Impact of 18F-FDG PET/CT on Clinical Management of Suspected Radio-Iodine Refractory Differentiated Thyroid Cancer (RAI-R-DTC)
Abstract
:1. Introduction
2. Materials and Methods
Statistical Analysis
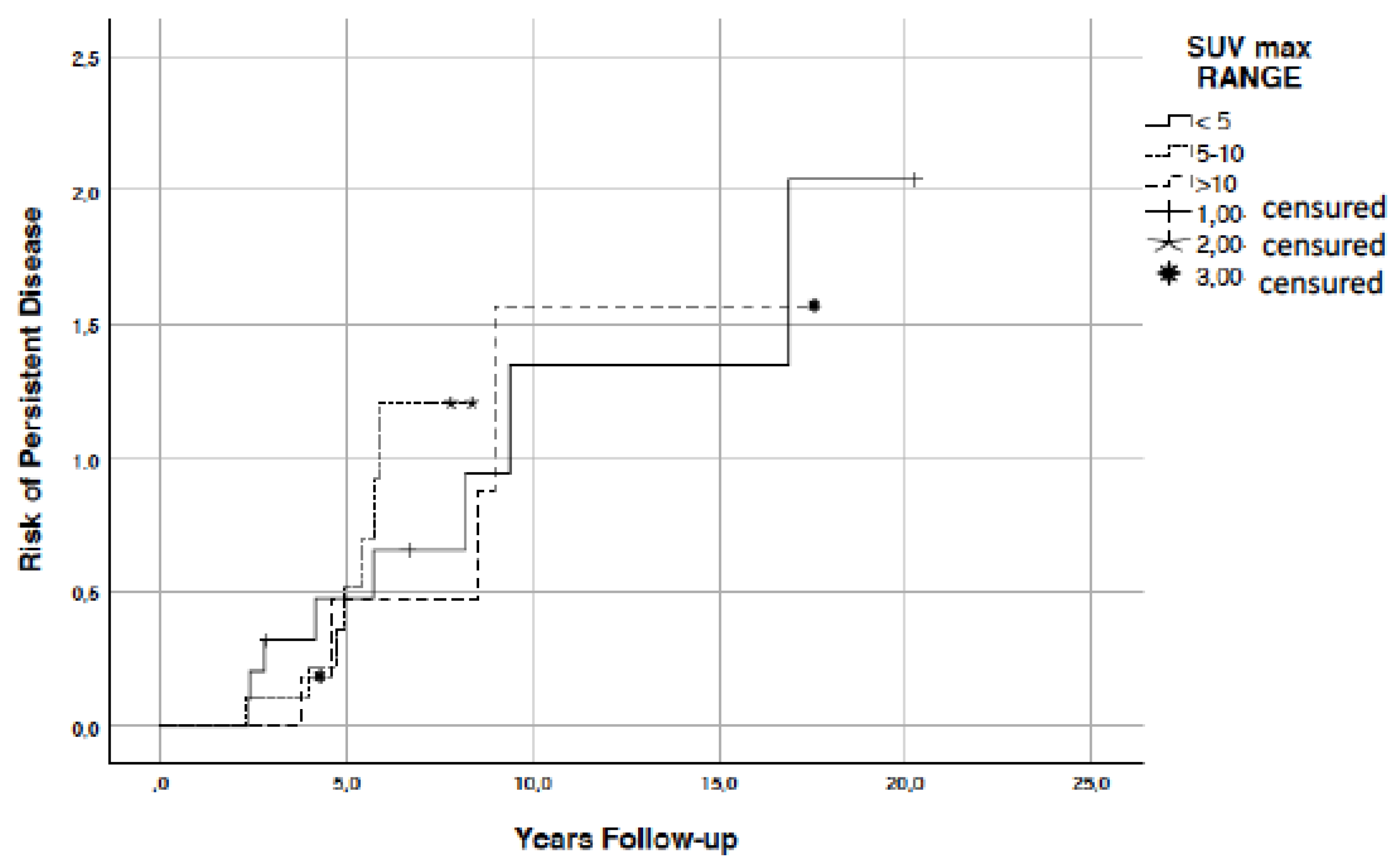
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: The Amercan Thyroid Association guidelines task force on thyroid nodules and diferentiated thyroid cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casara, D.; Rubello, D.; Saladini, G.; Masarotto, G.; Favero, A.; Girelli, M.E.; Busnardo, B. Different features of pulmonary metastases in differentiated thyroid cancer: Natural history and multivariate statistical analysis of prognostic variables. J. Nucl. Med. 1993, 34, 1626–1631. [Google Scholar]
- Kitamura, Y.; Shimizu, K.; Nagahama, M.; Sugino, K.; Ozaki, O.; Mimura, T.; Ito, K.; Ito, K.; Tanaka, S. Immediate causes of death in thyroid carcinoma: Clinicopathological analysis of 161 fatal cases. J. Clin. Endocrinol. Metab. 1999, 84, 4043–4049. [Google Scholar] [CrossRef]
- Durante, C.; Haddy, N.; Baudin, E.; Leboulleux, S.; Hartl, D.; Travagli, J.P.; Caillou, B.; Ricard, M.; Lumbroso, J.D.; De Vathaire, F.; et al. Long-term outcome of 444 patients with distant metastases from papillary and follicu-lar thyroid carcinoma: Benefits and limits of radioiodine therapy. J. Clin. Endocrinol. Metab. 2006, 91, 2892–2899. [Google Scholar] [CrossRef] [PubMed]
- Silberstein, E.B. The problem of the patient with thyroglobulin elevation but negative iodine scintigraphy: The TENIS syndrome. Semin. Nucl. Med. 2011, 41, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Qichang, W.; Lin, B.; Gege, Z.; Youjia, Z.; Qingjie, M.; Renjie, W.; Bin, J.; Wan, Q.; Bai, L.; Zhao, G.; et al. Diagnostic performance of 18F-FDG-PET/CT in DTC patients with thyroglobulin elevation and negative iodine scintigraphy: A meta-analysis. Eur. J. Endocrinol. 2019, 181, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Weber, T.; Ohlhauser, D.; Hillenbrand, A.; Henne-Bruns, D.; Reske, S.N.; Luster, M. Impact of FDG-PET computed tomography for surgery of recurrent or persistent differentiated thyroid carcinoma. Horm. Metab. Res. 2012, 44, 904–908. [Google Scholar] [CrossRef]
