Left Ventricular Summit—Concept, Anatomical Structure and Clinical Significance
Abstract
1. Introduction
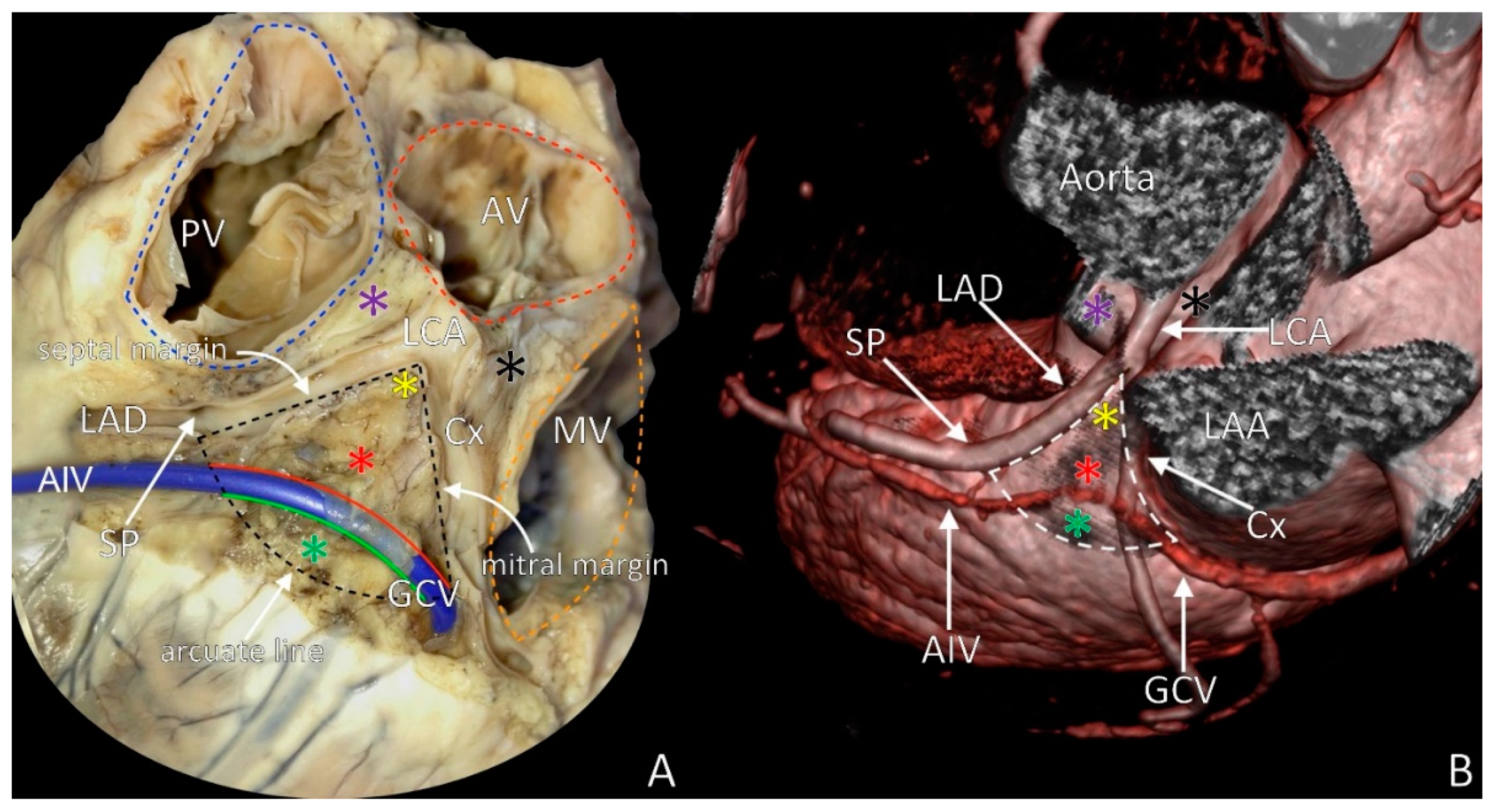
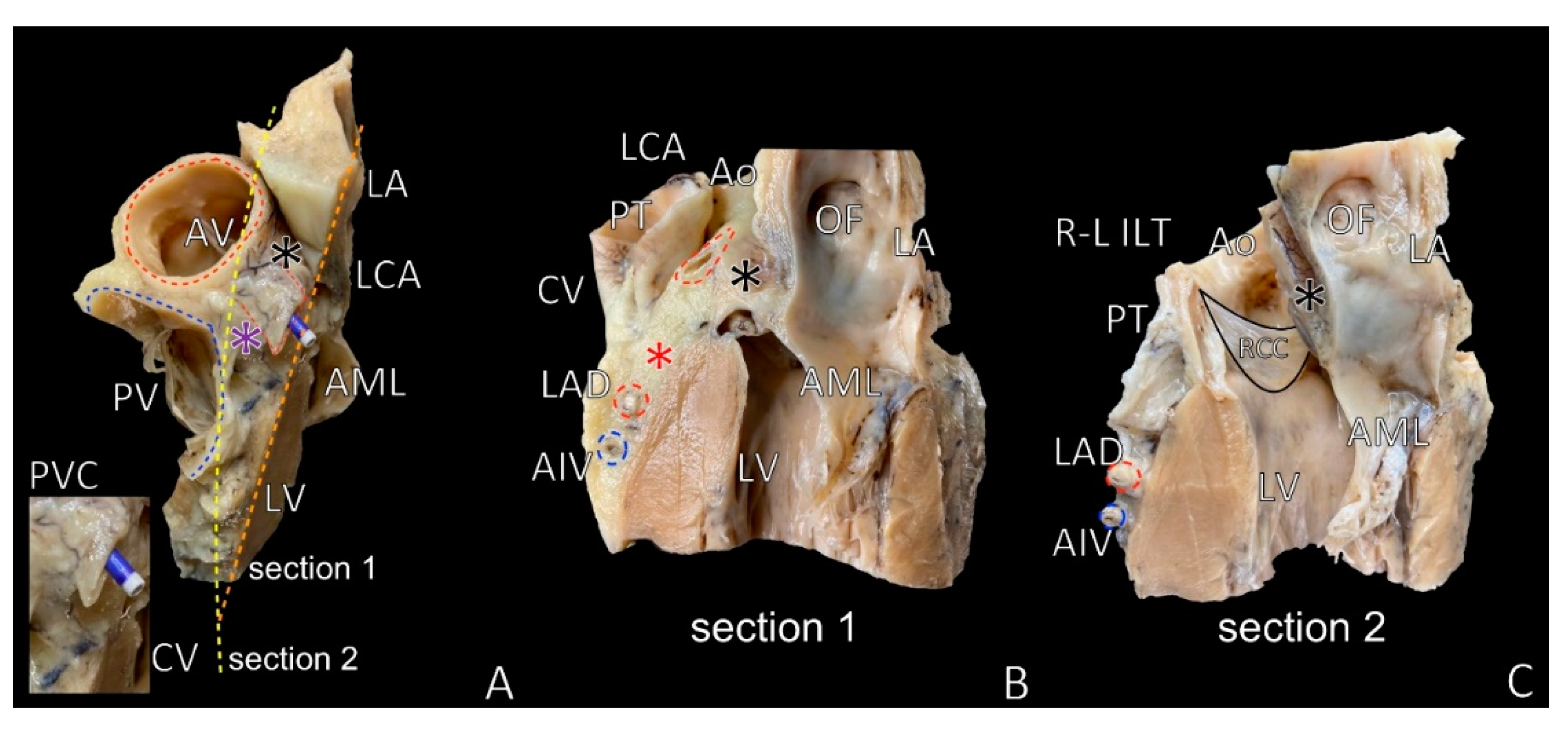
2. LVS Definition
3. LVS Apex
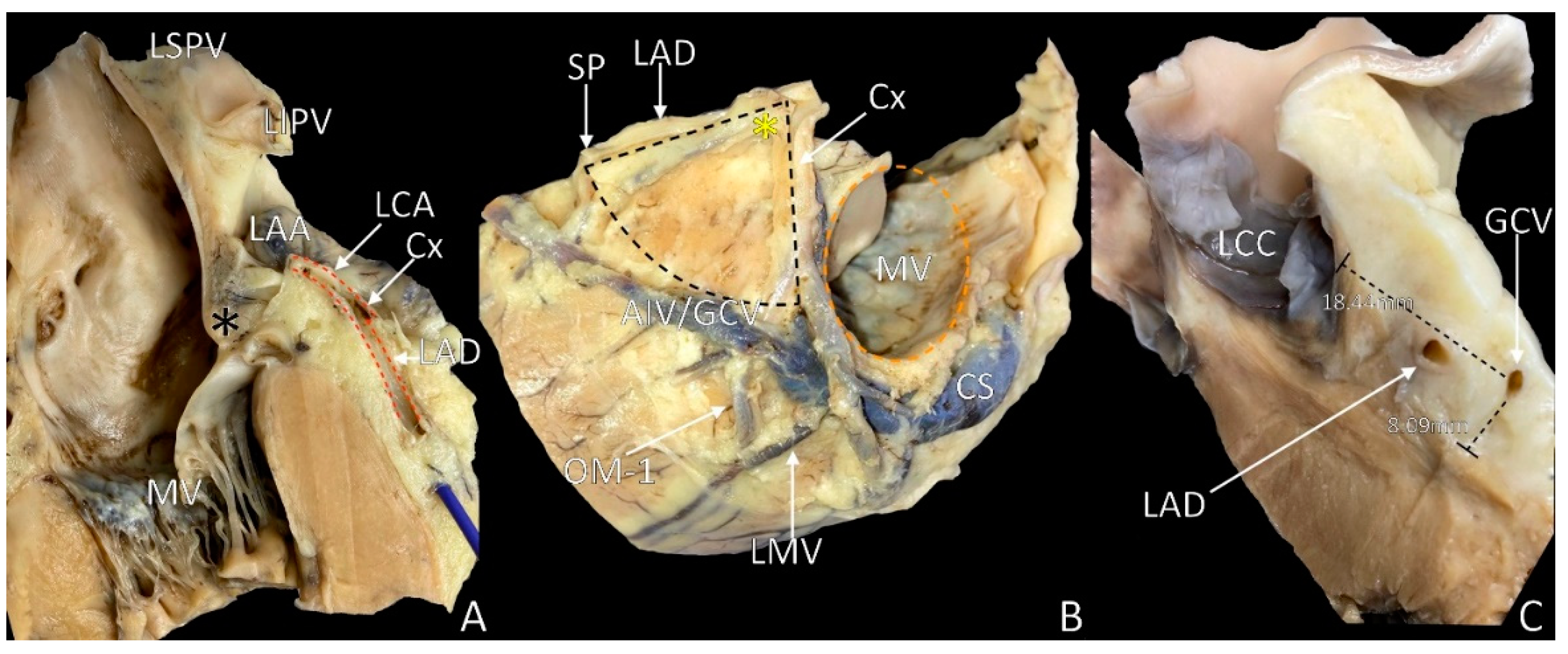
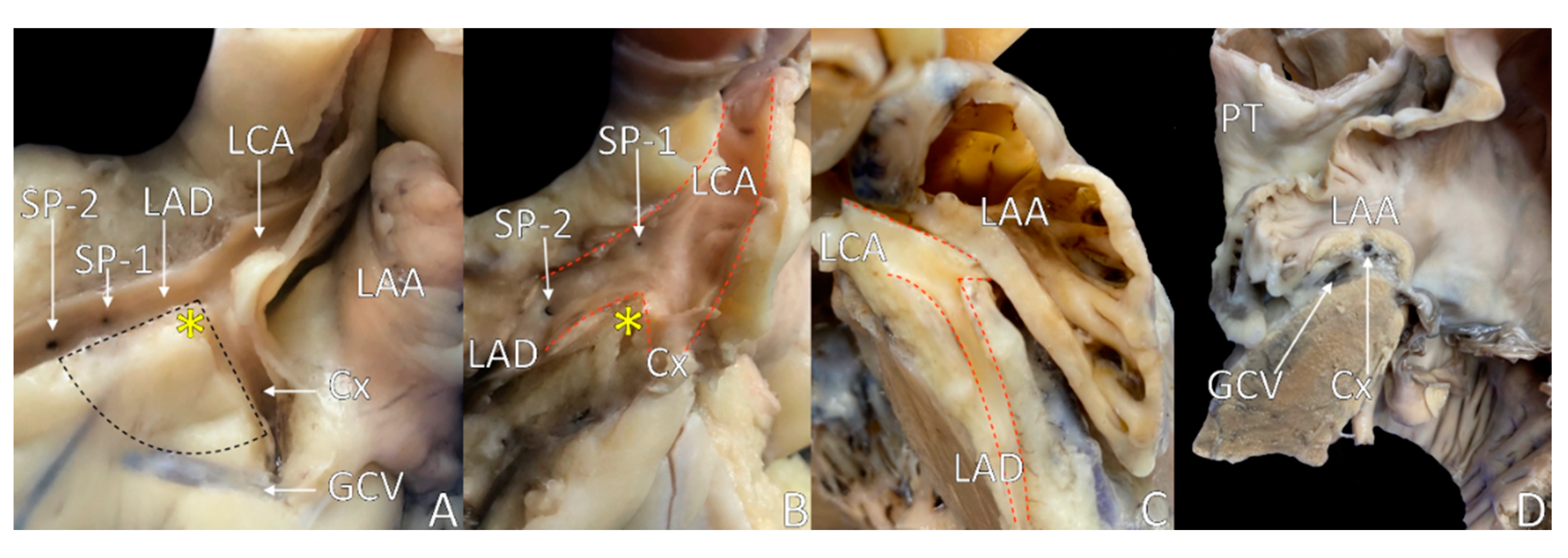
4. The Septal Margin of LVS
5. Mitral Margin of LVS
6. The Base of the LVS: Arcuate Line
7. LVS Size and Content
8. LVS Nourishment and Innervation
9. LVS Accessibility and Ablations
10. Septal Summit/Septal Aspect of the LVS
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roberts, W.; Salandy, S.; Mandal, G.; Holda, M.K.; Tomaszewski, K.A.; Gielecki, J.; Tubbs, R.S.; Loukas, M. Across the centuries: Piecing together the anatomy of the heart. Transl. Res. Anat. 2019, 17, 100051. [Google Scholar] [CrossRef]
- Dudkiewicz, D.; Słodowska, K.; Jasińska, K.A.; Dobrzynski, H.; Hołda, M.K. The clinical anatomy of the left atrial structures used as landmarks in ablation of arrhythmogenic substrates and cardiac invasive procedures. Transl. Res. Anat. 2021, 23, 100102. [Google Scholar] [CrossRef]
- Whiteman, S.; Alimi, Y.; Carrasco, M.; Gielecki, J.; Zurada, A.; Loukas, M. Anatomy of the cardiac chambers: A review of the left ventricle. Transl. Res. Anat. 2021, 23, 100095. [Google Scholar] [CrossRef]
- Kucybała, I.; Ciuk, K.; Klimek-Piotrowska, W. Clinical anatomy of the human heart atria and internal septum—Anatomical basis for interventional cardiologists and elektrocardiologists. Part 1: Right atrium and interatrial septum. Kardiol. Pol. 2018, 76, 499–509. [Google Scholar] [CrossRef]
- Ciuk, S.; Janas, P.; Klimek-Piotrowska, W. Clinical anatomy of the human heart atria and internal septum—anatomical basis for interventional cardiologists and elektrocardiologists. Part 2: Left atrium. Kardiol. Pol. 2018, 76, 510–519. [Google Scholar] [CrossRef]
- Kumagai, K. Idiopathic ventricular arrhythmias arising from the left ventricular outflow tract: Tips and tricks. J. Arrhythmia 2014, 30, 211–221. [Google Scholar] [CrossRef]
- Enriquez, A.; Malavassi, F.; Saenz, L.C.; Supple, G.; Santangeli, P.; Marchlinski, F.E.; Garcia, F.C. How to map and ablate left ventricular summit arrhythmias. Heart Rhythm. 2017, 14, 141–148. [Google Scholar] [CrossRef]
- Yamada, T.; McElderry, H.T.; Doppalapudi, H.; Okada, T.; Murakami, Y.; Yoshida, Y.; Yoshida, N.; Inden, Y.; Murohara, T.; Plumb, V.J.; et al. Idiopathic ventricular arrhythmias originating from the left ventricular summit: Anatomic concepts relevant to ablation. Circ. Arrhythmia Electrophysiol. 2010, 3, 616–623. [Google Scholar] [CrossRef]
- McAlpine, W.A. Heart and Coronary Arteries: An Anatomical Atlas for Clinical Diagnosis, Radiological Investigation, and Surgical Treatment; Springer: New York, NY, USA, 1975; pp. 160–178. [Google Scholar]
- Loukas, M.; Bilinsky, S.; Bilinsky, E.; el-Sedfy, A.; Anderson, R.H. Cardiac veins: A review of the literature. Clin. Anat. 2009, 22, 129–145. [Google Scholar] [CrossRef] [PubMed]
- Mazur, M.; Żabówka, A.; Bolechała, F.; Kopacz, P.; Klimek-Piotrowska, W.; Hołda, M.K. Variations and angulation of the coronary sinus tributaries: Implications for left ventricular pacing. Pacing Clin. Electrophysiol. 2019, 42, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Frankel, D.S.; Mountantonakis, S.E.; Dahu, M.I.; Marchlinski, F.E. Elimination of ventricular arrhythmias originating from the anterior interventricular vein with ablation in the right ventricular outflow tract. Circ. Arrhythmia Electrophysiol. 2014, 7, 984–985. [Google Scholar] [CrossRef]
