Diagnostic Accuracy of B-Mode- and Contrast-Enhanced Ultrasound in Differentiating Malignant from Benign Pleural Effusions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ultrasound Examinations
2.2. B-Mode Lung Ultrasound Parameters
- The presence of pleural thickening (>3 mm), classified as nodular or flat thickening.
- The pleural-effusion volume, classified as >1000 mL or <1000 mL. The volume was measured using the following formula: Pleural effusion volume (mL) = 70 × (basal lung-diaphragm distance (cm) + max. effusion height (cm)) [18].
- The presence of a septated PE or a solid mass within the PE.
- The homogeneity of the associated pulmonary consolidation, classified as homogeneous or inhomogeneous.
2.3. Contrast-Enhanced Ultrasound Parameters
- The presence of enhancement of septa or a solid mass within the PE [16].
2.4. Statistical Analysis
3. Results
3.1. Characteristics of the Participants
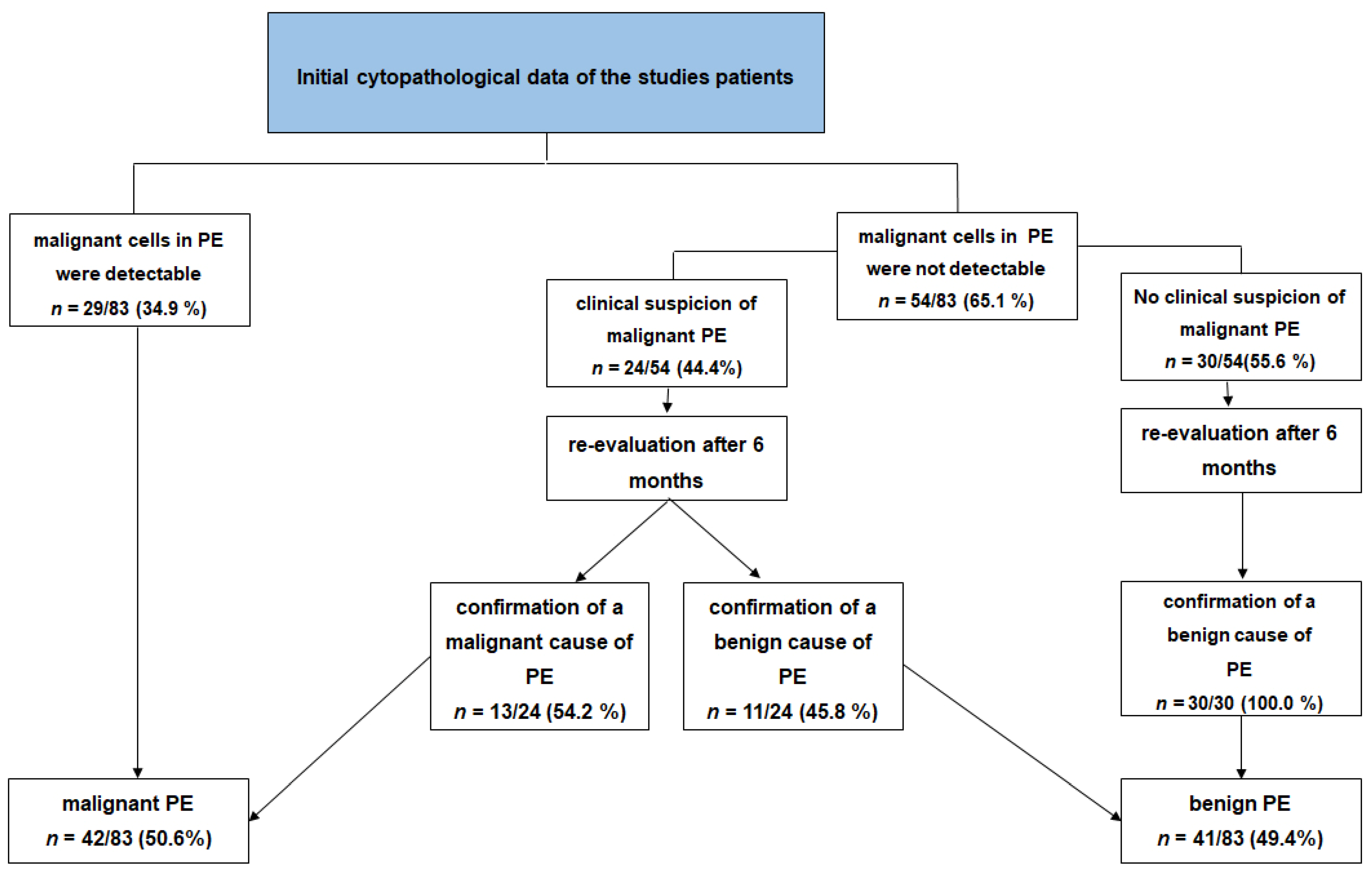
3.2. Initial Cytopathological Data of the Patients
3.3. Ultrasound Data
3.4. B-Mode Thoracic Ultrasound
3.5. Contrast-Enhanced Thoracic Ultrasound
| Variable |
All Patients n = 83 |
Malignant PE n = 42 |
Benign PE n = 41 | p-Value |
|---|---|---|---|---|
| Pathological ultrasound findings with CEUS | ||||
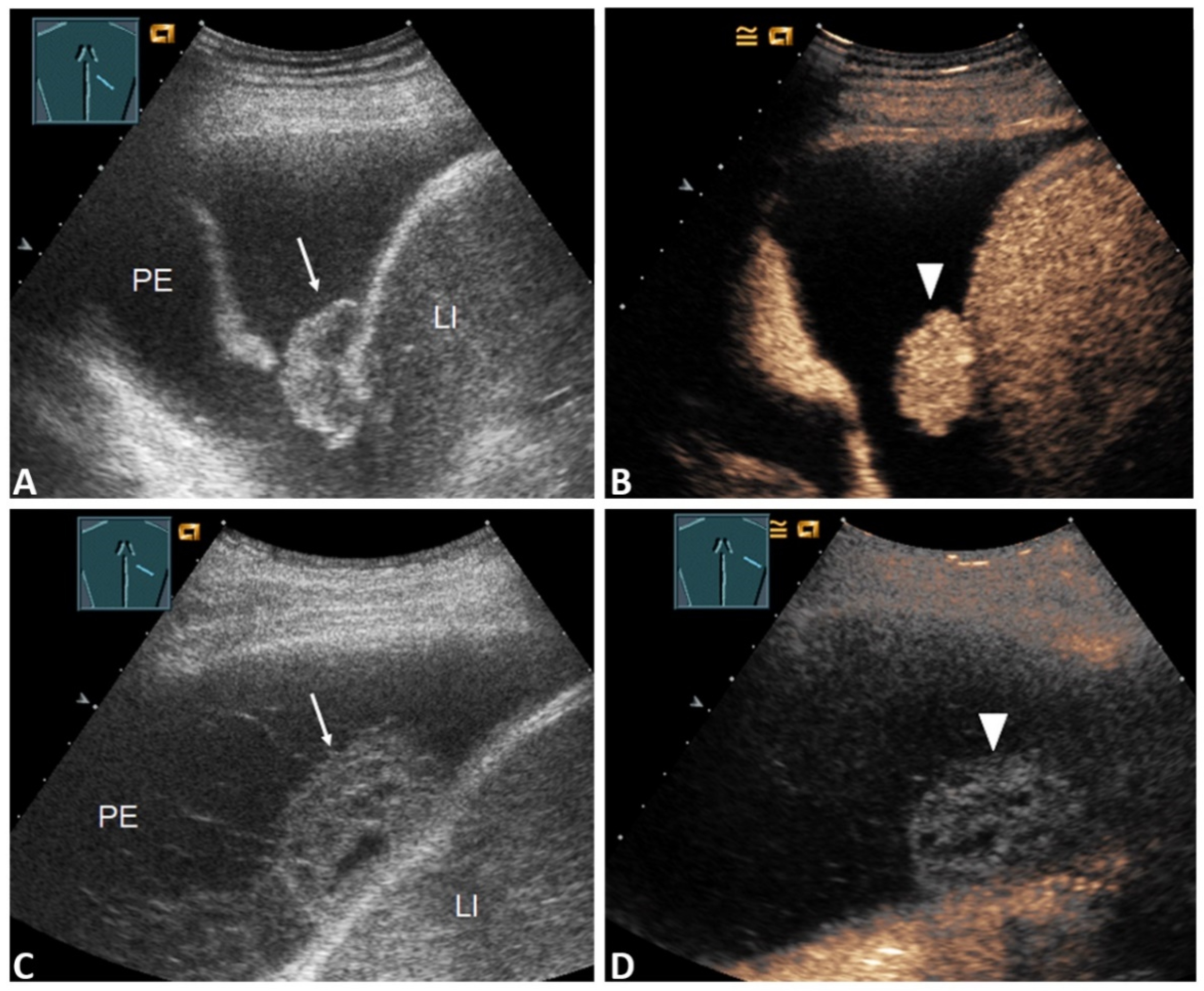
| Pleural thickening with marked enhancement (Figure 2B and Figure 3D) | 27 (32.5%) | 20 (47.6%) | 7 (17.1%) | 0.005 * |
| Pleural thickening with absent or reduced enhancement or no pleural thickening (Figure 3B) | 56 (67.5%) | 22 (52.4%) | 34 (82.9%) | |
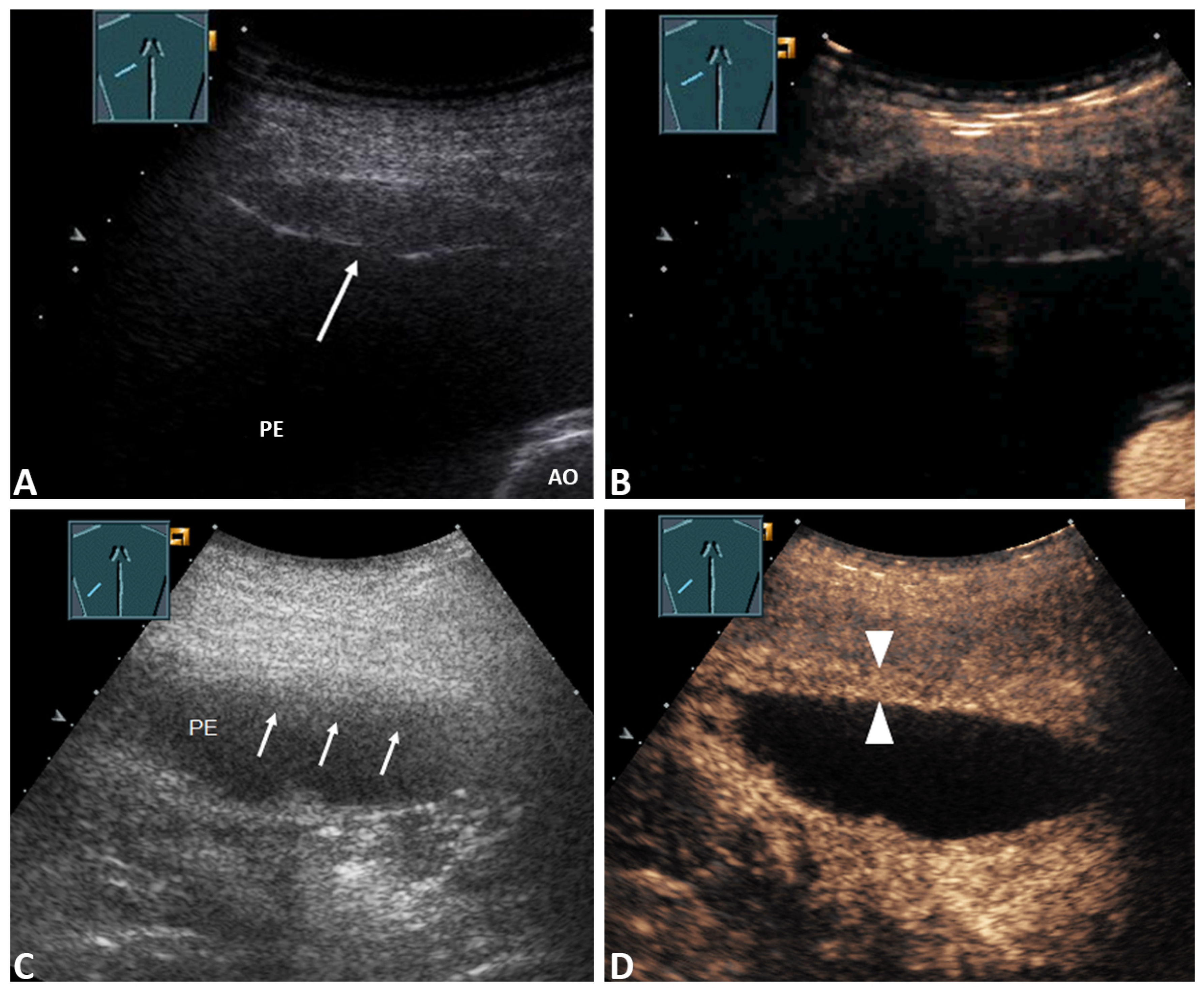
| Enhancement of septa or solid formation (Figure 4D) | 2 (2.4%) | 2 (4.8%) | 0 (0.0%) | 0.494 * |
| No enhancement of septa or solid formation (Figure 5C) | 81 (97.6%) | 40 (95.2%) | 41(100.0%) | |
| Inhomogeneous enhancement of lung consolidation (Figure 5B–D) | 25 (30.1%) | 19 (45.2%) | 6 (14.6%) | 0.004 * |
| Homogeneous enhancement of lung consolidation | 58 (69.9%) | 23 (54.8%) | 35 (85.4%) | |
