Effect of Liraglutide on Vascular Inflammation Evaluated by [64Cu]DOTATATE
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Blood and Urine Analysis
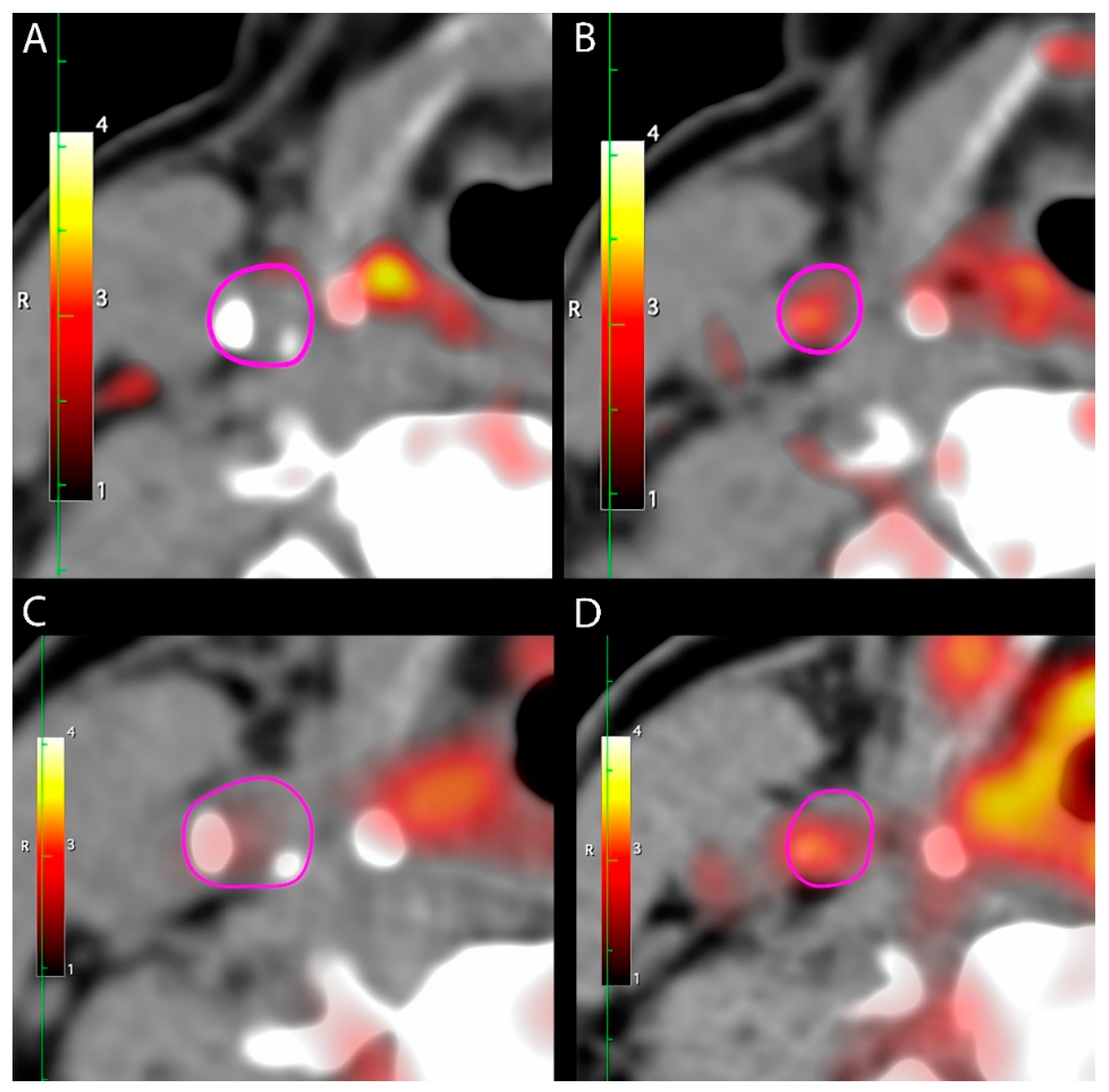
2.3. [64Cu]DOTATATE PET/CT
2.4. [18F]FDG PET/CT
2.5. Statistical Analysis
3. Results
3.1. Patients
3.2. [64Cu]DOTATATE Uptake in Carotid Arteries
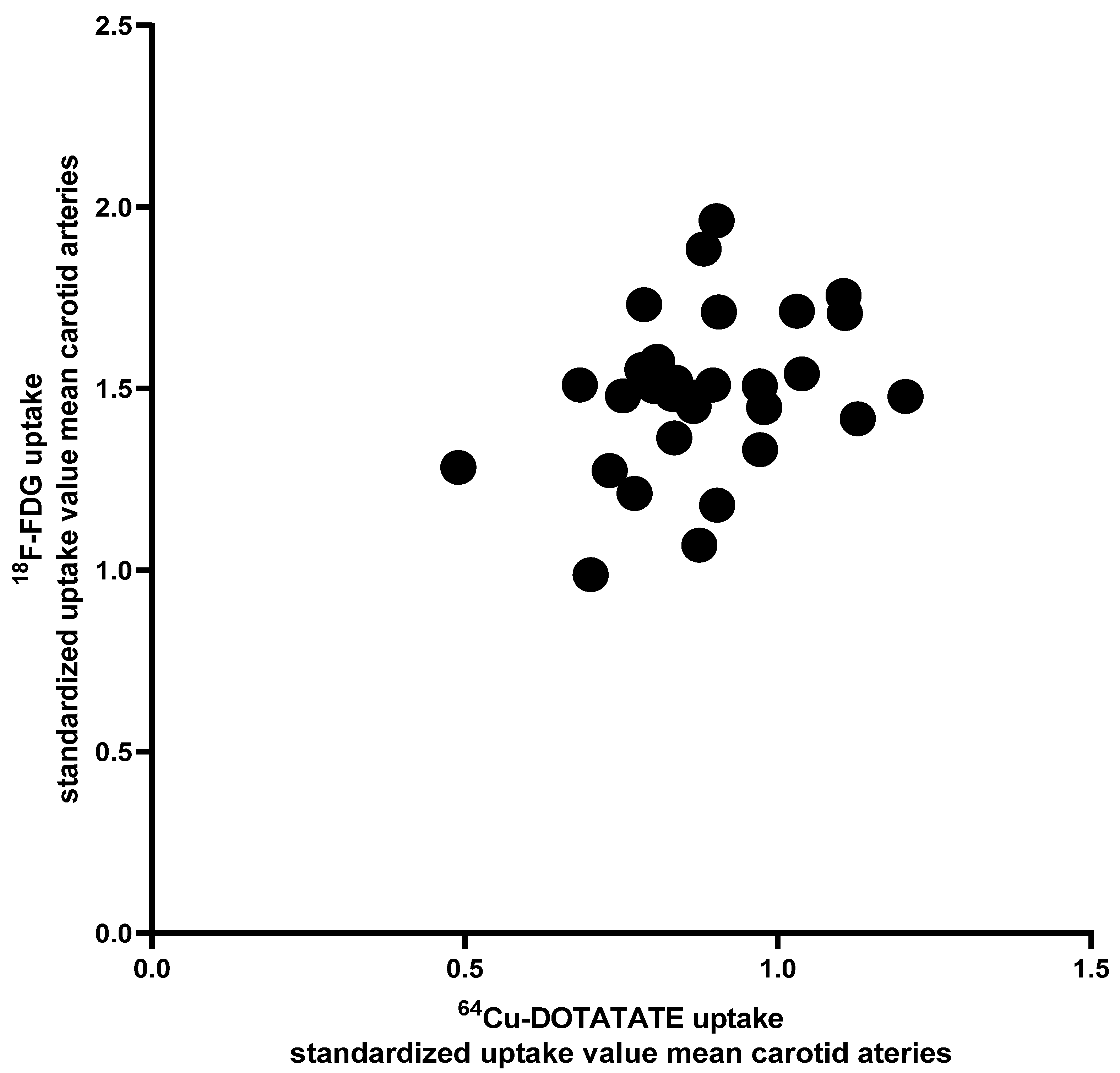
3.3. [64Cu]DOTATATE Uptake vs. [18F]FDG Uptake in Carotid Arteries
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rudd, J.H.; Myers, K.S.; Bansilal, S.; Machac, J.; Rafique, A.; Farkouh, M.; Fuster, V.; Fayad, Z.A. 18Fluorodeoxyglucose positron emission tomography imaging of atherosclerotic plaque inflammation is highly reproducible: Implications for atherosclerosis therapy trials. J. Am. Coll. Cardiol. 2007, 50, 892–896. [Google Scholar] [CrossRef] [Green Version]
- Tahara, N.; Kai, H.; Ishibashi, M.; Nakaura, H.; Kaida, H.; Baba, K.; Hayabuchi, N.; Imaizumi, T. Simvastatin attenuates plaque inflammation: Evaluation by fluorodeoxyglucose positron emission tomography. J. Am. Coll. Cardiol. 2006, 48, 1825–1831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Bauer, W.; Kreissl, M.C.; Weirather, J.; Bauer, E.; Israel, I.; Richter, D.; Riehl, G.; Buck, A.; Samnick, S. Specific somatostatin receptor II expression in arterial plaque: 68Ga-DOTATATE autoradiographic, immunohistochemical and flow cytometric studies in apoE-deficient mice. Atherosclerosis 2013, 230, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Samnick, S.; Lapa, C.; Israel, I.; Buck, A.K.; Kreissl, M.C.; Bauer, W. 68Ga-DOTATATE PET/CT for the detection of inflammation of large arteries: Correlation with18F-FDG, calcium burden and risk factors. EJNMMI Res. 2012, 2, 52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malmberg, C.; Ripa, R.S.; Johnbeck, C.B.; Knigge, U.; Langer, S.W.; Mortensen, J.; Oturai, P.; Loft, A.; Hag, A.M.; Kjaer, A. 64Cu-DOTATATE for noninvasive assessment of atherosclerosis in large arteries and its correlation with risk factors: Head-to-head comparison with 68Ga-DOTATOC in 60 patients. J. Nucl. Med. 2015, 56, 1895–1900. [Google Scholar] [CrossRef] [Green Version]