- Mazur, M.; Holda, M.; Koziej, M.; Klimek-Piotrowska, W.; Kuniewicz, M.; Matuszyk, A.; Konarska, M.; Jaworek, J.; Mróz, I. Morphology of tributaries of coronary sinus in humans. Folia Med. Crac. 2015, 55, 5–13. [Google Scholar]
- Kassem, M.W.; Lake, S.; Roberts, W.; Salandy, S.; Loukas, M. Cardiac veins, an anatomical review. Transl. Res. Anat. 2021, 23, 100096. [Google Scholar] [CrossRef]
- Kuniewicz, M.; Krupiński, M.; Gosnell, M.; Budnicka, K.; Jakob, N.; Karkowski, G.; Urbańczyk-Zawadzka, M.; Lelakowski, J.; Walocha, J. Applicability of computed tomography preoperative assessment of the LAA in LV summit ablations. J. Interv. Card. Electrophysiol. 2020, 14. [Google Scholar] [CrossRef]
- Michałowska, I.M.; Hryniewiecki, T.; Kwiatek, P.; Stokłosa, P.; Swoboda-Rydz, U.; Szymański, P. Coronary Artery Variants and Anomalies in Patients With Bicuspid Aortic Valve. J. Thorac. Imaging 2016, 31, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.; Wei, W.; Tanager, K.S.; Miele, F.; Upadhyay, G.A.; Beaser, A.D.; Aziz, Z.; Nayak, H.M.; Ozcan, C.; Nishimura, T.; et al. Left ventricular summit arrhythmias with an abrupt V3 transition: Anatomy of the aortic interleaflet triangle vantage point. Heart Rhythm. 2021, 18, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Zuberi, Z.; Bradfield, J.S.; Zarif, J.K.; Ward, D.E.; Anderson, R.H.; Shivkumar, K.; Saba, M.M. Endocardial ablation of ventricular ectopic beats arising from the basal inferoseptal process of the left ventricle. Heart Rhythm. 2018, 15, 1356–1362. [Google Scholar] [CrossRef]
- Ishizawa, A.; Fumon, M.; Zhou, M.; Suzuki, R.; Abe, H. Intersection patterns of human coronary veins and arteries. Anat. Sci. Int. 2008, 83, 26–30. [Google Scholar] [CrossRef]
- Pauza, D.H.; Skripka, V.; Pauziene, N.; Stropus, R. Morphology, distribution, and variability of the epicardiac neural ganglionated subplexuses in the human heart. Anat. Rec. 2000, 259, 353–382. [Google Scholar] [CrossRef]
- Loukas, M.; Patel, S.; Cesmebasi, A.; Muresian, H.; Tubbs, R.S.; Spicer, D.; Dabrowski, M. The clinical anatomy of the conal artery. Clin. Anat. 2016, 29, 371–379. [Google Scholar] [CrossRef]
- von Lüdinghausen, M. The venous drainage of the human myocardium. Adv. Anat. Embryol. Cell Biol. 2003, 168, 1–104. [Google Scholar] [CrossRef]
- Roberts, W.; Charles, S.M.; Ang, C.; Holda, M.K.; Walocha, J.; Lachman, N.; Tubbs, R.S.; Loukas, M. Myocardial bridges: A meta-analysis. Clin. Anat. 2021, 34, 685–709. [Google Scholar] [CrossRef]
- Kosiński, A.; Grzybiak, M.; Skwarek, M.; Hreczecha, J. Distribution of muscular bridges in the adult human heart. Folia Morphol. 2004, 63, 491–498. [Google Scholar]
- Janes, R.D.; Brandys, J.C.; Hopkins, D.A.; Johnstone, D.E.; Murphy, D.A.; Armour, J.A. Anatomy of human extrinsic cardiac nerves and ganglia. Am. J. Cardiol. 1986, 57, 299–309. [Google Scholar] [CrossRef]
- Slodowska, K.; Szczepanek, E.; Dudkiewicz, D.; Holda, J.; Bolechała, F.; Strona, M.; Lis, M.; Batko, J.; Koziej, M.; Holda, M.K. Morphology of the lest atrial appendage—introduction of a new simplified shape-based classification system. Heart Lung Circ. 2021, 30, 1014–1022. [Google Scholar] [CrossRef]
