.L. Organ injury scaling: Spleen, liver, and kidney. J. Trauma 1989, 29, 1664–1666. [Google Scholar] [CrossRef] [PubMed]
- Kozar, R.A.; Crandall, M.; Shanmuganathan, K.; Zarzaur, B.L.; Coburn, M.; Cribari, C.; Kaups, K.; Schuster, K.; Tominaga, G.T.; The AAST Patient Assessment Committee. Organ injury scaling 2018 update: Spleen, liver, and kidney. J. Trauma Acute Care Surg. 2018, 85, 1119–1122. [Google Scholar] [CrossRef] [PubMed]
- Morell-Hofert, D.; Primavesi, F.; Fodor, M.; Gassner, E.; Kranebitter, V.; Braunwarth, E.; Haselbacher, M.; Nitsche, U.P.; Schmid, S.; Blauth, M.; et al. Validation of the revized 2018 AAST-OIS classification and the CT severity index for prediction of operative management and survival in patients with blunt spleen and liver injuries. Eur. Radiol. 2020, 30, 6570–6581. [Google Scholar] [CrossRef] [PubMed]
- Fodor, M.; Primavesi, F.; Morell-Hofert, D.; Kranebitter, V.; Palaver, A.; Braunwarth, E.; Haselbacher, M.; Nitsche, U.; Schmid, S.; Blauth, M.; et al. Non-operative management of blunt hepatic and splenic injury: A time-trend and outcome analysis over a period of 17 years. World J. Emerg. Surg. 2019, 14, 29. [Google Scholar] [CrossRef] [Green Version]
- Abajas Bustillo, R.; Leal Costa, C.; Ortego Mate, M.D.C.; Zonfrillo, M.R.; Seguí Gómez, M.; Durá Ros, M.J. Classification of the severe trauma patient with the Abbreviated Injury Scale: Degree of correlation between versions 98 and 2005 (2008 update). Emergencias 2018, 30, 41–44. [Google Scholar]
- Baker, S.P.; O’Neill, B.; Haddon, W., Jr.; Long, W.B. The injury severity score: A method for describing patients with multiple injuries and evaluating emergency care. J. Trauma 1974, 14, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Yendamuri, S.; Fulda, G.J.; Tinkoff, G.H. Admission Hyperglycemia as a Prognostic Indicator in Trauma. J. Trauma 2003, 55, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Laird, A.M.; Miller, P.R.; Kilgo, P.D.; Meredith, J.W.; Chang, M.C. Relationship of early hyperglycemia to mortality in trauma patients. J. Trauma 2004, 56, 1058–1062. [Google Scholar] [CrossRef] [PubMed]
- Bochicchio, G.V.; Salzano, L.; Joshi, M.; Bochicchio, K.; Scalea, T.M. Admission preoperative glucose is predictive of morbidity and mortality in trauma patients who require immediate operative intervention. Am. Surg. 2005, 71, 171–174. [Google Scholar] [CrossRef]
- Kreutziger, J.; Wenzel, V.; Kurz, A.; Constantinescu, M.A. Admission blood glucose is an independent predictive factor for hospital mortality in polytraumatised patients. Intensive Care Med. 2009, 35, 1234–1239. [Google Scholar] [CrossRef] [PubMed]
- Kreutziger, J.; Schlaepfer, J.; Wenzel, V.; Constantinescu, M.A. The role of admission blood glucose in outcome prediction of surviving patients with multiple trauma. J. Trauma 2009, 67, 704–708. [Google Scholar]