- Kiess, A.P.; Agrawal, N.; Brierley, J.D.; Duvvuri, U.; Ferris, R.L.; Genden, E.M.; Wong, R.J.; Tuttle, R.M.; Lee, N.Y.; Randolph, G.W. External-beam radiotherapy for differentiated thyroid cancer locoregional control: A statement of the American Head and Neck Society. Head Neck 2016, 38, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Wahl, R.L.; Jacene, H.; Kasamon, Y.; Lodge, M.A. From RECIST to PERCIST: Evolving considerations for PET response criteria in solid tumors. J. Nucl. Med. 2009, 50, 122S–150S. [Google Scholar] [CrossRef] [Green Version]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumors: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef] [PubMed]
- Boellaard, R.; Delgado-Bolton, R.; Oyen, W.J.; Giammarile, F.; Tatsch, K.; Eschner, W.; Verzijlbergen, F.J.; Barrington, S.F.; Pike, L.C.; Weber, W.A.; et al. FDG PET/CT: EANM procedure guidelines for tumor imaging: Version 2.0. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 328–354. [Google Scholar] [CrossRef] [PubMed]
- Na, S.J.; Yoo, l.R.; O, J.H.; Lin, C.; Lin, Q.; Kim, S.H.; Chung, S.K. Diagnostic accuracy of (18)F-fluorodeoxyglucose positron emission tomograpy/computed tomog-raphy in differentiated thyroid cancer patients with elevated thyroglobulin and negative (131)I whole body scan: Evaluation by thyroglobulin level. Ann. Nucl. Med. 2012, 26, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Pacini, F.; Ito, Y.; Luster, M.; Pitoia, F.; Robinson, B.; Wirth, L. Radioactive iodine-refractory differentiated thyroid cancer: Unmet needs and future directions. Expert Rev. Endocrinol. Metab. 2012, 7, 541–554. [Google Scholar] [CrossRef]
- Salvatori, M.; Biondi, B.; Rufini, V. Imaging in endocrinology: 2-18F-fluoro-2-deoxy-D-glucose positron emission tomogra-phy/computed tomography in differentiated thyroid carcinoma: Clinical indications and controversies in diagnosis and follow-up. Eur. J. Endocrinol. 2015, 173, 115–130. [Google Scholar] [CrossRef] [Green Version]
- Dong, M.-J.; Liu, Z.-F.; Zhao, K.; Ruan, L.-X.; Wang, G.-L.; Yang, S.-Y.; Sun, F.; Luo, X.-G. Value of 18F-FDG-PET/PET-CT in differentiated thyroid carcinoma with radioiodine-negative whole-body scan: A meta-analysis. Nucl. Med. Commun. 2009, 30, 639–650. [Google Scholar] [CrossRef]
