Laboratory Diagnosis of SARS-CoV-2 Pneumonia
Abstract
:1. Introduction
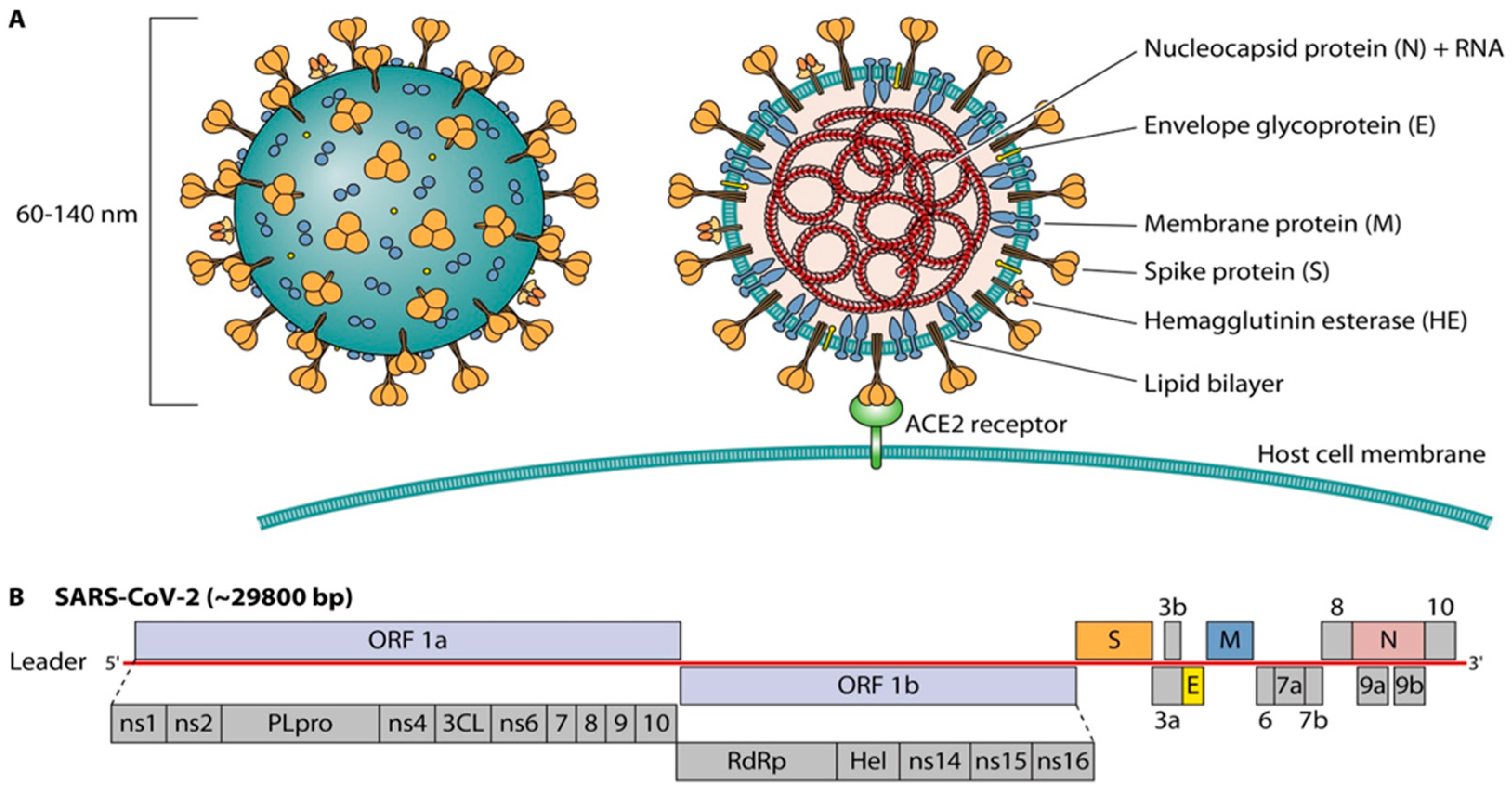
2. Viral Structure
3. Whom to Test
4. What Specimen to Collect
5. Types of Diagnostic Testing
5.1. Nucleic Acid Amplification Tests (NAAT)
5.2. Rapid Antigen Tests (RATs)
5.3. SARS CoV-2 Antibody Tests
6. Conclusions
Funding
Conflicts of Interest
References
- European Centre for Disease Prevention and Control. Cluster of Pneumonia Cases Caused by a Novel Coronavirus, Wuhan, China; 17 January 2020; ECDC: Stockholm, Sweden, 2020. [Google Scholar]
- Astuti, I. Ysrafil Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2): An overview of viral structure and host response. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Andersen, K.G.; Rambaut, A.; Lipkin, W.I.; Holmes, E.C.; Garry, R.F. The proximal origin of SARS-CoV-2. Nat. Med. 2020, 26, 450–452. [Google Scholar] [CrossRef] [Green Version]
- Wrapp, D.; Wang, N.; Corbett, K.S.; Goldsmith, J.A.; Hsieh, C.-L.; Abiona, O.; Graham, B.S.; McLellan, J.S. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science 2020, 367, 1260–1263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, R.; Zhang, Y.; Li, Y.; Xia, L.; Guo, Y.; Zhou, Q. Structural basis for the recognition of the SARS-CoV-2 by full-length human ACE2. Science 2020, 367, 1444–1448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ortiz-Prado, E.; Simbana-Rivera, K.; Gomez-Barreno, L.; Rubio-Neira, M.; Guaman, L.P.; Kyriakidis, N.C.; Muslin, C.; Jaramillo, A.M.G.; Barba-Ostria, C.; Cevallos-Robalino, D.; et al. Clinical, molecular, and epidemiological characterization of the SARS-CoV-2 virus and the Coronavirus Disease 2019 (COVID-19), a comprehensive literature review. Diagn. Microbiol. Infect. Dis. 2020, 98, 115094. [Google Scholar] [CrossRef] [PubMed]
- Gralinski, L.E.; Menachery, V.D. Return of the Coronavirus: 2019-nCoV. Viruses 2020, 12, 135. [Google Scholar] [CrossRef] [Green Version]
- Safiabadi Tali, S.H.; LeBlanc, J.J.; Sadiq, Z.; Oyewunmi, O.D.; Camargo, C.; Nikpour, B.; Armanfard, N.; Sagan, S.M.; Jahanshahi-Anbuhi, S. Tools and Techniques for Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2)/COVID-19 Detection. Clin. Microbiol. Rev. 2021, 34, e00228-20. [Google Scholar] [CrossRef]
- Hatfield, K.M.; Reddy, S.C.; Forsberg, K.; Korhonen, L.; Garner, K.; Gulley, T.; James, A.; Patil, N.; Bezold, C.; Rehman, N.; et al. Facility-Wide Testing for SARS-CoV-2 in Nursing Homes—Seven U.S. Jurisdictions, March–June 2020. Morb. Mortal. Wkly. Rep. 2020, 69, 1095–1099. [Google Scholar] [CrossRef]