- Rau, C.S.; Wu, S.C.; Chen, Y.C.; Chien, P.C.; Hsieh, H.Y.; Kuo, P.J.; Hsieh, C.H. Higher Mortality in Trauma Patients Is Associated with Stress-Induced Hyperglycemia, but Not Diabetic Hyperglycemia: A Cross-Sectional Analysis Based on a Propensity-Score Matching Approach. Int. J. Environ. Res. Public Health 2017, 14, 1161. [Google Scholar] [CrossRef] [Green Version]
- Stumvoll, M.; Meyer, C.; Perriello, G.; Kreider, M.; Welle, S.; Gerich, J. Human Kidney and Liver Gluconeogenesis: Evidence for organ substrate selectivity. Am. J. Physiol. 1998, 274, E817–E826. [Google Scholar] [CrossRef]
- Rau, C.S.; Wu, S.C.; Chen, Y.C.; Chien, P.C.; Hsieh, H.Y.; Kuo, P.J.; Hsieh, C.H. Stress-Induced Hyperglycemia in Diabetes: A Cross-Sectional Analysis to Explore the Definition Based on the Trauma Registry Data. Int. J. Environ. Res. Public Health 2017, 14, 1527. [Google Scholar] [CrossRef] [Green Version]
- Kerby, J.D.; Griffin, R.L.; MacLennan, P.; Rue, L.W. Stress-Induced Hyperglycemia, Not Diabetic Hyperglycemia, Is Associated with Higher Mortality in Trauma. Ann. Surg. 2012, 256, 446–452. [Google Scholar] [CrossRef]
- Meyer, C.; Stumvoll, M.; Welle, S.; Woerle, H.J.; Haymond, M.; Gerich, J. Relative importance of liver, kidney, and substrates in epinephrine-induced increased gluconeogenesis in humans. Am. J. Physiol. Endocrinol. Metab. 2003, 285, E819–E826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frank, S.M.; Fleisher, L.A.; Olson, K.F.; Gorman, R.B.; Higgins, M.S.; Breslow, M.J.; Sitzmann, J.V.; Beattie, C. Multivariate determinats of early postoperative oxygen consumption in elderly patients. Effects of shivering, body temperature, and gender. Anesthesiology 1995, 83, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.H.; Michiue, T.; Inamori-Kawamoto, O.; Ikeda, S.; Ishikawa, T.; Maeda, H. Comprehensive investigation of postmortem glucose levels in blood and body fluids with regard to the cause of death in forensic autopsy cases. Leg. Med. 2015, 17, 475–482. [Google Scholar] [CrossRef] [PubMed]

| AAST | AIS | Liver | Spleen | Kidney | |
|---|---|---|---|---|---|
| I | 2 | Hematoma | Subcapsular, <10% surface area | Subcapsular, <10% surface area | Subcapsular hematoma and/or parenchymal contusion without laceration |
| Laceration | Capsular tear, <1% parenchymal depth | Capsular tear, <1% parenchymal depth | |||
| II | 2 | Hematoma | Subcapsular, 10–50% surface area, intra-parenchymal, <10 cm in diameter | Subcapsular, 10–50% surface area Intra-parenchymal, <5 cm in diameter | Perirenal hematoma confined to Gerota fascia |
| Laceration | Capsular tear, 1–3 cm parenchymal depth, <10 cm length | 1–3 cm parenchymal depth | Renal parenchymal laceration ≤1 cm depth without urinary extravasation | ||
| III | 3 | Hematoma | Subcapsular, >50% surface area of ruptured subcapsular or parenchymal hematoma; intraparenchymal >10 cm | Subcapsular, >50% surface area Ruptured subcapsular or parenchymal hematoma ≥5 cm | |
