Muscle Thickness and Echo Intensity by Ultrasonography and Cognitive and Physical Dimensions in Older Adults
Abstract
:1. Introduction
2. Methods
2.1. Study Participants
2.2. Procedures
2.3. Anthropometric Measurements
2.4. Cognitive Assessment
2.5. Physical Performance
2.6. Muscle Strength
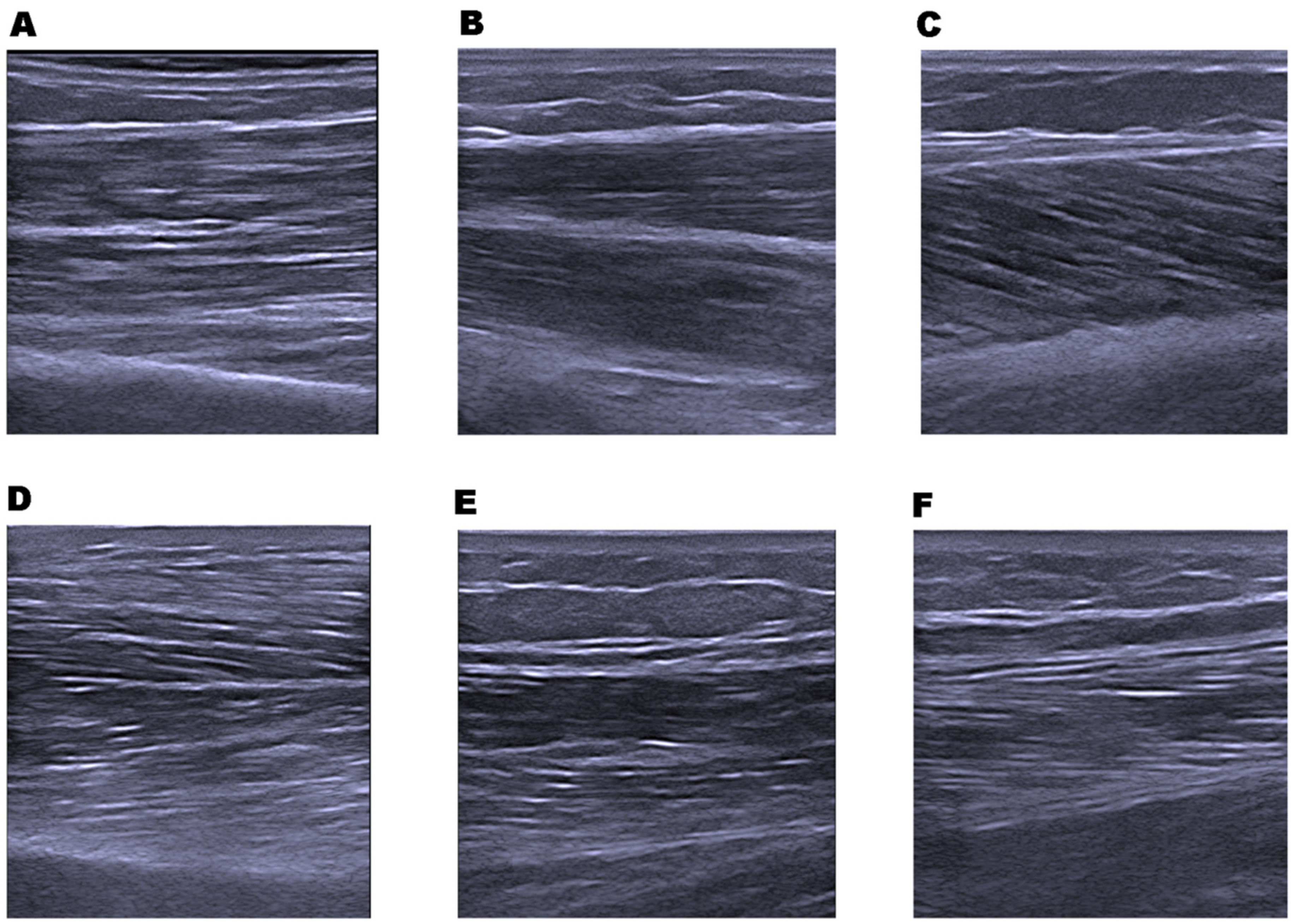
2.7. Muscle Architecture; Muscle Thickness and Echo Intensity
2.8. Statistical Analysis
2.9. Sample Size
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Consent for Publication
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Clark, B.C.; Manini, T.M. Sarcopenia ≠ Dynapenia. J. Gerontol. Ser. A 2008, 63, 829–834. [Google Scholar] [CrossRef]
- Rosenberg, I.H. Summary Comments. Am. J. Clin. Nutr. 1989, 50, 1231–1233. [Google Scholar] [CrossRef]
- Doherty, T.J. Invited Review: Aging and Sarcopenia. J. Appl. Physiol. 2003, 95, 1717–1727. [Google Scholar] [CrossRef] [Green Version]
- Saka, B.; Ozkaya, H.; Karisik, E.; Akin, S.; Akpinar, T.S.; Tufan, F.; Bahat, G.; Dogan, H.; Horasan, Z.; Cesur, K.; et al. Malnutrition and Sarcopenia Are Associated with Increased Mortality Rate in Nursing Home Residents: A Prospective Study. Eur. Geriatr. Med. 2016, 7, 232–238. [Google Scholar] [CrossRef]
- Goodpaster, B.H.; Krishnaswami, S.; Resnick, H.; Kelley, D.E.; Haggerty, C.; Harris, T.B.; Schwartz, A.V.; Kritchevsky, S.; Newman, A.B. Association Between Regional Adipose Tissue Distribution and Both Type 2 Diabetes and Impaired Glucose Tolerance in Elderly Men and Women. Diabetes Care 2003, 26, 372–379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European Consensus on Definition and Diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbat-Artigas, S.; Rolland, Y.; Zamboni, M.; Aubertin-Leheudre, M. How to Assess Functional Status: A New Muscle Quality Index. J. Nutr. Health Aging 2012, 16, 67–77. [Google Scholar] [CrossRef]
- Fragala, M.S.; Kenny, A.M.; Kuchel, G.A. Muscle Quality in Aging: A Multi-Dimensional Approach to Muscle Functioning with Applications for Treatment. Sports Med. Auckl. 2015, 45, 641–658. [Google Scholar] [CrossRef]
- Pillen, S.; van Dijk, J.P.; Weijers, G.; Raijmann, W.; de Korte, C.L.; Zwarts, M.J. Quantitative Gray-Scale Analysis in Skeletal Muscle Ultrasound: A Comparison Study of Two Ultrasound Devices. Muscle Nerve 2009, 39, 781–786. [Google Scholar] [CrossRef]