- Pedersen, S.F.; Sandholt, B.V.; Keller, S.H.; Hansen, A.E.; Clemmensen, A.E.; Sillesen, H.; Hojgaard, L.; Ripa, R.S.; Kjaer, A. 64Cu-DOTATATE PET/MRI for detection of activated macrophages in carotid atherosclerotic plaques: Studies in patients undergoing endarterectomy. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 1696–1703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tarkin, J.M.; Joshi, F.R.; Evans, N.R.; Chowdhury, M.M.; Figg, N.L.; Shah, A.V.; Starks, L.T.; Martin-Garrido, A.; Manavaki, R.; Yu, E.; et al. Detection of atherosclerotic inflammation by (68)Ga-DOTATATE PET compared to [(18)F]FDG PET imaging. J. Am. Coll. Cardiol. 2017, 69, 1774–1791. [Google Scholar] [CrossRef]
- Marso, S.P.; Bain, S.C.; Consoli, A.; Eliaschewitz, F.G.; Jodar, E.; Leiter, L.A.; Lingvay, I.; Rosenstock, J.; Seufert, J.; Warren, M.L.; et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N. Engl. J. Med. 2016, 375, 1834–1844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marso, S.P.; Daniels, G.H.; Brown-Frandsen, K.; Kristensen, P.; Mann, J.F.; Nauck, M.A.; Nissen, S.E.; Pocock, S.; Poulter, N.R.; Ravn, L.S.; et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N. Engl. J. Med. 2016, 375, 311–322. [Google Scholar] [CrossRef] [Green Version]
- Hernandez, A.F.; Green, J.B.; Janmohamed, S.; D’Agostino, R.B., Sr.; Granger, C.B.; Jones, N.P.; Leiter, L.A.; Rosenberg, A.E.; Sigmon, K.N.; Somerville, M.C.; et al. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (harmony outcomes): A double-blind, randomised placebo-controlled trial. Lancet 2018, 392, 1519–1529. [Google Scholar] [CrossRef] [Green Version]
- Gerstein, H.C.; Colhoun, H.M.; Dagenais, G.R.; Diaz, R.; Lakshmanan, M.; Pais, P.; Probstfield, J.; Riesmeyer, J.S.; Riddle, M.C.; Ryden, L.; et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): A double-blind, randomised placebo-controlled trial. Lancet 2019, 394, 121–130. [Google Scholar] [CrossRef]