- Whiteman, S.; Saker, E.; Courant, E.; Salandy, S.; Gielecki, J.; Zurada, A.; Loukas, M. An anatomical review of the left atrium. Transl. Res. Anat. 2019, 17, 100052. [Google Scholar] [CrossRef]
- Huemer, M.; Wutzler, A.; Parwani, A.S.; Attanasio, P.; Haverkamp, W.; Boldt, L.H. Mapping of the left-sided phrenic nerve course in patients undergoing left atrial catheter ablations. Pacing Clin. Electrophysiol. 2014, 37, 1141–1148. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, M.; Ellenbogen, K.A.; Koneru, J.N. Prevention of phrenic nerve injury during interventional electrophysiologic procedures. Heart Rhythm. 2014, 11, 1839–1844. [Google Scholar] [CrossRef] [PubMed]
- Fukumoto, K.; Takatsuki, S.; Jinzaki, M.; Yamada, M.; Tanimoto, K.; Nishiyama, N.; Aizawa, Y.; Hagiwara, Y.; Fukuda, Y.; Kimura, T.; et al. Three-dimensional imaging and mapping of the right and left phrenic nerves: Relevance to interventional cardiovascular therapy. Europace 2013, 15, 937–943. [Google Scholar] [CrossRef] [PubMed]
- Stoney, W.S.; Vernon, R.P.; Alford, W.C.; Burrus, G.R.; Thomas, C.S. Revascularization of the septal artery. Ann. Thorac. Surg. 1976, 21, 2–6. [Google Scholar] [CrossRef]
- Spencer, J.; Anderson, S.; Iaizzo, P. Human Coronary Venous Anatomy for Interventions. J. Card. Trans. Res. 2013, 6, 208–217. [Google Scholar] [CrossRef]
- Obel, O.A.; d’Avila, A.; Neuzil, P.; Saad, E.B.; Ruskin, J.N.; Reddy, V.Y. Ablation of left ventricular epicardial outflow tract tachycardia from the distal great cardiac vein. J. Am. Coll. Cardiol. 2006, 48, 1813–1817. [Google Scholar] [CrossRef]
- Holda, M.K.; Koziej, M.; Holda, J.; Tyrak, K.; Piatek, K.; Krawczyk-Ozóg, A.; Klimek-Piotrowska, W. Spatial relationship of blood vessels within the mitral isthmus line. Europace 2018, 20, 706–711. [Google Scholar] [CrossRef]
- Holda, M.K.; Holda, J.; Strona, M.; Koziej, M.; Klimek-Piotrowska, W. Blood vessels and myocardial thickness within the left atrial appendage isthmus line. Clin. Anat. 2018, 31, 1024–1030. [Google Scholar] [CrossRef] [PubMed]
- Bales, G.S. Great cardiac vein variations. Clin. Anat. 2004, 17, 436–443. [Google Scholar] [CrossRef] [PubMed]
- Pejkovic, B.; Bogdanovic, D. The great cardiac vein. Surg. Radiol. Anat. 1992, 14, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Villasante Fricke, A.C.; Lacobellis, G. Epicardial Adipose Tissue: Clinical Biomarker of Cardio-Metabolic Risk. Int. J. Mol. Sci. 2019, 20, 5989. [Google Scholar] [CrossRef]
- Rabkin, S.W. The relationship between epicardial fat and indices of obesity and the metabolic syndrome: A systematic review and meta-analysis. Metab. Syndr. Relat. Disord. 2014, 12, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Couselo-Seijas, M.; Rodríguez-Mañero, M.; González-Juanatey, J.R.; Eiras, S. Updates on epicardial adipose tissue mechanisms on atrial fibrillation. Obes. Rev. 2021, 17, e13277. [Google Scholar] [CrossRef]
- Samanta, R.; Pouliopoulos, J.; Thiagalingam, A.; Kovoor, P. Role of adipose tissue in the pathogenesis of cardiac arrhythmias. Heart Rhythm. 2016, 13, 311–320. [Google Scholar] [CrossRef]
- Babakr, A.A.; Fomison-Nurse, I.C.; van Hout, I.; Aitken-Buck, H.M.; Sugunesegran, R.; Davis, P.J.; Bunton, R.W.; Williams, M.J.A.; Coffey, S.; Stiles, M.K.; et al. Acute interaction between human epicardial adipose tissue and human atrial myocardium induces arrhythmic susceptibility. Am. J. Physiol. Endocrinol. Metab. 2020, 318, E164–E172. [Google Scholar] [CrossRef]