- Overview of Testing for SARS-CoV-2 (COVID-19). Available online: https://www.cdc.gov/coronavirus/2019-ncov/hcp/testing-overview.html (accessed on 30 March 2021).
- Hanson, E.K.; Caliendo, A.M.; Arias, A.C.; Hayden, M.K.; Englund, A.J.; Lee, M.J.; Loeb, M.; Patel, R.; Alayli, E.A.; Altayar, O.; et al. The Infectious Diseases Society of America Guidelines on the Diagnosis of COVID-19: Molecular Diagnostic Testing. Clin. Infect. Dis. 2021. [Google Scholar] [CrossRef]
- Sullivan, C.B.; Schwalje, A.T.; Jensen, M.; Li, L.; Dlouhy, B.J.; Greenlee, J.D.; Walsh, J.E. Cerebrospinal Fluid Leak after Nasal Swab Testing for Coronavirus Disease 2019. JAMA Otolaryngol. Neck Surg. 2020, 146, 1179. [Google Scholar] [CrossRef]
- Péré, H.; Podglajen, I.; Wack, M.; Flamarion, E.; Mirault, T.; Goudot, G.; Hauw-Berlemont, C.; Le, L.; Caudron, E.; Carrabin, S.; et al. Nasal Swab Sampling for SARS-CoV-2: A Convenient Alternative in Times of Nasopharyngeal Swab Shortage. J. Clin. Microbiol. 2020, 58. [Google Scholar] [CrossRef] [Green Version]
- To, K.K.-W.; Tsang, O.T.-Y.; Yip, C.C.-Y.; Chan, K.-H.; Wu, T.-C.; Chan, J.M.-C.; Leung, W.-S.; Chik, T.S.-H.; Choi, C.Y.-C.; Kandamby, D.H.; et al. Consistent Detection of 2019 Novel Coronavirus in Saliva. Clin. Infect. Dis. 2020, 71, 841–843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wyllie, A.L.; Fournier, J.; Casanovas-Massana, A.; Campbell, M.; Tokuyama, M.; Vijayakumar, P.; Warren, J.L.; Geng, B.; Muenker, M.C.; Moore, A.J.; et al. Saliva or Nasopharyngeal swab specimens for detection of SARS-CoV-2. N. Engl. J. Med. 2020, 383, 1283–1286. [Google Scholar] [CrossRef]
- Jamal, A.J.; Mohammad, M.; Coomes, E.; Powis, J.; Li, A.; Paterson, A.; Anceva-Sami, S.; Barati, S.; Crowl, G.; Faheem, A.; et al. Sensitivity of nasopharyngeal swabs and saliva for the detection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Clin. Infect. Dis. 2020, ciaa848. [Google Scholar] [CrossRef]
- Huang, N.; Pérez, P.; Kato, T.; Mikami, Y.; Okuda, K.; Gilmore, R.C.; Conde, C.D.; Gasmi, B.; Stein, S.; Beach, M.; et al. SARS-CoV-2 infection of the oral cavity and saliva. Nat. Med. 2021, 27, 892–903. [Google Scholar] [CrossRef] [PubMed]