- Nagashima, M.; Watanabe, T.; Terasaki, M.; Tomoyasu, M.; Nohtomi, K.; Kim-Kaneyama, J.; Miyazaki, A.; Hirano, T. Native incretins prevent the development of atherosclerotic lesions in apolipoprotein E knockout mice. Diabetologia 2011, 54, 2649–2659. [Google Scholar] [CrossRef] [Green Version]
- Rakipovski, G.; Rolin, B.; Nohr, J.; Klewe, I.; Frederiksen, K.S.; Augustin, R.; Hecksher-Sorensen, J.; Ingvorsen, C.; Polex-Wolf, J.; Knudsen, L.B. The GLP-1 analogs liraglutide and semaglutide reduce atherosclerosis in ApoE(−/−) and LDLr(−/−) mice by a mechanism that includes inflammatory pathways. JACC Basic Transl. Sci. 2018, 3, 844–857. [Google Scholar] [CrossRef] [PubMed]
- Tashiro, Y.; Sato, K.; Watanabe, T.; Nohtomi, K.; Terasaki, M.; Nagashima, M.; Hirano, T. A glucagon-like peptide-1 analog liraglutide suppresses macrophage foam cell formation and atherosclerosis. Peptides 2014, 54, 19–26. [Google Scholar] [CrossRef]
- Arakawa, M.; Mita, T.; Azuma, K.; Ebato, C.; Goto, H.; Nomiyama, T.; Fujitani, Y.; Hirose, T.; Kawamori, R.; Watada, H. Inhibition of monocyte adhesion to endothelial cells and attenuation of atherosclerotic lesion by a glucagon-like peptide-1 receptor agonist, exendin-4. Diabetes 2010, 59, 1030–1037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ripa, R.S.; Zobel, E.H.; von Scholten, B.J.; Jensen, J.K.; Binderup, T.; Diaz, L.J.; Curovic, V.R.; Hansen, T.W.; Rossing, P.; Kjaer, A. Effect of liraglutide on arterial inflammation assessed as [(18)F]FDG uptake in patients with type 2 diabetes: A randomized, double-blind, placebo-controlled trial. Circ. Cardiovasc. Imaging 2021, 14, e012174. [Google Scholar] [CrossRef]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F., 3rd; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef] [PubMed]
- von Scholten, B.J.; Persson, F.; Rosenlund, S.; Eugen-Olsen, J.; Pielak, T.; Faber, J.; Hansen, T.W.; Rossing, P. Effects of liraglutide on cardiovascular risk biomarkers in patients with type 2 diabetes and albuminuria: A sub-analysis of a randomized, placebo-controlled, double-blind, crossover trial. Diabetes Obes. Metab. 2017, 19, 901–905. [Google Scholar] [CrossRef]