- Saremi, F.; Muresian, H.; Sánchez-Quintana, D. Coronary veins: Comprehensive CT-anatomic classification and review of variants and clinical implications. Radiographics 2012, 32, E1–E32. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.J.; Bogun, F. Coronary Venous Mapping and Catheter Ablation for Ventricular Arrhythmias. Methodist Debakey Cardiovasc. J. 2021, 17, 13–18. [Google Scholar] [CrossRef]
- Sirajuddin, A.; Chen, M.Y.; White, C.S.; Arai, A.E. Coronary venous anatomy and anomalies. J. Cardiovasc. Comput. Tomogr. 2020, 14, 80–86. [Google Scholar] [CrossRef]
- Briceño, D.F.; Enriquez, A.; Liang, J.J.; Shirai, Y.; Santangeli, P.; Guandalini, G.; Supple, G.E.; Schaller, R.; Arkles, J.; Frankel, D.S.; et al. Septal Coronary Venous Mapping to Guide Substrate Characterization and Ablation of Intramural Septal Ventricular Arrhythmia. JACC Clin. Electrophysiol. 2019, 5, 789–800. [Google Scholar] [CrossRef]
- Alam, M.; Dokainish, H.; Lakkis, N. Alcohol septal ablation for hypertrophic obstructive cardiomyopathy: A systematic review of published studies. J. Interv. Cardiol. 2006, 19, 319–327. [Google Scholar] [CrossRef]
- Betensky, B.P.; Kapa, S.; Desjardins, B.; Garcia, F.C.; Callans, D.J.; Dixit, S.; Frankel, D.S.; Hutchinson, M.D.; Supple, G.E.; Zado, E.S.; et al. Characterization of trans-septal activation during septal pacing: Criteria for identification of intramural ventricular tachycardia substrate in nonischemic cardiomyopathy. Circ. Arrhythmia Electrophysiol. 2013, 6, 1123–1130. [Google Scholar] [CrossRef]
- Pothineni, N.V.K.; Garcia, F.C.; Santangeli, P. Radiofrequency Ablation Strategies for Intramural Ventricular Arrhythmias. Methodist Debakey Cardiovasc. J. 2021, 17, 8–12. [Google Scholar] [CrossRef]
- Ortale, J.R.; Marquez, C.Q. Anatomy of the intramural venous sinuses of the right atrium and their tributaries. Surg. Radiol. Anat. 1998, 20, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, Y.; Nogami, A.; Shinoda, Y.; Masuda, K.; Machino, T.; Kuroki, K.; Yamasaki, H.; Sekiguchi, Y.; Aonuma, K. Idiopathic Ventricular Arrhythmias Originating From the Vicinity of the Communicating Vein of Cardiac Venous Systems at the Left Ventricular Summit. Circ. Arrhythmia Electrophysiol. 2018, 11, e005386. [Google Scholar] [CrossRef] [PubMed]
- Wink, J.; van Delft, R.; Notenboom, R.G.E.; Wouters, P.F.; DeRuiter, M.C.; Plevier, J.W.M.; Jongbloed, M.R.M. Human adult cardiac autonomic innervation: Controversies in anatomical knowledge and relevance for cardiac neuromodulation. Auton. Neurosci. 2020, 227, 102674. [Google Scholar] [CrossRef]
- Wallis, D.; Watson, A.H.; Mo, N. Cardiac neurones of autonomic ganglia. Microsc. Res. Tech. 1996, 35, 69–79. [Google Scholar] [CrossRef]
- Kawashima, T. The autonomic nervous system of the human heart with special reference to its origin, course, and peripheral distribution. Anat. Embryol. 2005, 209, 425–438. [Google Scholar] [CrossRef] [PubMed]
- Saburkina, I.; Rysevaite, K.; Pauziene, N.; Mischke, K.; Schauerte, P.; Jalife, J.; Pauza, D.H. Epicardial neural ganglionated plexus of ovine heart: Anatomic basis for experimental cardiac electrophysiology and nerve protective cardiac surgery. Heart Rhythm. 2010, 7, 942–950. [Google Scholar] [CrossRef] [PubMed]