3.6. Negative Cytology and Clinical Suspicion
4. Discussion
Interpretation
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dietrich, C.F.; Mathis, G.; Cui, X.-W. Ultrasound of the pleurae and lungs. Ultrasound Med. Biol. 2015, 41, 351–365. [Google Scholar] [CrossRef] [Green Version]
- Feller-Kopman, D. Ultrasound-Guided Thoracentesis. Chest 2006, 129, 1709–1714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jany, B.; Welte, T. Pleural Effusion in Adults—Etiology, Diagnosis, and Treatment. Dtsch. Aerzteblatt Online 2019, 116, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Loveland, P.; Christie, M.; Hammerschlag, G.; Irving, L.; Steinfort, D. Diagnostic yield of pleural fluid cytology in malignant effusions: An Australian tertiary centre experience. Intern. Med. J. 2018, 48, 1318–1324. [Google Scholar] [CrossRef] [PubMed]
- Bielsa, S.; Panadés, M.J.; Egido, R. Rentabilidad del estudio citológico del líquido pleural en el derrame maligno. Anales de medicina interna. Madr. Spain 1984 2008, 25, 173–177. [Google Scholar]
- Rodríguez-Panadero, F.; Janssen, J.P.; Astoul, P. Thoracoscopy: General overview and place in the diagnosis and management of pleural effusion. Eur. Respir. J. 2006, 28, 409–422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pandit, S.; Chaudhuri, A.D.; Datta, S.B.S.; Dey, A.; Bhanja, P. Role of pleural biopsy in etiological diagnosis of pleural effusion. Lung India 2010, 27, 202–204. [Google Scholar] [CrossRef] [PubMed]
- Cantey, E.P.; Walter, J.M.; Corbridge, T. Complications of thoracentesis: Incidence, risk factors, and strategies for prevention. Curr. Opin. Pulm. Med. 2016, 22, 378–385. [Google Scholar] [CrossRef]
- Görg, C.; Restrepo, I.; Schwerk, W.B. Sonography of malignant pleural effusion. Eur. Radiol. 1997, 7, 1195–1198. [Google Scholar] [CrossRef]
- Traill, Z.C.; Davies, R.J.; Gleeson, F.V. Thoracic Computed Tomography in Patients with Suspected Malignant Pleural Effusions. Clin. Radiol. 2001, 56, 193–196. [Google Scholar] [CrossRef]