- Gaspari, T.; Welungoda, I.; Widdop, R.E.; Simpson, R.W.; Dear, A.E. The GLP-1 receptor agonist liraglutide inhibits progression of vascular disease via effects on atherogenesis, plaque stability and endothelial function in an ApoE(−/−) mouse model. Diab. Vasc. Dis. Res. 2013, 10, 353–360. [Google Scholar] [CrossRef]
- Helmstadter, J.; Frenis, K.; Filippou, K.; Grill, A.; Dib, M.; Kalinovic, S.; Pawelke, F.; Kus, K.; Kroller-Schon, S.; Oelze, M.; et al. Endothelial GLP-1 (glucagon-like peptide-1) receptor mediates cardiovascular protection by liraglutide in mice with experimental arterial hypertension. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 145–158. [Google Scholar] [CrossRef]
- Hogan, A.E.; Gaoatswe, G.; Lynch, L.; Corrigan, M.A.; Woods, C.; O’Connell, J.; O’Shea, D. Glucagon-like peptide 1 analogue therapy directly modulates innate immune-mediated inflammation in individuals with type 2 diabetes mellitus. Diabetologia 2014, 57, 781–784. [Google Scholar] [CrossRef] [PubMed]
- Balestrieri, M.L.; Rizzo, M.R.; Barbieri, M.; Paolisso, P.; D’Onofrio, N.; Giovane, A.; Siniscalchi, M.; Minicucci, F.; Sardu, C.; D’Andrea, D.; et al. Sirtuin 6 expression and inflammatory activity in diabetic atherosclerotic plaques: Effects of incretin treatment. Diabetes 2015, 64, 1395–1406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tawakol, A.; Fayad, Z.A.; Mogg, R.; Alon, A.; Klimas, M.T.; Dansky, H.; Subramanian, S.S.; Abdelbaky, A.; Rudd, J.H.; Farkouh, M.E.; et al. Intensification of statin therapy results in a rapid reduction in atherosclerotic inflammation: Results of a multicenter fluorodeoxyglucose-positron emission tomography/computed tomography feasibility study. J. Am. Coll. Cardiol. 2013, 62, 909–917. [Google Scholar] [CrossRef] [Green Version]
- Mizoguchi, M.; Tahara, N.; Tahara, A.; Nitta, Y.; Kodama, N.; Oba, T.; Mawatari, K.; Yasukawa, H.; Kaida, H.; Ishibashi, M.; et al. Pioglitazone attenuates atherosclerotic plaque inflammation in patients with impaired glucose tolerance or diabetes a prospective, randomized, comparator-controlled study using serial FDG PET/CT imaging study of carotid artery and ascending aorta. JACC Cardiovasc. Imaging 2011, 4, 1110–1118. [Google Scholar] [CrossRef] [Green Version]