- Spach, M.S.; Huang, S.; Armstrong, S.I.; Canent, R.V., Jr. Demonstration of peripheral conduction system in human hearts. Circulation 1963, 28, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Elizari, M.V. Fascicular Blocks: Update 2019. Curr. Cardiol. Rev. 2021, 17, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Elizari, M.V.; Acunzo, R.S.; Ferreiro, M. Hemiblocks revisited. Circulation 2007, 115, 1154–1163. [Google Scholar] [CrossRef]
- Elizari, M.V. The normal variants in the left bundle branch system. J. Electrocardiol. 2017, 50, 389–399. [Google Scholar] [CrossRef]
- Pérez-Riera, A.R.; Baranchuk, A. Unusual conduction disorder: Left posterior fascicular block + left septal fascicular block. Ann. Noninvasive Electrocardiol. 2015, 20, 187–188. [Google Scholar] [CrossRef]
- Chen, S.; Lu, X.; Peng, S.; Xue, Y.; Zhou, G.; Ling, Z.; Wei, Y.; Yang, K.; Fu, W.; Cai, L.; et al. Ablation at Right Coronary Cusp as an Alternative and Favorable Approach to Eliminate Premature Ventricular Complexes Originating From the Proximal Left Anterior Fascicle. Circ. Arrhythmia Electrophysiol. 2020, 13, e008173. [Google Scholar] [CrossRef]
- Ito, S.; Tada, H.; Naito, S.; Kurosaki, K.; Ueda, M.; Hoshizaki, H.; Miyamori, I.; Oshima, S.; Taniguchi, K.; Nogami, A. Development and validation of an ECG algorithm for identifying the optimal ablation site for idiopathic ventricular outflow tract tachycardia. J. Cardiovasc. Electrophysiol. 2003, 14, 1280–1286. [Google Scholar] [CrossRef]
- Andrade, F.M.; Ribeiro, D.C.; Babinski, M.A.; Cisne, R.; Góes, M.L. Triangle of Brocq and Mouchet: Anatomical study in brazilian cadavers and clinical implications. J. Morphol. Sci. 2010, 27, 3–4. [Google Scholar]
- Agarwal, J.; Agrawal, A.; Sinha, D.N.; Virendra. Study of BROCQ and Mouchet triangles in human hearts—A cadaveric study and its clinical implication. IP Indian J. Anat. Surg. Head Neck Brain 2020, 6, 36–39. [Google Scholar] [CrossRef]
- Dubey, A. Triangle of brocq and mouchet: An anatomicals in human cadaveric heart and its clinical significance. Int. J. Anat. Res. 2016, 4, 2266–2268. [Google Scholar] [CrossRef][Green Version]
- Brocq, P.; Mouchet, A. Etude Anatomique Des Artères Coronaires Du Cœur; Maloine et Fils: Paris, French, 1920. [Google Scholar]
- Mandarim-De-Lacerda, C.A. Anatomia Do Coração: Clínica e Cirúrgica; Revinter: Rio de Janeiro, Brasil, 1990. [Google Scholar]
- Benhayon, D.; Cogan, J.; Young, M. Left atrial appendage as a vantage point for mapping and ablating premature ventricular contractions originating in the epicardial left ventricular summit. Clin. Case Rep. 2018, 6, 1124–1127. [Google Scholar] [CrossRef] [PubMed]
- Altmann, D.R.; Knecht, S.; Sticherling, C.; Ammann, P.; Osswald, S.; Kühne, M. Ventricular tachycardia originating from the “Bermuda Triangle”. Cardiovasc. Med. 2013, 16, 208–210. [Google Scholar]
- Yakubov, A.; Salayev, O.; Hamrayev, R.; Sultankhonov, S. A case of successful ablation of ventricular tachycardia focus in the left ventricular summit through the left atrial appendage: A case report. Eur. Heart J. Case Rep. 2018, 2, yty110. [Google Scholar] [CrossRef] [PubMed]
- Marai, I.; Boulos, M.; Lessick, J.; Abadi, S.; Blich, M.; Suleiman, M. Outflow tract ventricular arrhythmia originating from the aortic cusps: Our approach for challenging ablation. J. Interv. Card. Electrophysiol. 2016, 45, 57–62. [Google Scholar] [CrossRef]