- Maskell, N.A.; Gleeson, F.V.; Davies, R.J.O. Standard pleural biopsy versus CT-guided cutting-needle biopsy for diagno-sis of malignant disease in pleural effusions: A randomised controlled trial. Lancet 2003, 361, 1326–1330. [Google Scholar] [CrossRef]
- Shkolnik, B.; Judson, M.A.; Austin, A. Diagnostic Accuracy of Thoracic Ultrasonography to Differentiate Tran-sudative From Exudative Pleural Effusion. Chest 2020, 158, 692–697. [Google Scholar] [CrossRef] [PubMed]
- Shiroshita, A.; Nozaki, S.; Tanaka, Y.; Luo, Y.; Kataoka, Y. Thoracic ultrasound for malignant pleural effusion: A systematic review and meta-analysis. ERJ Open Res. 2020, 6, 464–2020. [Google Scholar] [CrossRef] [PubMed]
- Görg, C.; Bert, T.; Kring, R.; Dempfle, A. Transcutaneous contrast enhanced sonography of the chest for evaluation of pleural based pulmonary lesions: Experience in 137 patients. Ultraschall der Med. Eur. J. Ultrasound 2006, 27, 437–444. [Google Scholar] [CrossRef]
- Mathis, G. (Ed.) Bildatlas der Lungensonographie. 6., Vollständig Überarbeitete und Aktualisierte Auflage; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Safai Zadeh, E.; Görg, C.; Dietrich, C.F. Contrast-Enhanced Ultrasound for Evaluation of Pleural Effusion: A Pictorial Essay. J. ultrasound Med. Off. J. Am. Inst. Ultrasound Med. 2021. online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Sidhu, P.S.; Cantisani, V.; Dietrich, C.F. Die EFSUMB-Leitlinien und Empfehlungen für den klinischen Einsatz des kontrastverstärkten Ultraschalls (CEUS) bei nicht-hepatischen Anwendungen: Update 2017 (Langversion). Ultraschall Med. Stuttg. Ger. 1980 2018, 39, e2–e44. [Google Scholar]
- Goecke, W.; Schwerk, W.B. Die Real-Time Sonographie in der Diagnostik von Pleuraergüssen; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 1990; pp. 385–387. [Google Scholar]