- Tawakol, A.; Migrino, R.Q.; Bashian, G.G.; Bedri, S.; Vermylen, D.; Cury, R.C.; Yates, D.; LaMuraglia, G.M.; Furie, K.; Houser, S.; et al. In vivo 18F-fluorodeoxyglucose positron emission tomography imaging provides a noninvasive measure of carotid plaque inflammation in patients. J. Am. Coll. Cardiol. 2006, 48, 1818–1824. [Google Scholar] [CrossRef] [Green Version]
- Rudd, J.H.; Warburton, E.A.; Fryer, T.D.; Jones, H.A.; Clark, J.C.; Antoun, N.; Johnstrom, P.; Davenport, A.P.; Kirkpatrick, P.J.; Arch, B.N.; et al. Imaging atherosclerotic plaque inflammation with [18F]-fluorodeoxyglucose positron emission tomography. Circulation 2002, 105, 2708–2711. [Google Scholar] [CrossRef] [Green Version]
- van der Valk, F.M.; Verweij, S.L.; Zwinderman, K.A.; Strang, A.C.; Kaiser, Y.; Marquering, H.A.; Nederveen, A.J.; Stroes, E.S.; Verberne, H.J.; Rudd, J.H. Thresholds for Arterial Wall Inflammation Quantified by (18)F-FDG PET Imaging: Implications for Vascular Interventional Studies. JACC Cardiovasc. Imaging 2016, 9, 1198–1207. [Google Scholar] [CrossRef]
- Sadeghi, M.M. (18)F-FDG PET and vascular inflammation: Time to refine the paradigm? J. Nucl. Cardiol. 2015, 22, 319–324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Mashhadi, R.H.; Tolbod, L.P.; Bloch, L.O.; Bjorklund, M.M.; Nasr, Z.P.; Al-Mashhadi, Z.; Winterdahl, M.; Frokiaer, J.; Falk, E.; Bentzon, J.F. 18Fluorodeoxyglucose accumulation in arterial tissues determined by pet signal analysis. J. Am. Coll. Cardiol. 2019, 74, 1220–1232. [Google Scholar] [CrossRef]


| Total (n = 30) | Liraglutide (n = 15) | Placebo (n = 15) | p Value | |
|---|---|---|---|---|
| Sex (Women) | 5 (16.7%) | 2 (13.3%) | 3 (20.0%) | 1.0 |
| Age (years) | 66.4 (7.2) | 65.9 (8.3) | 66.9 (6.3) | 0.69 |
| Body mass index (kg/m2) | 28.9 (4.3) | 29.5 (4.0) | 28.2 (4.7) | 0.41 |
| Type 2 diabetes | ||||
| Known duration of DM (years) | 12.3 [5.7–19.8] | 13.9 [5.9–20.9] | 8.8 [5.5–17.2] | 0.43 |
| HbA1c (mmol/mol) | 56.4 (9.2) | 59.1 (10.4) | 53.7 (7.0) | 0.11 |