- Daniels, D.V.; Lu, Y.Y.; Morton, J.B.; Santucci, P.A.; Akar, J.G.; Green, A.; Wilber, D.J. Idiopathic epicardial left ventricular tachycardia originating remote from the sinus of Valsalva: Electrophysiological characteristics, catheter ablation, and identification from the 12-lead electrocardiogram. Circulation 2006, 113, 1659–1666. [Google Scholar] [CrossRef]
- Kumagai, K.; Fukuda, K.; Wakayama, Y.; Sugai, Y.; Hirose, M.; Yamaguchi, N.; Takase, K.; Yamauchi, Y.; Takahashi, A.; Aonuma, K.; et al. Electrocardiographic characteristics of the variants of idiopathic left ventricular outflow tract ventricular tachyarrhythmias. J. Cardiovasc. Electrophysiol. 2008, 19, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; Santangeli, P.; Pathak, R.K.; Muser, D.; Liang, J.J.; Castro, S.A.; Garcia, F.C.; Hutchinson, M.D.; Supple, G.E.; Frankel, D.S.; et al. Outcomes of Catheter Ablation of Idiopathic Outflow Tract Ventricular Arrhythmias With an R Wave Pattern Break in Lead V2: A Distinct Clinical Entity. J. Cardiovasc. Electrophysiol. 2017, 28, 504–514. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; McElderry, H.T.; Doppalapudi, H.; Murakami, Y.; Yoshida, Y.; Yoshida, N.; Okada, T.; Tsuboi, N.; Inden, Y.; Murohara, T.; et al. Idiopathic ventricular arrhythmias originating from the aortic root prevalence, electrocardiographic and electrophysiologic characteristics, and results of radiofrequency catheter ablation. J. Am. Coll. Cardiol. 2008, 52, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Stavrakis, S.; Jackman, W.M.; Nakagawa, H.; Sun, Y.; Xu, Q.; Beckman, K.J.; Lockwood, D.; Scherlag, B.J.; Lazzara, R.; Po, S.S. Risk of coronary artery injury with radiofrequency ablation and cryoablation of epicardial posteroseptal accessory pathways within the coronary venous system. Circ. Arrhythmia Electrophysiol. 2014, 7, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Aoyama, H.; Nakagawa, H.; Pitha, J.V.; Khammar, G.S.; Chandrasekaran, K.; Matsudaira, K.; Yagi, T.; Yokoyama, K.; Lazzara, R.; Jackman, W.M. Comparison of cryothermia and radiofrequency current in safety and efficacy of catheter ablation within the canine coronary sinus close to the left circumflex coronary artery. J. Cardiovasc Electrophysiol. 2005, 16, 1218–1226. [Google Scholar] [CrossRef]
- Paul, T.; Bökenkamp, R.; Mahnert, B.; Trappe, H.J. Coronary artery involvement early and late after radiofrequency current application in young pigs. Am. Heart J. 1997, 133, 436–440. [Google Scholar] [CrossRef]
- Makimoto, H.; Zhang, Q.; Tilz, R.R.; Wissner, E.; Cuneo, A.; Kuck, K.H.; Ouyang, F. Aborted sudden cardiac death due to radiofrequency ablation within the coronary sinus and subsequent total occlusion of the circumflex artery. J. Cardiovasc. Electrophysiol. 2013, 24, 929–932. [Google Scholar] [CrossRef]
- Alazard, M.; Lacotte, J.; Horvilleur, J.; Ait-Said, M.; Salerno, F.; Manenti, V.; Piechaud, J.F.; Garot, J.; Bonnet, D.; Maltret, A. Prventing the risk of coronary injury in posteroseptal accessory pathway ablation in children: Different strategies and advantages of fluoroscopy integrated 3D-mapping system (CARTO-UNIVU™). J. Interv. Card. Electrophysiol. 2018, 52, 127–135. [Google Scholar] [CrossRef]
- Correia, M.; Maresca, D.; Goudot, G.; Villemain, O.; Bizé, A.; Sambin, L.; Tanter, M.; Ghaleh, B.; Pernot, M. Quantitative imaging of coronary flows using 3D ultrafast Doppler coronary angiography. Phys. Med. Biol. 2020, 65, 105013. [Google Scholar] [CrossRef]