- Yamada, Y.; Schoeller, D.A.; Nakamura, E.; Morimoto, T.; Kimura, M.; Oda, S. Extracellular Water May Mask Actual Muscle Atrophy during Aging. J. Gerontol. A. Biol. Sci. Med. Sci. 2010, 65, 510–516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arts, I.M.P.; Pillen, S.; Schelhaas, H.J.; Overeem, S.; Zwarts, M.J. Normal Values for Quantitative Muscle Ultrasonography in Adults. Muscle Nerve 2010, 41, 32–41. [Google Scholar] [CrossRef]
- Caresio, C.; Molinari, F.; Emanuel, G.; Minetto, M.A. Muscle Echo Intensity: Reliability and Conditioning Factors. Clin. Physiol. Funct. Imaging 2015, 35, 393–403. [Google Scholar] [CrossRef]
- Goodpaster, B.H.; Carlson, C.L.; Visser, M.; Kelley, D.E.; Scherzinger, A.; Harris, T.B.; Stamm, E.; Newman, A.B. Attenuation of Skeletal Muscle and Strength in the Elderly: The Health ABC Study. J. Appl. Physiol. Bethesda Md 1985 2001, 90, 2157–2165. [Google Scholar] [CrossRef] [PubMed]
- Fukumoto, Y.; Ikezoe, T.; Yamada, Y.; Tsukagoshi, R.; Nakamura, M.; Mori, N.; Kimura, M.; Ichihashi, N. Skeletal Muscle Quality Assessed from Echo Intensity Is Associated with Muscle Strength of Middle-Aged and Elderly Persons. Eur. J. Appl. Physiol. 2012, 112, 1519–1525. [Google Scholar] [CrossRef] [Green Version]
- Rech, A.; Radaelli, R.; Goltz, F.R.; da Rosa, L.H.T.; Schneider, C.D.; Pinto, R.S. Echo Intensity Is Negatively Associated with Functional Capacity in Older Women. Age 2014, 36, 9708. [Google Scholar] [CrossRef]
- Zaslavsky, O.; Cochrane, B.B.; Thompson, H.J.; Woods, N.F.; Herting, J.R.; LaCroix, A. Frailty: A Review of the First Decade of Research. Biol. Res. Nurs. 2013, 15, 422–432. [Google Scholar] [CrossRef]
- Tuerk, C.; Zhang, H.; Sachdev, P.; Lord, S.R.; Brodaty, H.; Wen, W.; Delbaere, K. Regional Gray Matter Volumes Are Related to Concern About Falling in Older People: A Voxel-Based Morphometric Study. J. Gerontol. A. Biol. Sci. Med. Sci. 2016, 71, 138–144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Functional Outcomes for Clinical Trials in Frail Older Persons: Time to Be Moving. J. Gerontol. A. Biol. Sci. Med. Sci. 2008, 63, 160–164. [CrossRef] [PubMed] [Green Version]
- Cadore, E.L.; Izquierdo, M.; Conceição, M.; Radaelli, R.; Pinto, R.S.; Baroni, B.M.; Vaz, M.A.; Alberton, C.L.; Pinto, S.S.; Cunha, G.; et al. Echo Intensity Is Associated with Skeletal Muscle Power and Cardiovascular Performance in Elderly Men. Exp. Gerontol. 