- Lim, A.K.P.; Patel, N.; Eckersley, R.J. Evidence for spleen-specific uptake of a microbubble contrast agent: A quantitative study in healthy volunteers. Radiology 2004, 231, 785–788. [Google Scholar] [CrossRef]
- Trenker, C.; Dohse, M.; Ramaswamy, A.; Michel, C.; Görg, C. Histological validation of pulmonary infarction detected with contrast-enhanced ultrasound in patients with negative computed tomography pulmonary angiogram: A case series. J. Clin. Ultrasound 2019, 47, 461–465. [Google Scholar] [CrossRef]
- Hooper, C.; Lee, Y.C.G.; Maskell, N. Investigation of a unilateral pleural effusion in adults: British Thoracic Society pleural disease guideline 2010. Thorax 2010, 65 (Suppl. 2), ii4–ii17. [Google Scholar] [CrossRef] [Green Version]
- Qureshi, N.R.; Rahman, N.M.; Gleeson, F. Thoracic ultrasound in the diagnosis of malignant pleural effusion. Thorax 2009, 64, 139–143. [Google Scholar] [CrossRef] [Green Version]
- Bugalho, A.; Ferreira, D.; Dias, S.S.; Schuhmann, M.; Branco, J.C.; Gomes, M.J.M.; Eberhardt, R. The Diagnostic Value of Transthoracic Ultrasonographic Features in Predicting Malignancy in Undiagnosed Pleural Effusions: A Prospective Observational Study. Respiration 2014, 87, 270–278. [Google Scholar] [CrossRef]
- Porcel, J.M.; Pardina, M.; Bielsa, S.; González, A.; Light, R.W. Derivation and validation of a CT scan scoring system for discriminating malignant from benign pleural effusions. Chest 2015, 147, 513–519. [Google Scholar] [CrossRef]
- Mathis, G. Thoraxsonography—Part II: Peripheral pulmonary consolidation. Ultrasound Med. Biol. 1997, 23, 1141–1153. [Google Scholar] [CrossRef]
- Saito, A.; Hakamata, Y.; Yamada, Y.; Sunohara, M.; Tarui, M.; Murano, Y.; Mitani, A.; Tanaka, K.; Nagase, T.; Yanagimoto, S. Pleural thickening on screening chest X-rays: A single institutional study. Respir. Res. 2019, 20, 1–7. [Google Scholar] [CrossRef]
- Sack, U.; Hoffmann, M.; Zhao, X.J.; Chan, K.S.; Hui, D.S.C.; Gosse, H.; Engelmann, L.; Schauer, J.; Emmrich, F.; Hoheisel, G. Vascular endothelial growth factor in pleural effusions of different origin. Eur. Respir. J. 2005, 25, 600–604. [Google Scholar] [CrossRef] [Green Version]