- Karkowski, G.; Kuniewicz, M.; Kozluk, E.; Chyzy, T.; Zabek, A.; Dusza, M.; Lelakowski, J. Non-fluoroscopic radiofrequency catheter ablation of right- and left-sided ventricular arrhythmias. Postepy Kardiol. Interwencyjnej 2020, 16, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, M.; Nogami, A.; Fukamizu, S.; Sekiguchi, Y.; Nitta, J.; Sakamoto, N.; Sakamoto, Y.; Kurosaki, K.; Takahashi, Y.; Kimata, A.; et al. Acute and long-term results of bipolar radiofrequency catheter ablation of refractory ventricular arrhythmias of deep intramural origin. Heart Rhythm. 2020, 17, 1500–1507. [Google Scholar] [CrossRef]
- Santangeli, P.; Marchlinski, F.E.; Zado, E.S.; Benhayon, D.; Hutchinson, M.D.; Lin, D.; Frankel, D.S.; Riley, M.P.; Supple, G.E.; Garcia, F.C.; et al. Percutaneous epicardial ablation of ventricular arrhythmias arising from the left ventricular summit: Outcomes and electrocardiogram correlates of success. Circ. Arrhythmia Electrophysiol. 2015, 8, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Sacher, F.; Roberts-Thomson, K.; Maury, P.; Tedrow, U.; Nault, I.; Steven, D.; Hocini, M.; Koplan, B.; Leroux, L.; Derval, N.; et al. Epicardial ventricular tachycardia ablation a multicenter safety study. J. Am. Coll. Cardiol. 2010, 55, 2366–2372. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; Maddox, W.R.; McElderry, H.T.; Doppalapudi, H.; Plumb, V.J.; Kay, G.N. Radiofrequency catheter ablation of idiopathic ventricular arrhythmias originating from intramural foci in the left ventricular outflow tract: Efficacy of sequential versus simultaneous unipolar catheter ablation. Circ. Arrhythmia Electrophysiol. 2015, 8, 344–352. [Google Scholar] [CrossRef]
- Yamada, T.; Yoshida, N.; Doppalapudi, H.; Litovsky, S.H.; McElderry, H.T.; Kay, G.N. Efficacy of an Anatomical Approach in Radiofrequency Catheter Ablation of Idiopathic Ventricular Arrhythmias Originating from the Left Ventricular Outflow Tract. Circ. Arrhythmia Electrophysiol. 2017, 10, e004959. [Google Scholar] [CrossRef]
- Candemir, B.; Baskovski, E.; Duzen, V.; Coskun, F.; Vurgun, K.; Goksuluk, H.; Ozyuncu, N.; Kurklu, S.T.; Altin, T.; Akyurek, O.; et al. Late elimination of challenging idiopathic ventricular arrhythmias originating from left ventricular summit by anatomical ablation. Indian Pacing Electrophysiol. J. 2019, 19, 114–118. [Google Scholar] [CrossRef]
- Chung, F.P.; Lin, C.Y.; Shirai, Y.; Futyma, P.; Santangeli, P.; Lin, Y.J.; Chang, S.L.; Lo, L.W.; Hu, Y.F.; Chang, H.Y.; et al. Outcomes of catheter ablation of ventricular arrhythmia originating from the left ventricular summit: A multicenter study. Heart Rhythm. 2020, 17, 1077–1083. [Google Scholar] [CrossRef] [PubMed]
- Aly, I.; Rizvi, A.; Roberts, W.; Khalid, S.; Kassem, M.W.; Salandy, S.; du Plessis, M.; Tubbs, R.S.; Loukas, M. Cardiac ultrasound: An Anatomical and Clinical Review. Transl. Res. Anat. 2021, 22, 100083. [Google Scholar] [CrossRef]
- Nogami, A. Behind the Valsalva: What Else Is Hiding? Circ. Arrhythmia Electrophysiol. 2020, 13, e008611. [Google Scholar] [CrossRef]
| Approach to LVS | Reachable LVS Region | References |
|---|---|---|
| Right ventricular outflow track | Septal margin, right aspect of LVS accessible area, lower right septal summit | [12,70] |