| Kidney function | ||||
| Estimated glomerular filtration rate (mL/min/1.73 m2) | 85.8 (13.3) | 86.2 (14.4) | 85.4 (12.6) | 0.88 |
| Urinary albumin creatinine ratio (mg/g) | 5.5 [4.5–12.5] | 5.5 [2.5–12.5] | 5.5 [5.0–14.5] | 0.74 |
| Cardiovascular risk factors | ||||
| Systolic blood pressure (mmHg) | 134 (19) | 133 (14) | 136 (24) | 0.68 |
| LDL cholesterol (mmol/L) | 2.2 (0.51) | 2.2 (0.54) | 2.1 (0.49) | 0.70 |
| Triglycerides (mmol/L) | 1.7 (0.88) | 2.1 (0.97) | 1.3 (0.55) | 0.01 |
| Current smoker | 1 (3.3%) | 1 (6.7%) | 0 (0%) | 1.0 |
| Hypertension | 22 (73.3%) | 12 (80.0%) | 10 (66.7%) | 0.68 |
| High-sensitivity C-reactive protein (mg/L) | 1.4 [0.9–3.1] | 1.4 [1.0–3.3] | 2.0 [0.8–2.8] | 0.88 |
| History of cardiovascular disease * | 8 (26.7%) | 6 (40.0%) | 2 (13.3%) | 0.21 |
| Glucose-lowering medication | ||||
| Insulin use | 12 (40.0%) | 8 (53.3%) | 4 (26.7%) | 0.14 |
| SGLT2 inhibitors | 6 (20.0%) | 3 (20.0%) | 3 (20.0%) | 1.0 |
| Cardiovascular medication | ||||
| Aspirin treatment | 10 (33.3%) | 4 (26.7%) | 6 (40.0%) | 0.44 |
| Lipid-lowering treatment | 27 (90.0%) | 14 (93.3%) | 13 (86.7%) | 1.0 |
| Included in Substudy (n = 30) | Not Included in Substudy (n = 72) | p-Values | |
|---|---|---|---|
| Sex (Women) | 5 (16.7%) | 11 (15.3%) | 1.0 |
| Age (years) | 66.4 (7.2) | 66.4 (8.6) | 1.0 |
| Body mass index (kg/m2) | 28.9 (4.3) | 30.3 (4.7) | 0.15 |
| Type 2 diabetes | |||
| Known duration of DM (years) | 12.3 [5.7–19.8] | 10.6 [5.7–18.2] | 0.30 |
| HbA1c (mmol/mol) | 56.4 (9.2) | 57.0 [52.0-64.0] | 0.20 |
| Kidney function | |||
| Estimated glomerular filtration rate (mL/min/1.73 m2) | 85.8 (13.3) | 82.1 (17.3) | 0.30 |
| Urinary albumin creatinine ratio (mg/g) | 5.5 [4.5–12.5] | 6.3 [3.5–16.0] | 0.30 |
| Cardiovascular risk factors | |||
| Systolic blood pressure (mmHg) | 134 (19) | 136 (17) | 0.61 |
| LDL cholesterol (mmol/L) | 2.2 (0.51) | 2.1 (0.73) | 0.48 |
| Triglycerides (mmol/L) | 1.7 (0.88) | 1.9 (1.1) | 0.33 |
| Current smoker | 1 (3.3%) | 13 (18.1%) | 0.06 |
| Hypertension | 22 (73.3%) | 57 (79.2%) | 0.52 |