2012, 47, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, Y.; Yamada, Y.; Fukumoto, Y.; Ishihara, T.; Yokoyama, K.; Yoshida, T.; Miyake, M.; Yamagata, E.; Kimura, M. Echo Intensity Obtained from Ultrasonography Images Reflecting Muscle Strength in Elderly Men. Clin. Interv. Aging 2013, 8, 993–998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopez, P.; Wilhelm, E.N.; Rech, A.; Minozzo, F.; Radaelli, R.; Pinto, R.S. Echo Intensity Independently Predicts Functionality in Sedentary Older Men. Muscle Nerve 2017, 55, 9–15. [Google Scholar] [CrossRef]
- Wilhelm, E.N.; Rech, A.; Minozzo, F.; Radaelli, R.; Botton, C.E.; Pinto, R.S. Relationship between Quadriceps Femoris Echo Intensity, Muscle Power, and Functional Capacity of Older Men. Age 2014, 36, 9625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osawa, Y.; Arai, Y.; Oguma, Y.; Hirata, T.; Abe, Y.; Azuma, K.; Takayama, M.; Hirose, N. Relationships between Anterior Thigh Muscle Echo Intensity and Physical Activity and Functional Mobility in a Very Old Population. J. Aging Phys. Act. 2016, 1–25. [Google Scholar] [CrossRef]
- Strasser, E.M.; Draskovits, T.; Praschak, M.; Quittan, M.; Graf, A. Association between Ultrasound Measurements of Muscle Thickness, Pennation Angle, Echogenicity and Skeletal Muscle Strength in the Elderly. Age 2013, 35, 2377–2388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ross, W.; Hebbelinck, M.; Faulkner, R. Kinantropometry terminology and landmarks. In Shepard R, and Lavalle, H. Physical Fitness Assessment; Charles Thomas: Springfield, IL, USA, 1978; pp. 44–50. [Google Scholar]
- Pfeiffer, E. A Short Portable Mental Status Questionnaire for the Assessment of Organic Brain Deficit in Elderly Patients. J. Am. Geriatr. Soc. 1975, 23, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Martínez de la Iglesia, J.; Dueñas Herrero, R.; Onís Vilches, M.C.; Aguado Taberné, C.; Albert Colomer, C.; Luque Luque, R. Spanish language adaptation and validation of the Pfeiffer’s questionnaire (SPMSQ) to detect cognitive deterioration in people over 65 years of age. Med. Clínica 2001, 117, 129–134. [Google Scholar]
- Rey Cao, A.; Canales Lacruz, I.; Táboas País, I.; Cancela Carra, J.M. “Memoria En Movimiento” Programa De Estimulación Cognitiva a Través De La Motricidad Para Personas Mayores De La Obra Social Caixa Galicia. “Memory in Motion”. Cognitive Stimulation Program with Motor Skills Oriented to Elderly People (Social Programs, Caixa Galicia). 2008. Available online: http://hdl.handle.net/11268/3281 (accessed on 12 August 2021).