- Deandreis, D.; Al Ghuzlan, A.; Leboulleux, S.; Lacroix, L.; Garsi, J.P.; Talbot, M.; Lumbroso, J.; Baudin, E.; Caillou, B.; Bidart, J.M.; et al. Do histological, immunohistochemical, and metabolic (radioiodine and fluorodeoxyglucose uptakes) patterns of metastatic thyroid cancer correlate with patient outcome? Endocr. Relat. Cancer 2010, 18, 159–169. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Larson, S.M.; Fazzari, M.; Tickoo, S.K.; Kolbert, K.; Sgouros, G.; Yeung, H.; Macapinlac, H.; Rosai, J.; Robbins, R.J. Prognostic value of 18F-fluorodeoxyglucose positron emission tomographic scanning in patients with thyroid cancer. J. Clin. Endocrinol. Metab. 2000, 85, 1107–1113. [Google Scholar] [CrossRef]
- Masson-Deshayes, S.; Schvartz, C.; Dalban, C.; Guendouzen, S.; Pochart, J.-M.; Dalac, A.; Fieffe, S.; Bruna-Muraille, C.; Dabakuyo-Yonli, T.S.; Papathanassiou, D. Prognostic value of 18F-FDG PET/CT metabolic parameters in metastatic differentiated thyroid cancers. Clin. Nucl. Med. 2015, 40, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Manohar, P.M.; Beesley, L.J.; Bellile, E.L.; Worden, F.P.; Avram, A.M. Prognostic value of FDG-PET/CT metabolic parameters in metastatic radioio-dine-refractory differentiated thyroid cancer. Clin. Nucl. Med. 2018, 43, 641–647. [Google Scholar] [CrossRef] [PubMed]
- Treglia, G.; Annunziata, S.; Muoio, B.; Salvatori, M.; Ceriani, L.; Giovanella, L. The role of fluorine-18-fluorodeoxyglucose positron emission tomography in aggres-sive histological subtypes of thyroid cancer: An overview. Int. J Endocrinol. 2013, 2013, 856189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pomerri, F.; Cervino, A.R.; Al Bunni, F.; Evangelista, L.; Muzzio, P.C. Therapeutic impact of 18F-FDG PET/CT in recurrent differentiated thyroid carcinoma. Radiol. Med. 2014, 119, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Giovanella, L. Positron emission tomography/computed tomography in patients treated for differentiated thyroid carcinomas. Expert Rev. Endocrinol. Metab. 2012, 7, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Ciarallo, A.; Marcus, C.; Taghipour, M.; Subramaniam, R.M. Value of fluorodeoxyglucose PET/computed tomography patient management and outcomes in thyroid cancer. PET Clin. 2015, 10, 265–278. [Google Scholar] [CrossRef] [PubMed]

| No of Patients | % | ||
|---|---|---|---|
| SEX | FEMALE MALE | 31 22 | 58.5 41.5 |