- Safai Zadeh, E.; Keber, C.U.; Dietrich, C.F. Perfusion Patterns of Peripheral Pulmonary Granulomatous Lesions Using Contrast-Enhanced Ultrasound (CEUS) and Their Correlation with Immunohistochemically Detected Vascularization Patterns. J. ultrasound Med. Off. J. Am. Inst. Ultrasound Med. 2021. online ahead of print. [Google Scholar] [CrossRef]
- Safai Zadeh, E.; Beutel, B.; Dietrich, C.F. Perfusion patterns of peripheral pulmonary lesions in COVID-19 pa-tients using contrast-enhanced ultrasound (CEUS): A case series. J. Ultrasound Med. Off. J. Am. Inst. Ultrasound Med. 2021. online ahead of print. [Google Scholar] [CrossRef]
- Bartelt, S.; Trenker, C.; Görg, C. Contrast-enhanced ultrasound of embolic consolidations in patients with pulmonary embolism: A pilot study. J. Clin. Ultrasound JCU 2016, 44, 129–135. [Google Scholar] [CrossRef]
- Cao, B.-S.; Wu, J.-H.; Li, X.-L. Sonographically guided transthoracic biopsy of peripheral lung and mediastinal lesions: Role of contrast-enhanced sonography. J. Ultrasound Med. Off. J. Am. Inst. Ultrasound Med. 2011, 30, 1479–1490. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Zhang, Y.-Y.; Cui, L.-G.; Tan, S.; Sun, Y. Ultrasound-Guided Biopsy of Pleural-Based Pulmonary Lesions by Injection of Contrast-Enhancing Drugs. Front. Pharmacol. 2019, 10, 960. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Cause of Benign PE |
Number (%) of Patients | Cause of Malignant PE |
Number (%) of Patients |
|---|---|---|---|
| Parapneumonic PE | 18 (43.9) | Pulmonary metastasis of solid carcinomas | 17 (40.5) |
| Pleura empyema | 7 (17.1) | Primary bronchial carcinoma | 17 (40.5) |
| Congestive heart failure | 9 (22.0) | Pleural mesothelioma | 4 (9.5) |
| Pulmonary artery embolism | 3 (7.3) | Lung infiltration by malignant lymphoma | 2 (4.8) |