| Laceration | Capsular tear, >3 cm parenchymal depth | >3 cm parenchymal depth or involving trabecular vessels | Renal parenchymal laceration >1 cm depth without collecting system rupture or urinary extravasation | ||
| Vascular | Vascular injury with active bleeding contained within liver parenchyma | Any injury in the presence of a kidney vascular injury or active bleeding contained within Gerota fascia | |||
| IV | 4 | Laceration | Parenchymal disruption involving 25–75% of hepatic lobe or 1–3 segments | Parenchymal laceration involving segmental or hilar vessels producing >25% devascularization | Parenchymal laceration extending into urinary collecting system with urinary extravasation Renal pelvis laceration and/or complete ureteropelvic disruption |
| Vascular | Vascular injury with active bleeding breaching the liver parenchyma into the peritoneum | Any injury in the presence of a splenic vascular injury or active bleeding confined within splenic capsule | Segmental renal vein or artery injury Active bleeding beyond Gerota fascia into the retroperitoneum or peritoneum Segmental or complete kidney infarction(s) due to vessel thrombosis without active bleeding | ||
| V | 5 | Laceration | Parenchymal disruption involving >75% of hepatic lobe | Shattered spleen | Shattered kidney with loss of identifiable parenchymal renal anatomy |
| Vascular | Juxtavenous hepatic injuries; i.e., retrohepatic vena cava/central major hepatic veins | Any injury in the presence of splenic vascular injury with active bleeding extending beyond the spleen into the peritoneum | Main renal artery or vein laceration or avulsion of hilum Devascularized kidney with active bleeding |
| Characteristics | Median/Number | Range/Percent |
|---|---|---|
| Age | 29 | 1–89 |
| female/male | 238/534 | 30.8/69.2 |
| Body mass index (BMI) | 23.0 | 13.1–43.2 |
| Deceased | 26 | 3.4 |
| Hospital length of stay (d) | 13 | 0–112 |
| Blood glucose (mmol/L) 1 | 7.16 | 2.33–27.19 1 |
| ISS 2 | 21 | 1–75 |
| Accident mechanism | ||
| Accidents with skis, sleigh, or snowboard | 301 | 39.0 |
| Accident with motor vehicle | 148 | 19.2 |
| Accident with moped or motorbike | 81 | 10.5 |
| Accident with bike, scooter, skateboard | 71 | 9.2 |
| Fall > 3 m | 67 | 8.7 |
| Occupational injury | 28 | 3.7 |
| Unknown mechanism | 19 | 2.5 |
| Accident with large animal | 18 | 2.3 |
| Accident as pedestrian | 14 | 1.8 |
| Fall < 3 m | 11 | 1.4 |
| Accident with flex wing, parachute or similar | 6 | 0.8 |
| Fall down stairs | 5 | 0.6 |
| Brawl | 3 | 0.4 |
| Co-morbidities | ||
| Arterial hypertension | 36 | 4.7 |
| Other disease | 35 | 4.5 |
| Psychiatric disease | 25 | 3.2 |
| Allergy/atopic dermatitis | 22 | 2.8 |
| Neurologic disease | 18 | 2.3 |
| Cardiac insufficiency and other cardiac diseases | 16 | 2.1 |
| Diabetes mellitus | 13 | 1.7 |
| Alcohol abuse | 14 | 1.8 |
| Adiposity | 12 | 1.6 |
| Endocrinologic disease | 11 | 1.4 |
| Malign disease | 10 | 1.3 |