- Motor-Cognitive Stimulation of the Elderly: Educational Gerontology: Vol 37, No 2. Available online: https://www.tandfonline.com/doi/abs/10.1080/03601270903547020 (accessed on 12 August 2021).
- Guralnik, J.M.; Simonsick, E.M.; Ferrucci, L.; Glynn, R.J.; Berkman, L.F.; Blazer, D.G.; Scherr, P.A.; Wallace, R.B. A Short Physical Performance Battery Assessing Lower Extremity Function: Association with Self-Reported Disability and Prediction of Mortality and Nursing Home Admission. J. Gerontol. 1994, 49, M85–M94. [Google Scholar] [CrossRef]
- Clegg, A.; Rogers, L.; Young, J. Diagnostic Test Accuracy of Simple Instruments for Identifying Frailty in Community-Dwelling Older People: A Systematic Review. Age Ageing 2015, 44, 148–152. [Google Scholar] [CrossRef] [Green Version]
- Tinetti, M.E. Performance-Oriented Assessment of Mobility Problems in Elderly Patients. J. Am. Geriatr. Soc. 1986, 34, 119–126. [Google Scholar] [CrossRef]
- Mahoney, F.I.; Barthel, D.W. FUNCTIONAL EVALUATION: THE BARTHEL INDEX. Md. State Med. J. 1965, 14, 61–65. [Google Scholar]
- Reis, M.M.; Arantes, P.M.M. Assessment of Hand Grip Strength- Validity and Reliability of the Saehan Dynamometer. Fisioter. E Pesqui. 2011, 18, 176–181. [Google Scholar] [CrossRef] [Green Version]
- Fess E Grip Strength. Clinical assessment recommendations; Casanova, J.S., Ed.; American Society of Hand Therapists: Chicago, IL, USA, 1992; pp. 41–45. [Google Scholar]
- Roy, J.-S.; MacDermid, J.C.; Orton, B.; Tran, T.; Faber, K.J.; Drosdowech, D.; Athwal, G.S. The Concurrent Validity of a Hand-Held versus a Stationary Dynamometer in Testing Isometric Shoulder Strength. J. Hand Ther. 2009, 22, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Bandinelli, S.; Benvenuti, E.; Del Lungo, I.; Baccini, M.; Benvenuti, F.; Di Iorio, A.; Ferrucci, L. Measuring Muscular Strength of the Lower Limbs by Hand-Held Dynamometer: A Standard Protocol. Aging Milan Italy 1999, 11, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Volpato, S.; Cavalieri, M.; Sioulis, F.; Guerra, G.; Maraldi, C.; Zuliani, G.; Fellin, R.; Guralnik, J.M. Predictive Value of the Short Physical Performance Battery Following Hospitalization in Older Patients. J. Gerontol. A. Biol. Sci. Med. Sci. 2011, 66A, 89–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ayers, E.; Verghese, J. Diagnosing Motoric Cognitive Risk Syndrome to Predict Progression to Dementia. Neurodegener. Dis. Manag. 2014, 4, 339–342. [Google Scholar] [CrossRef] [Green Version]
| Variable | Mean | SD | Variable | Mean | SD |
|---|---|---|---|---|---|
| Age (years) | 85.4 | 7 | GM MT (cm) | 1.16 | 0.32 |
| Height (m) | 1.59 | 0.05 | GM MVC MT (cm) | 1.21 | 0.33 |
| Weight (kg) | 65.2 | 9.7 | RF MT (cm) | 1.26 | 0.27 |
| BMI (kg/m2) | 25.6 | 3.8 | RF MVC MT (cm) | 1.56 | 0.36 |
| Mid-upper arm circumference (cm) | 28.4 | 3.6 | RL MT (cm) | 1.21 | 0.21 |