| HsCRP (mg/L) | 1.4 [0.9–3.1] | 1.6 [0.86–4.2] | 0.10 |
| History of cardiovascular disease ** | 8 (26.7%) | 15 (20.8%) | 0.52 |
| Glucose-lowering medication | |||
| Insulin use | 12 (40.0%) | 27 (37.5%) | 0.81 |
| SGLT2 inhibitors | 6 (20.0%) | 14 (19.4%) | 0.95 |
| Cardiovascular medication | |||
| Aspirin treatment | 10 (33.3%) | 27 (37.5%) | 0.69 |
| Lipid-lowering treatment *** | 27 (90.0%) | 61 (84.7%) | 0.75 |
| Baseline Mean (SD) | End-of-Treatment Mean (SD) | p Value | ∆ (95% CI) | p Value | |
|---|---|---|---|---|---|
| [64Cu]DOTATATE uptake | |||||
| Carotid SUVmean | |||||
| Liraglutide, n = 15 | 0.90 (0.15) | 0.79 (0.15) | 0.01 | −0.11 (−0.19; −0.03) | 0.44 |
| Placebo, n = 15 | 0.86 (0.15) | 0.80 (0.11) | 0.08 | −0.07 (−0.14; 0.01) | |
| [18F]FDG uptake | |||||
| Carotid SUVmean | |||||
| Liraglutide, n = 15 | 1.4 (0.24) | 1.4 (0.23) | 0.28 | −0.04 (−0.12; 0.04) | 0.83 |
| Placebo, n = 15 | 1.5 (0.20) | 1.5 (0.21) | 0.25 | −0.06 (−0.16; 0.04) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zobel, E.H.; Ripa, R.S.; von Scholten, B.J.; Curovic, V.R.; Diaz, L.J.; Hansen, T.W.; Rossing, P.; Kjaer, A. Effect of Liraglutide on Vascular Inflammation Evaluated by [64Cu]DOTATATE. Diagnostics 2021, 11, 1431. https://doi.org/10.3390/diagnostics11081431
Zobel EH, Ripa RS, von Scholten BJ, Curovic VR, Diaz LJ, Hansen TW, Rossing P, Kjaer A. Effect of Liraglutide on Vascular Inflammation Evaluated by [64Cu]DOTATATE. Diagnostics. 2021; 11(8):1431. https://doi.org/10.3390/diagnostics11081431
Chicago/Turabian StyleZobel, Emilie H., Rasmus S. Ripa, Bernt J. von Scholten, Viktor Rotbain Curovic, Lars Jorge Diaz, Tine W. Hansen, Peter Rossing, and Andreas Kjaer. 2021. "Effect of Liraglutide on Vascular Inflammation Evaluated by [64Cu]DOTATATE" Diagnostics 11, no. 8: 1431. https://doi.org/10.3390/diagnostics11081431
APA StyleZobel, E. H., Ripa, R. S., von Scholten, B. J., Curovic, V. R., Diaz, L. J., Hansen, T. W., Rossing, P., & Kjaer, A. (2021). Effect of Liraglutide on Vascular Inflammation Evaluated by [64Cu]DOTATATE. Diagnostics, 11(8), 1431. https://doi.org/10.3390/diagnostics11081431