| Chronic obstructive pulmonary disease | 8 | 1.0 |
| Renal insufficiency | 8 | 1.0 |
| Autoimmune disease | 8 | 1.0 |
| Metabolic disease | 6 | 0.8 |
| Coronary heart disease | 5 | 0.6 |
| Bronchial asthma | 5 | 0.6 |
| Laboratory findings 1 | ||
| Hemoglobin mg/dL | 121 | 34–187 |
| Thrombocytes G/L | 198 | 14–835 |
| Leukocytes G/L | 13.1 | 1.5–39.1 |
| Lactate mg/dL | 15.4 | 4–176 |
| pH | 7.367 | 6.882–7.642 |
| Bicarbonate standardized mmol/L | 22.1 | 8.3–27.0 |
| Base excess mmol/L | −2.0 | −15.6–11.0 |
| Potassium mmol/L | 3.8 | 2.32–7.62 |
| Anion gap mmol/L | 21.8 | 8.3–27.0 |
| AAST I | AAST II | AAST III | AAST IV | AAST V | p | AAST I | AAST II | AAST III | AAST IV | AAST V | p | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Liver | All patients with liver injuries | Patients with liver injuries without co-injuries AIS 4–6 | ||||||||||
| Median | 6.58 | 6.88 | 6.94 | 8.60 | 5.77 | <0.0001 | 6.16 | 6.22 | 6.41 | 8.96 | 5.58 | <0.0001 |
| Min-max | 4.50–24.64 | 4.33–22.42 | 3.77–16.32 | 5.55–27.19 | 2.33–7.83 | 4.50–15.26 | 4.33–9.05 | 3.77–14.87 | 5.55–27.19 | 3.83–6.94 | ||
| IQR | 5.70–7.95 | 5.77–8.21 | 6.05–8.12 | 7.31–10.91 | 4.36–6.33 | 5.63–6.63 | 5.34–7.44 | 5.87–7.56 | 7.99–11.20 | 5.01–6.06 | ||
| CI 95% | 6.90–8.39 | 7.05–8.04 | 7.11–7.71 | 8.82–10.80 | 4.32–6.29 | 5.90–7.12 | 6.15–6.71 | 6.54–7.19 | 9.07–11.75 | 4.71–6.28 | ||
| Patients with liver injuries without diabetes mellitus | Patients with liver injuries without co-injuries AIS 4–6 without diabetes mellitus | |||||||||||
| Median | 6.52 | 6.80 | 6.94 | 8.52 | 5.77 | <0.0001 | 6.08 | 6.22 | 6.41 | 8.91 | 5.58 | <0.0001 |
| Min-max | 4.50–24.64 | 4.33–22.42 | 3.77–16.32 | 5.55–27.19 | 2.33–7.83 | 4.50–7.77 | 4.33–9.05 | 3.77–14.87 | 5.55–27.19 | 3.83–6.94 | ||
| IQR | 5.67–7.83 | 5.77–8.21 | 6.05–8.13 | 7.17–10.75 | 4.36–6.33 | 5.58–6.59 | 5.37–7.44 | 5.87–7.56 | 7.88–11.07 | 5.01–6.06 | ||
| CI 95% | 6.77–8.24 | 7.04–8.04 | 7.11–7.71 | 8.59–10.36 | 4.42–6.19 | 5.85–6.40 | 6.14–6.70 | 6.54–7.19 | 8.75–11.15 | 4.71–6.28 | ||
| Spleen | All patients with spleen injuries | Patients with spleen injuries without co-injuries AIS 4–6 | ||||||||||
| Median | 7.13 | 6.88 | 7.08 | 8.32 | 10.71 | <0.0001 | 6.72 | 6.55 | 6.88 | 8.21 | 9.41 | <0.0001 |
| Min-max | 4.83–24.81 | 3.88–24.25 | 4.83–13.10 | 5.05–22.42 | 5.61–18.48 | 4.83–9.43 | 4.83–21.48 | 4.83–10.32 | 5.05–13.65 | 5.61–12.43 | ||
| IQR | 5.90–8.57 | 5.80–8.69 | 6.10–8.03 | 6.94–9.55 | 9.16–12.43 | 5.83–7.49 | 5.72–7.38 | 5.88–7.44 | 6.91–9.42 | 7.42–11.17 | ||
| CI 95% | 6.98–8.39 | 7.17–8.58 | 6.96–7.46 | 8.23–9.92 | 9.74–12.16 | 6.34–7.28 | 6.18–7.97 | 6.58–7.07 | 7.96–8.61 | 8.09–10.40 | ||
| Patients with spleen injuries without diabetes mellitus | Patients with spleen injuries without co-injuries AIS AIS 4–6without diabetes mellitus | |||||||||||
| Median | 6.99 | 6.88 | 7.10 | 8.32 | 10.60 | <0.0001 | 6.66 | 6.49 | 6.88 | 8.21 | 9.41 | <0.0001 |