| Non-infectious granulomatous diseases | 2 (4.9) | Pulmonary metastasis of sarcoma | 2 (4.8) |
| Liver cirrhosis | 1 (2.4) | - | - |
| IgG4-related pleuritis | 1 (2.4) | - | - |
| Total number of patients with benign PE | 41 (100) | Total number of patients with malignant PE | 42 (100) |
| Variable |
All Patients n = 83 |
Malignant PE n = 42 |
Benign PE n = 41 | p-Value |
|---|---|---|---|---|
| Pathological ultrasound findings with B-TUS | ||||
| Pleural thickening | 28 (33.7%) | 20 (47.6%) | 8 (19.5%) | 0.010 * |
| No pleural thickening | 55 (66.3%) | 22 (52.4%) | 33 (80.5%) | |
| PE volume ≤ 1000 | 51 (61.4%) | 21 (50.0%) | 30 (73.2%) | 0.042 * |
| PE volume > 1000 | 32 (38.6%) | 21 (50.0%) | 11 (26.8%) | |
| Septa or solid formation in PE | 24 (28.9%) | 8 (19.0%) | 16 (39.0%) | 0.055 * |
| No septa or solid formation in PE | 59 (71.1%) | 34 (81.0%) | 25 (61.0%) | |
| Inhomogeneous lung consolidation | 12 (14.5%) | 9 (21.4%) | 3 (7.3%) | 0.116 * |
| Homogeneous lung consolidation | 71 (85.5%) | 33 (78.6%) | 38 (92.7%) | |
| Variable | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Diagnostic Accuracy (%) |
|---|---|---|---|---|---|
| Initial cytopathological examination | 69.0 | 100.0 | 100.0 | 75.9 | 84.3 |
| B-TUS data associated with malignancy (volume of PE > 1000 mL, pleural thickening) | 69.1 | 58.5 | 63.0 | 64.7 | 63.9 |
| CEUS data associated with malignancy (pleural thickening with marked enhancement and inhomogeneous enhancement of pulmonary consolidations) | 73.8 | 70.7 | 72.1 | 72.5 | 72.3 |
| B-TUS and CEUS data associated with malignancy | 88.1 | 46.3 | 62.7 | 79.2 | 67.5 |
| Variable | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Diagnostic Accuracy (%) |
|---|---|---|---|---|---|
| B-TUS data associated with malignancy (volume of PE > 1000 mL, pleural thickening) | 69.2 | 63.4 | 69.2 | 63.4 | 66.7 |
| CEUS data associated with malignancy (pleural thickening with marked and inhomogeneous enhancement of pulmonary consolidations) | 92.3 | 90.0 | 92.3 | 90.0 | 87.5 |