| HISTOLOGY | CLASSICALPAPILLARY FOLLICULAR PAPILLARY FOLLICULAR WIDELY INVASIVE HURTLE CARCINOMA PAPILLARY TALL CELL VAR. PAPILLARY MIXED VAR. INSULAR PAPILLARY DIFFUSE SCLEROSANT PAPILLARY SOLID VARIANT PAPILLARY COLUMNAR CELL VAR FOLLICULAR MIN INVASIVE | 30 8 4 2 2 2 1 1 1 1 1 | 56.6 15.1 7.5 3.8 3.8 3.8 1.9 1.9 1.9 1.9 1.9 |
| Stage T at diagnosis (AJCC7) | pT1a pT1b pT2 pT3 pT4a pT4b | 6 5 6 27 6 3 | 11.3 9.4 11.3 50.9 11.3 5.7 |
| Stage N at diagnosis (AJCC7) | pN0 pN1a pN1b pNx | 9 18 14 12 | 17.0 34.0 26.4 22.6 |
| Stage M at diagnosis (AJCC7) | M0 M1 | 49 4 | 92.5 7.5 |
| LVI | Yes No | 21 32 | 39.6 60.4 |
| EC | Yes No | 6 47 | 11.3 88.7 |
| R1 | Yes No | 20 33 | 37.7 62.3 |
| R2 | Yes No | 4 49 | 7.5 92.5 |
| Risk Stratification (ATA 2015) | LOW LOW/INTERMEDIATE INTERMEDIATE INTERMEDIATE/HIGH HIGH | 7 4 11 15 16 | 13.2 7.5 20.8 28.3 30.2 |
| THYROGEN use at the first RAIT | Yes No UNKNOWN | 28 24 1 | 45.3 52.8 1.9 |
| Rx-WBS first course of RAI | NEGATIVE RESIDUAL UPTAKE LYMPH NODE UPTAKE DISTANT LESION UPTAKE | 2 42 3 6 | 3.8 77.4 5.7 11.3 |
| THERAPEUTIC APPROACH BEFORE second RAIT | OBSERVATION SURGERY RTE BIOPSY SURGERY + EBRT | 36 9 4 3 1 | 67.9 17.0 7.5 5.7 1.9 |
| THYROGEN use at the second RAIT | Yes No | 8 45 | 15.1 84.9 |
| Rx-WBS at second RAIT | NEGATIVE RESIDUAL UPTAKE LYMPH NODE UPTAKE DISTANT LESION UPTAKE | 30 14 2 7 | 56.6 24.6 3.8 13.2 |
| No of Patients | % | ||
|---|---|---|---|
| 131I-FDG | 131I−/FDG− 131I+/FDG− 131I+/FDG+ 131I−/FDG+ | 24 2 7 20 | 45.3 3.8 13.2 37.7 |
| Hypermetabolic Lesion | NONE LYMPH NODES BONE LUNG MULTIPLE SITES | 26 15 3 3 6 | 49.1 28.3 5.7 5.7 11.3 |
| SUVmax range | <5 5–10 >10 | 11 10 6 | 20.8 18.9 11.3 |
| SUVmean | 27 | 50.9 | |
| MTV | 27 | 50.9 | |
| TLG | 27 | 50.9 | |
| SULpeak | 27 | 50.9 | |
| PERCIST RESPONSE | Not available CMR SMD PMR PMD | 13/27 7/27 2/27 1/27 4/27 | 48.1 25.9 7.4 3.7 14.8 |
| Therapeutic FDG PET/CT guided approach | OBSERVATION SURGERY EBRT RAI SURGERY + EBRT | 16/27 4/27 4/27 2/27 1/27 | 59.2 14.8 14.8 7.4 3.7 |
| FIRST RESTAGING | EXCELLENT RESPONSE BIOCHEMICAL PERSISTENCE STRUCTURAL PERSISTENCE | 4 34 15 | 7.5 64.0 28.0 |
| FINAL RESTAGING | EXCELLENT RESPONSE BIOCHEMICAL PERSISTENCE STRUCTURAL PERSISTENCE | 15 25 13 | 28.3 42.7 24.5 |
| PFS range (months) | <12 12–24 >12 | 8 11 34 | 15.1 20.8 64.2 |
| OS | ALIVE DEATH DISEASE RELATED DEATH NON DISEASE RELATED | 47 2 4 | 88.7 3.7 7.5 |
| 131I−/FDG− | 131I+/FDG− | 131I+/FDG+ | 131I−/FDG+ | p Value | ||