| Calf circumference (cm) | 34.03 | 2.8 | RL MVC MT (cm) | 1.37 | 0.24 |
| SPMSQ (0–12) | 2.8 | 3.1 | TA MT (cm) | 2.3 | 0.29 |
| Motor memory (0–10) | 5.9 | 2.6 | TA MVC MT (cm) | 2.5 | 0.32 |
| Tinetti (0–28) | 19.2 | 6.6 | WF MT (cm) | 1.25 | 0.24 |
| Barthel (0–100) | 75.3 | 19.2 | WF MVC MT (cm) | 1.41 | 0.24 |
| TUG (seconds) | 20.8 | 11.5 | BB EI (0–255) | 82.46 | 12.65 |
| GS (m/s) | 0.6 | 0.1 | GM EI (0–255) | 69.36 | 11.86 |
| SPPB (0–12) | 5.3 | 3.1 | RF EI (0–255) | 69.62 | 8.51 |
| HGS (kg) | 16.5 | 4.7 | RL EI (0–255) | 79.68 | 11.66 |
| Knee Extensors Strength (N) | 135.1 | 62.9 | TA EI (0–255) | 87.93 | 7.47 |
| BB MT (cm) | 1.96 | 0.38 | WF EI (0–255) | 65.68 | 12.25 |
| BB MVC MT (cm) | 2.77 | 0.41 |
| UL EI | LL EI | UL MT | LL MT | |
|---|---|---|---|---|
| SPPB | −0.323 | −0.576 † | 0.432 | 0.541 * |
| SPMSQ | −0.062 | −0.274 | 0.030 | 0.007 |
| Motor Memory | −0.280 | −0.180 | 0.378 | 0.431 |
| Barthel | −0.118 | −0.307 | 0.344 | 0.582 † |
| Tinetti | −0.052 | −0.444 * | 0.307 | 0.522 * |
| TUG (s) | 0.211 | 0.344 | −0.107 | −0.266 |
| GS (m/s) | −0.141 | −0.556 * | 0.219 | 0.327 |
| HGS (kg) | −0.037 | −0.268 | 0.562 † | 0.528 * |
| Knee Extensor Strength (N) | −0.523 * | −0.681 † | 0.515 * | 0.495 * |
| Dependent Variable | Predictor Variables | Standardized β | Adjusted R2 |
|---|---|---|---|
| SPPB | |||
| Step 1 | RF EI | −0.575 † | 0.293 † |
| Step 2 | RF EI | −0.481 * | 0.449 † |
| HGS | 0.431 * | ||
| TUG | |||
| Step 1 | MG EI | 0.567 * | 0.265 * |
| Gait Speed | |||
| Step 1 | LLEI | −0.556 * | 0.256 * |
| Step 2 | LL EI | −0.426 * | 0.585 † |
| HGS | 0.594 † | ||
| Step 3 | LLEI | −0.500 † | 0.731 † |
| HGS | 0.532 † | ||
| BMI | −0.390 * | ||
| Motor memory | |||
| Step 1 | TA_MCV | 0.634 † | 0.362 † |
| SPMSQ | |||
| Step 1 | RF EI | −0.459 * | 0.167 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mateos-Angulo, A.; Galán-Mercant, A.; Cuesta-Vargas, A.I. Muscle Thickness and Echo Intensity by Ultrasonography and Cognitive and Physical Dimensions in Older Adults. Diagnostics 2021, 11, 1471. https://doi.org/10.3390/diagnostics11081471
Mateos-Angulo A, Galán-Mercant A, Cuesta-Vargas AI. Muscle Thickness and Echo Intensity by Ultrasonography and Cognitive and Physical Dimensions in Older Adults. Diagnostics. 2021; 11(8):1471. https://doi.org/10.3390/diagnostics11081471
Chicago/Turabian StyleMateos-Angulo, Alvaro, Alejandro Galán-Mercant, and Antonio Ignacio Cuesta-Vargas. 2021. "Muscle Thickness and Echo Intensity by Ultrasonography and Cognitive and Physical Dimensions in Older Adults" Diagnostics 11, no. 8: 1471. https://doi.org/10.3390/diagnostics11081471
APA StyleMateos-Angulo, A., Galán-Mercant, A., & Cuesta-Vargas, A. I. (2021). Muscle Thickness and Echo Intensity by Ultrasonography and Cognitive and Physical Dimensions in Older Adults. Diagnostics, 11(8), 1471. https://doi.org/10.3390/diagnostics11081471








