To the Question on the Use of Multivariate Analysis and 2D Visualisation of Synchrotron ATR-FTIR Chemical Imaging Spectral Data in the Diagnostics of Biomimetic Sound Dentin/Dental Composite Interface
Abstract
:1. Introduction
2. Materials and Methods
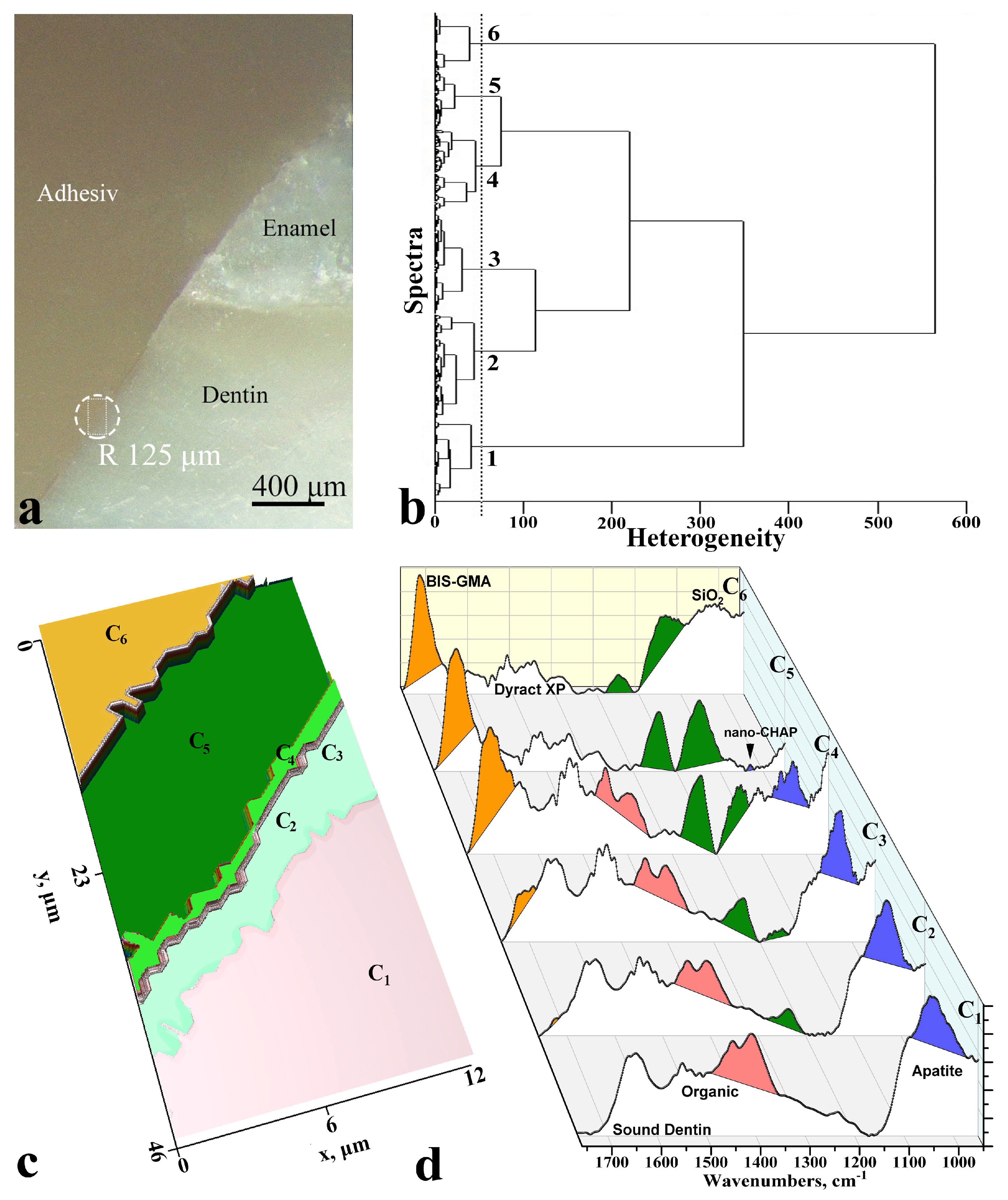
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Golubnitschaja, O.; Costigliola, V. Dental health: EPMA recommendations for innovative strategies. EPMA J. 2014, 5, A119. [Google Scholar] [CrossRef] [Green Version]
- Chinelatti, M.; Amaral, T.H.A.D.; Borsatto, M.C.; Palma-Dibb, R.G.; Corona, S.A.M. Adhesive interfaces of enamel and dentin prepared by air-abrasion at different distances. Appl. Surf. Sci. 2007, 253, 4866–4871. [Google Scholar] [CrossRef]
- Stape, T.H.S.; Seseogullari-Dirihan, R.; Tjäderhane, L.; Abuna, G.; Martins, L.R.M.; Tezvergil-Mutluay, A. A novel dry-bonding approach to reduce collagen degradation and optimize resin-dentin interfaces. Sci. Rep. 2018, 8, 16890. [Google Scholar] [CrossRef]
- Liu, Y.; Tjäderhane, L.; Breschi, L.; Mazzoni, A.; Li, N.; Mao, J.; Pashley, D.; Tay, F. Limitations in Bonding to Dentin and Experimental Strategies to Prevent Bond Degradation. J. Dent. Res. 2011, 90, 953–968. [Google Scholar] [CrossRef] [PubMed]
- Perdigão, J. Current perspectives on dental adhesion: (1) Dentin adhesion – not there yet. Jpn. Dent. Sci. Rev. 2020, 56, 190–207. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhao, Y.; Tian, Z.; Zhu, J.; Shi, Z.; Cui, Z.; Zhu, S. Enhancement performance of application mussel-biomimetic adhesive primer for dentin adhesives. RSC Adv. 2020, 10, 12035–12046. [Google Scholar] [CrossRef] [Green Version]
- Ding, Q.; Cui, J.; Shen, H.; He, C.; Wang, X.; Shen, S.G.F.; Lin, K. Advances of nanomaterial applications in oral and maxillofacial tissue regeneration and disease treatment. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnology 2021, 13, e1669. [Google Scholar] [CrossRef]
- Asiri, A.M.; Mohammad, A. Applications of Nanocomposite Materials in Dentistry, 1st ed.; Woodhead Publishing series in biomaterials; Woodhead Publishing: Duxford, UK, 2019; ISBN 978-0-12-813742-0. [Google Scholar]
- Seredin, P.; Goloshchapov, D.; Prutskij, T.; Ippolitov, Y. Fabrication and characterisation of composites materials similar optically and in composition to native dental tissues. Results Phys. 2017, 7, 1086–1094. [Google Scholar] [CrossRef]