|---|---|---|---|---|---|---|
| Age | 1RAI | 49.7 ± 14.6 | 63.7 ± 13.3 | 63.8 ± 16.4 | 49.8 ± 25.5 | 0.187 |
| 2RAI | 51.2 ± 14.4 | 63.5 ± 13.4 | 65.5 ± 16.2 | 58.4 ± 20.8 | 0.127 | |
| Basal TG | 1RAI | 14.6 ± 42.7 | 1512.8 ± 1994 | 1646.2 ± 2014.0 | 4.7 ± 8.4 | 0.013 |
| 2RAI | 3.5 ± 4.8 | 160.0 ± 217.7 | 950.0 ± 1816.0 | 38.9 ± 100.5 | 0.234 | |
| Stimulated TG | 1RAI | 47.3 ± 83.9 | 3000.0 ± 0.0 | 1830.0 ± 1809.0 | 109.4 ± 319.2 | 0.004 |
| 2RAI | 19.2 ± 22.1 | 968.6 ± 1305 | 1459.0 ± 1929.0 | 295 ± 1030 | 0.028 | |
| DeltastimhTG | 53.9 ± 259.8 | −67.7 ± 43.5 | −26.4 ± 77.9 | 727.9 ± 2585 | 0.334 | |
| Cumulative 131I dose | 232.9 ± 52.8 | 297.9 ± 1.8 | 313.7 ± 74.2 | 227.2 ± 57.9 | 0.020 | |
| MTV | 50.7 ± 118.4 | 3.5 ± 1.7 | 0.361 | |||
| TLG | 78.3 ± 155.4 | 19.8 ± 22.3 | 0.213 | |||
| SUVmax | 10.8 ± 12.0 | 6.9 ± 4.0 | 0.825 | |||
| SUVmean | 4.4 ± 4.3 | 2.9 ± 1.3 | 0.618 | |||
| SULpeak | 6.1 ± 6.1 | 3.5 ± 1.7 | 0.912 | |||
| Follow up (years) | 6.2 ± 2.3 | 5.3 ± 1.0 | 5.2 ± 5.5 | 8.0 ± 4.9 | 0.076 | |
| PFS (months) | 42.5 ± 21.3 | 53.5 ± 14.8 | 21.2 ± 16.4 | 36.0 ± 22.2 | 0.079 | |
| VARIABLES | p Value | |
|---|---|---|
| 131I/FDG groups | SEX | 0.600 |
| HISTOLOGY | 0.127 | |
| T | 0.107 | |
| N | 0.039 | |
| M | 0.002 | |
| LVI | 0.502 | |
| EC | 0.097 | |
| R1 | 0.597 | |
| R2 | 0.114 | |
| RISK | 0.075 | |
| SUVmax range | 0.833 | |
| First restaging | 0.004 | |
| Final restaging | 0.098 | |
| PFS range | 0.364 | |
| SUVmax range | PET+/− GROUPS | 0.833 |
| Hypermetabolic lesion site | 0.109 | |
| PERCIST response | 0.714 | |
| Final restaging | 0.954 | |
| PFS range | 0.740 | |
| PFS range | PET+/− GROUPS | 0.149 |
| Hypermetabolic lesion site | 0.693 | |
| PERCIST response | 0.020 | |
| Final restaging | 0.050 | |
| Therapeutical PET/CT guided approach | PERCIST response | 0.091 |
| First restaging | 0.011 | |
| First and Final restaging | 0.012 | |
| FDG+/− groups | First and Final restaging | 0.070 |
| First Restaging | ||||
|---|---|---|---|---|
| Excellent Response | Biochemical Persistence | Structural Persistence | ||
| Persistence/recurrence treatment | Observation | 2.8% | 77.8% | 19.4% |
| Surgery | 40% | 0.0% | 60% | |
| EBRT | 20% | 60% | 20% | |
| RAI | 0.0% | 0.0% | 100% | |
| Surgery + EBRT | 0.0% | 100% | 0.0% | |
| 131I−/FDG− | 131I+/FDG− | 131I+/FDG+ | 131I−/FDG+ | ||