| B-TUS and CEUS data associated with malignancy | 92.3 | 54.6 | 70.6 | 85.7 | 75.0 |
| Imaging Modality | Cases | Year | Author | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Diagnostic Accuracy (%) |
|---|---|---|---|---|---|---|---|---|
| B-TUS | 52 | 2008 | Qureshi et al. [22] | 73 | 100 | 100 | 79 | NS |
| B-TUS | 133 | 2014 | Bugalho et al. [23] | 80.3 | 83.6 | 82.8 | 81.2 | 81.9 |
| B-TUS | 83 | 2021 | Present study | 69.1 | 58.5 | 63.0 | 64.7 | 63.9 |
| CEUS | 83 | 2021 | Present study | 73.8 | 70.7 | 72.1 | 72.5 | 72.3 |
| CECT | 40 | 2000 | Traill et al. [10] | 87.0 | 100 | 100 | 67.0 | 90.0 |
| CECT | 343 | 2015 | Porcel et al. [24] | 74.0 | 92.0 | NS | NS | NS |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Safai Zadeh, E.; Weide, J.; Dietrich, C.F.; Trenker, C.; Koczulla, A.R.; Görg, C. Diagnostic Accuracy of B-Mode- and Contrast-Enhanced Ultrasound in Differentiating Malignant from Benign Pleural Effusions. Diagnostics 2021, 11, 1293. https://doi.org/10.3390/diagnostics11071293
Safai Zadeh E, Weide J, Dietrich CF, Trenker C, Koczulla AR, Görg C. Diagnostic Accuracy of B-Mode- and Contrast-Enhanced Ultrasound in Differentiating Malignant from Benign Pleural Effusions. Diagnostics. 2021; 11(7):1293. https://doi.org/10.3390/diagnostics11071293
Chicago/Turabian StyleSafai Zadeh, Ehsan, Johanna Weide, Christoph Frank Dietrich, Corinna Trenker, Andreas Rembert Koczulla, and Christian Görg. 2021. "Diagnostic Accuracy of B-Mode- and Contrast-Enhanced Ultrasound in Differentiating Malignant from Benign Pleural Effusions" Diagnostics 11, no. 7: 1293. https://doi.org/10.3390/diagnostics11071293
APA StyleSafai Zadeh, E., Weide, J., Dietrich, C. F., Trenker, C., Koczulla, A. R., & Görg, C. (2021). Diagnostic Accuracy of B-Mode- and Contrast-Enhanced Ultrasound in Differentiating Malignant from Benign Pleural Effusions. Diagnostics, 11(7), 1293. https://doi.org/10.3390/diagnostics11071293