- Goloshchapov, D.; Ippolitov, Y.; Seredin, P. Mechanism of interaction among nanocrystalline carbonate-substituted hydroxyapatite and polar amino-acids for the biomimetic composite technology: Spectroscopic and structural study. Results Phys. 2020, 18, 103277. [Google Scholar] [CrossRef]
- Ye, Q.; Spencer, P. Analyses of material-tissue interfaces by Fourier transform infrared, Raman spectroscopy, and chemometrics. In Material-Tissue Interfacial Phenomena; Elsevier BV: Amsterdam, The Netherlands, 2017; pp. 231–251. [Google Scholar]
- Wang, Y.; Yao, X.; Parthasarathy, R. Characterization of interfacial chemistry of adhesive/dentin bond using FTIR chemical imaging with univariate and multivariate data processing. J. Biomed. Mater. Res. Part A 2009, 91, 251–262. [Google Scholar] [CrossRef] [Green Version]
- Oinas, J.; Rieppo, L.; Finnilä, M.A.; Valkealahti, M.; Lehenkari, P.; Saarakkala, S. Imaging of Osteoarthritic Human Articular Cartilage using Fourier Transform Infrared Microspectroscopy Combined with Multivariate and Univariate Analysis. Sci. Rep. 2016, 6, 30008. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Rastogi, S.; Bhati, K.K.; Srinivasan, A.; Roychoudhury, A.; Nikolajeff, F.; Kumar, S. Mapping the Inorganic and Proteomic Differences among Different Types of Human Teeth: A Preliminary Compositional Insight. Biomol. 2020, 10, 1540. [Google Scholar] [CrossRef] [PubMed]
- Honda, R.; Ryu, M.; Balčytis, A.; Vongsvivut, J.; Tobin, M.J.; Juodkazis, S.; Morikawa, J. Paracetamol micro-structure analysis by optical mapping. Appl. Surf. Sci. 2019, 473, 127–132. [Google Scholar] [CrossRef] [Green Version]
- Goloshchapov, D.; Kashkarov, V.; Rumyantseva, N.; Seredin, P.; Lenshin, A.; Agapov, B.; Domashevskaya, E. Synthesis of nanocrystalline hydroxyapatite by precipitation using hen’s eggshell. Ceram. Int. 2013, 39, 4539–4549. [Google Scholar] [CrossRef]
- Fugolin, A.P.; Sundfeld, D.; Ferracane, J.L.; Pfeifer, C.S. Toughening of Dental Composites with Thiourethane-Modified Filler Interfaces. Sci. Rep. 2019, 9, 2286. [Google Scholar] [CrossRef] [Green Version]
- Seredin, P.; Goloshchapov, D.; Prutskij, T.; Ippolitov, Y. Phase Transformations in a Human Tooth Tissue at the Initial Stage of Caries. PLoS ONE 2015, 10, e0124008. [Google Scholar] [CrossRef]
- Goloshchapov, D.; Kashkarov, V.; Ippolitov, Y.; Prutskij, T.; Seredin, P. Early screening of dentin caries using the methods of Micro-Raman and laser-induced fluorescence spectroscopy. Results Phys. 2018, 10, 346–347. [Google Scholar] [CrossRef]
- Jegova, G.; Titorenkova, R.; Rashkova, M.; Mihailova, B. Raman and IR reflection micro-spectroscopic study of Er:YAG laser treated permanent and deciduous human teeth. J. Raman Spectrosc. 2013, 44, 1483–1490. [Google Scholar] [CrossRef]