|---|---|---|---|---|---|
| N | pN0 | 22.2% | 0.0 | 0.0 | 77.8% |
| pN1a | 38.9% | 11.1% | 16.7% | 33.3% | |
| pN1b | 42.9% | 0.0 | 28.6% | 28.6% | |
| Nx | 75% | 0.0 | 0.0 | 25% | |
| M | Mx | 49% | 4.1% | 8.2% | 38.8% |
| M1 | 0.0% | 0.0% | 75.0% | 25% | |
| First restaging | Excellent response | 100% | 0.0% | 0.0% | 0.0% |
| Biochemical persistence | 64.5% | 6.5% | 3.2% | 25.8% | |
| Structural persistence | 14.3% | 0.0% | 28.6% | 57.1% | |
| PFS <12 Months | PFS 12–24 Months | PFS >24 Months | ||
|---|---|---|---|---|
| PERCIST response | NA | 40.0% | 25.0% | 64.3% |
| CMR | 0.0% | 37.5% | 28.6% | |
| SMD | 0.0% | 25.0% | 0.0% | |
| PMR | 0.0% | 0.0% | 7.1% | |
| PMD | 60% | 12.5% | 0.0% | |
| Final restaging | Excellent response | 25% | 9.1% | 35.3% |
| Biochemical persistence | 25% | 45.5% | 52.9% | |
| Structural persistence | 50% | 45.5% | 11.8% | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lodi Rizzini, E.; Repaci, A.; Tabacchi, E.; Zanoni, L.; Vicennati, V.; Cavicchi, O.; Pagotto, U.; Morganti, A.G.; Fanti, S.; Monari, F. Impact of 18F-FDG PET/CT on Clinical Management of Suspected Radio-Iodine Refractory Differentiated Thyroid Cancer (RAI-R-DTC). Diagnostics 2021, 11, 1430. https://doi.org/10.3390/diagnostics11081430
Lodi Rizzini E, Repaci A, Tabacchi E, Zanoni L, Vicennati V, Cavicchi O, Pagotto U, Morganti AG, Fanti S, Monari F. Impact of 18F-FDG PET/CT on Clinical Management of Suspected Radio-Iodine Refractory Differentiated Thyroid Cancer (RAI-R-DTC). Diagnostics. 2021; 11(8):1430. https://doi.org/10.3390/diagnostics11081430
Chicago/Turabian StyleLodi Rizzini, Elisa, Andrea Repaci, Elena Tabacchi, Lucia Zanoni, Valentina Vicennati, Ottavio Cavicchi, Uberto Pagotto, Alessio Giuseppe Morganti, Stefano Fanti, and Fabio Monari. 2021. "Impact of 18F-FDG PET/CT on Clinical Management of Suspected Radio-Iodine Refractory Differentiated Thyroid Cancer (RAI-R-DTC)" Diagnostics 11, no. 8: 1430. https://doi.org/10.3390/diagnostics11081430
APA StyleLodi Rizzini, E., Repaci, A., Tabacchi, E., Zanoni, L., Vicennati, V., Cavicchi, O., Pagotto, U., Morganti, A. G., Fanti, S., & Monari, F. (2021). Impact of 18F-FDG PET/CT on Clinical Management of Suspected Radio-Iodine Refractory Differentiated Thyroid Cancer (RAI-R-DTC). Diagnostics, 11(8), 1430. https://doi.org/10.3390/diagnostics11081430