| Pulmonary trunk/left pulmonary artery | Septal margin, right aspect of LVS inaccessible area, higher right septal summit | [6,73] |
| Aorta–left sinus of Valsalva/left coronary cusp | Septal summit, apex of LVS, aortic–mitral continuity | [70,71,73,75,88] |
| Aorta–L-R inter leaflet trigon | Septal summit, apex of LVS | [17,71,75,89] |
| Left atrial appendage | Mitral margin of LVS, accessible and inaccessible area (depends on the morphology and coverage of appendage) | [68,70,83] |
| Great cardiac vein/anterior interventricular vein | Mitral margin of LVS, between accessible and inaccessible areas (depends on the course of venous system) | [8,33,51,72,86,88,89] |
| Epicardial–subxiphoid access | Accessible area of LVS from septal to mitral margin | [8,38,51,70,72,73,89] |
| Study | LVS Access (if Specified) | Total Number of Cases | Number of Successful Cases | Number of Complications |
|---|---|---|---|---|
| Obel, 2006 [33] | Total GCV | 5 | 5 | 0 |
| Daniels, 2006 [72] | Total | 11 | 11 | 0 |
| GCV | 9 | |||
| Epi | 2 | |||
| Yamada, 2008 [75] | Total | 44 | 44 | 1 |
| LCC | 24 | |||
| RCC | 14 | |||
| NCC | 1 | |||
| R-L ILT | 5 | |||
| Kumagai, 2008 [73] | Total | 45 | 40 | 0 |
| AMC | 3 | |||
| MA | 8 | |||
| LCC/RCC | 32 | |||
| Epi | 2 | |||
| Sacher, 2010 [85] | Total Epi | 136 | 64 | 8 |
| Yamada, 2010 [8] | Total | 27 | 22 | 0 |
| GCV | 14 | |||
| Epi | 4 | |||
| Frankel, 2014 [12] | RVOT | 2 | 2 | 0 |
| Santangeli, 2015 [38] | Total Epi | 23 | 5 | no data |
| Yamada, 2015 [86] | Total | 64 | 58 (only inaccesabile area failure) | 0 |
| GCV | 36 | |||
| AMC | 28 | |||
| Marai, 2016 [71] | Total | 10 | 10 | 0 |
| R-L ILT | 5 | |||
| AIV-GCV | 1 | |||
| NCC | 1 | |||
| LCC | 2 | |||
| RCC | 1 | |||
| Hayashi, 2017 [70] | Total | 12 | 7 | 0 |
| RVOT | 7 | |||
| LCC | 1 | |||
| AIV | 2 | |||
| Epi | 2 | |||
| Yamada, 2017 [87] | not specified | 229 | 212 | no data |
| Komatsu, 2018 [51] | Total | 31 | ||
| GVC | 14 | 10 | 1 | |
| other | 17 | 17 | 0 | |
| Benhayon, 2018 [68] | LAA | 1 | 1 | 0 |
| Yakubov, 2018 [70] | LAA | 1 | 1 | 0 |
| Candemir, 2019 [88] | not specified | 21 | 15 | 0 |
| Liao, 2020 [17] | R-L ILT | 20 | 16 | 0 |
| Igarashi, 2020 [83] | not specified | 18 | 16 | 3 |
| Chung, 2020 [89] | Total | 238 | 199 | 7 |
| GCV | 91 | |||
| EPI | 6 | |||
| RL ILT | 139 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuniewicz, M.; Baszko, A.; Ali, D.; Karkowski, G.; Loukas, M.; Walocha, J.A.; Hołda, M.K. Left Ventricular Summit—Concept, Anatomical Structure and Clinical Significance. Diagnostics 2021, 11, 1423. https://doi.org/10.3390/diagnostics11081423
Kuniewicz M, Baszko A, Ali D, Karkowski G, Loukas M, Walocha JA, Hołda MK. Left Ventricular Summit—Concept, Anatomical Structure and Clinical Significance. Diagnostics. 2021; 11(8):1423. https://doi.org/10.3390/diagnostics11081423
Chicago/Turabian StyleKuniewicz, Marcin, Artur Baszko, Dyjhana Ali, Grzegorz Karkowski, Marios Loukas, Jerzy A. Walocha, and Mateusz K. Hołda. 2021. "Left Ventricular Summit—Concept, Anatomical Structure and Clinical Significance" Diagnostics 11, no. 8: 1423. https://doi.org/10.3390/diagnostics11081423
APA StyleKuniewicz, M., Baszko, A., Ali, D., Karkowski, G., Loukas, M., Walocha, J. A., & Hołda, M. K. (2021). Left Ventricular Summit—Concept, Anatomical Structure and Clinical Significance. Diagnostics, 11(8), 1423. https://doi.org/10.3390/diagnostics11081423