- Lopes, C.D.C.A.; Limirio, P.H.J.O.; Novais, V.R.; Dechichi, P. Fourier transform infrared spectroscopy (FTIR) application chemical characterization of enamel, dentin and bone. Appl. Spectrosc. Rev. 2018, 53, 747–769. [Google Scholar] [CrossRef]
- Seredin, P.; Goloshchapov, D.; Ippolitov, Y.; Vongsvivut, J. Development of a new approach to diagnosis of the early fluorosis forms by means of FTIR and Raman microspectroscopy. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef]
- Khan, A.S.; Khalid, H.; Sarfraz, Z.; Khan, M.; Iqbal, J.; Muhammad, N.; Fareed, M.A.; Rehman, I.U. Vibrational spectroscopy of selective dental restorative materials. Appl. Spectrosc. Rev. 2016, 52, 507–540. [Google Scholar] [CrossRef]
- Delgado, A.; Young, A. Modelling ATR-FTIR Spectra of Dental Bonding Systems to Investigate Composition and Polymerisation Kinetics. Materials 2021, 14, 760. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seredin, P.; Goloshchapov, D.; Kashkarov, V.; Ippolitov, Y.; Ippolitov, I.; Vongsvivut, J. To the Question on the Use of Multivariate Analysis and 2D Visualisation of Synchrotron ATR-FTIR Chemical Imaging Spectral Data in the Diagnostics of Biomimetic Sound Dentin/Dental Composite Interface. Diagnostics 2021, 11, 1294. https://doi.org/10.3390/diagnostics11071294
Seredin P, Goloshchapov D, Kashkarov V, Ippolitov Y, Ippolitov I, Vongsvivut J. To the Question on the Use of Multivariate Analysis and 2D Visualisation of Synchrotron ATR-FTIR Chemical Imaging Spectral Data in the Diagnostics of Biomimetic Sound Dentin/Dental Composite Interface. Diagnostics. 2021; 11(7):1294. https://doi.org/10.3390/diagnostics11071294
Chicago/Turabian StyleSeredin, Pavel, Dmitry Goloshchapov, Vladimir Kashkarov, Yuri Ippolitov, Ivan Ippolitov, and Jitraporn Vongsvivut. 2021. "To the Question on the Use of Multivariate Analysis and 2D Visualisation of Synchrotron ATR-FTIR Chemical Imaging Spectral Data in the Diagnostics of Biomimetic Sound Dentin/Dental Composite Interface" Diagnostics 11, no. 7: 1294. https://doi.org/10.3390/diagnostics11071294
APA StyleSeredin, P., Goloshchapov, D., Kashkarov, V., Ippolitov, Y., Ippolitov, I., & Vongsvivut, J. (2021). To the Question on the Use of Multivariate Analysis and 2D Visualisation of Synchrotron ATR-FTIR Chemical Imaging Spectral Data in the Diagnostics of Biomimetic Sound Dentin/Dental Composite Interface. Diagnostics, 11(7), 1294. https://doi.org/10.3390/diagnostics11071294