| Min-max | 4.83–12.71 | 3.88–24.25 | 4.83–13.10 | 5.05–22.42 | 5.61–18.09 | 4.83–9.43 | 4.83–9.60 | 4.83–10.32 | 5.05–13.65 | 5.61–12.43 | ||
| IQR | 5.88–8.30 | 5.80–8.69 | 6.10–8.05 | 6.94–9.55 | 8.91–11.58 | 5.83–7.38 | 5.72–7.30 | 5.88–7.44 | 6.91–9.42 | 7.42–11.17 | ||
| CI 95% | 6.88–7.41 | 7.13–8.39 | 6.96–7.46 | 8.23–9.29 | 9.74–12.16 | 6.27–7.19 | 6.23–7.10 | 6.58–7.07 | 7.72–8.85 | 8.09–10.40 | ||
| Kidney | All patients with kidney injuries | Patients with kidney injuries without co-injuries AIS 4–6 | ||||||||||
| Median | 6.49 | 8.21 | 7.60 | 8.02 | 9.60 | 0.006 | 6.02 | 7.44 | 6.94 | 8.10 | 9.10 | 0.0009 |
| Min-max | 5.27–11.16 | 4.94–24.81 | 5.11–24.25 | 5.44–19.59 | 8.88–18.48 | 5.27–9.60 | 5.94–11.99 | 5.11–12.54 | 6.05–19.59 | 8.88–10.60 | ||
| IQR | 5.88–8.27 | 6.44–11.16 | 6.55–8.88 | 6.94–9.84 | 8.94–10.48 | 5.66–7.21 | 6.33–7.88 | 5.88–7.60 | 6.98–10.05 | 8.99–9.85 | ||
| CI 95% | 6.52–7.85 | 7.35–11.27 | 7.26–9.24 | 7.87–9.36 | 8.29–13.73 | 5.87–7.30 | 6.67–8.16 | 6.45–7.81 | 7.82–9.80 | 8.66–10.39 | ||
| Patients with kidney injuries without diabetes mellitus | Patients with kidney injuries without co-injuries AIS 4–6without diabetes mellitus | |||||||||||
| Median | 6.49 | 7.94 | 7.60 | 7.99 | 9.10 | 0.02 | 6.02 | 7.44 | 6.94 | 8.10 | 9.10 | 0.001 |
| Min-max | 5.27–11.16 | 4.94–22.42 | 5.11–24.25 | 5.44–13.62 | 8.88–10.60 | 5.27–9.60 | 5.94–11.99 | 5.11–12.54 | 6.05–11.54 | 8.88–10.60 | ||
| IQR | 5.88–8.27 | 6.44–11.04 | 6.55–8.88 | 6.94–9.60 | 8.88–10.10 | 5.66–7.21 | 6.33–7.88 | 5.88–7.60 | 6.97–9.91 | 8.99–9.85 | ||
| CI 95% | 6.52–7.85 | 8.14–10.62 | 7.26–9.24 | 7.77–8.99 | 7.06–11.97 | 5.87–7.30 | 6.67–8.16 | 6.45–7.81 | 7.76–9.06 | 8.66–10.39 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kreutziger, J.; Fodor, M.; Morell-Hofert, D.; Primavesi, F.; Stättner, S.; Gassner, E.-M.; Schmid, S.; Rugg, C. Absence of Stress Hyperglycemia Indicates the Most Severe Form of Blunt Liver Trauma. Diagnostics 2021, 11, 1667. https://doi.org/10.3390/diagnostics11091667
Kreutziger J, Fodor M, Morell-Hofert D, Primavesi F, Stättner S, Gassner E-M, Schmid S, Rugg C. Absence of Stress Hyperglycemia Indicates the Most Severe Form of Blunt Liver Trauma. Diagnostics. 2021; 11(9):1667. https://doi.org/10.3390/diagnostics11091667
Chicago/Turabian StyleKreutziger, Janett, Margot Fodor, Dagmar Morell-Hofert, Florian Primavesi, Stefan Stättner, Eva-Maria Gassner, Stefan Schmid, and Christopher Rugg. 2021. "Absence of Stress Hyperglycemia Indicates the Most Severe Form of Blunt Liver Trauma" Diagnostics 11, no. 9: 1667. https://doi.org/10.3390/diagnostics11091667
APA StyleKreutziger, J., Fodor, M., Morell-Hofert, D., Primavesi, F., Stättner, S., Gassner, E.-M., Schmid, S., & Rugg, C. (2021). Absence of Stress Hyperglycemia Indicates the Most Severe Form of Blunt Liver Trauma. Diagnostics, 11(9), 1667. https://doi.org/10.3390/diagnostics11091667