- Savela, E.S.; Winnett, A.; Romano, A.E.; Porter, M.K.; Shelby, N.; Akana, R.; Ji, J.; Cooper, M.M.; Schlenker, N.W.; Reyes, J.A.; et al. SARS-CoV-2 is detectable using sensitive RNA saliva testing days before viral load reaches detection range of low-sensitivity nasal swab tests. medRxiv 2021. [Google Scholar] [CrossRef]
- Winichakoon, P.; Chaiwarith, R.; Liwsrisakun, C.; Salee, P.; Goonna, A.; Limsukon, A.; Kaewpoowat, Q. Negative Nasopharyngeal and Oropharyngeal Swabs Do Not Rule Out COVID-19. J. Clin. Microbiol. 2020, 58. [Google Scholar] [CrossRef] [Green Version]
- Menter, T.; Haslbauer, J.D.; Nienhold, R.; Savic, S.; Hopfer, H.; Deigendesch, N.; Frank, S.; Turek, D.; Willi, N.; Pargger, H.; et al. Post-mortem examination of COVID19 patients reveals diffuse alveolar damage with severe capillary congestion and variegated findings of lungs and other organs suggesting vascular dysfunction. Histopathology 2020, 77, 198–209. [Google Scholar] [CrossRef]
- Bosch, B.J.; Smits, S.L.; Haagmans, B.L. Membrane ectopeptidases targeted by human coronaviruses. Curr. Opin. Virol. 2014, 6, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, A.; Esmaeilzadeh, E.; Li, Y.; Bosch, R.J.; Li, J.Z. SARS-CoV-2 detection in different respiratory sites: A systematic review and meta-analysis. EBioMedicine 2020, 59, 102903. [Google Scholar] [CrossRef]
- Hou, Y.; Okuda, K.; Edwards, C.E.; Martinez, D.R.; Asakura, T.; Dinnon, K.H.; Kato, T.; Lee, R.E.; Yount, B.L.; Mascenik, T.M.; et al. SARS-CoV-2 Reverse Genetics Reveals a Variable Infection Gradient in the Respiratory Tract. Cell 2020, 182, 429–446. [Google Scholar] [CrossRef]
- Radbel, J.; Jagpal, S.; Roy, J.; Brooks, A.; Tischfield, J.; Sheldon, M.; Bixby, C.; Witt, D.; Gennaro, M.L.; Horton, D.B.; et al. Detection of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Is Comparable in Clinical Samples Preserved in Saline or Viral Transport Medium. J. Mol. Diagn. 2020, 22, 871–875. [Google Scholar] [CrossRef]
- Rodino, K.; Espy, M.J.; Buckwalter, S.P.; Walchak, R.C.; Germer, J.J.; Fernholz, E.; Boerger, A.; Schuetz, A.N.; Yao, J.D.; Binnicker, M.J. Evaluation of Saline, Phosphate-Buffered Saline, and Minimum Essential Medium as Potential Alternatives to Viral Transport Media for SARS-CoV-2 Testing. J. Clin. Microbiol. 2020, 58. [Google Scholar] [CrossRef] [Green Version]
- Bustin, S.A.; Nolan, T. RT-qPCR Testing of SARS-CoV-2: A Primer. Int. J. Mol. Sci. 2020, 21, 3004. [Google Scholar] [CrossRef]
- Kilic, T.; Weissleder, R.; Lee, H. Molecular and Immunological Diagnostic Tests of COVID-19: Current Status and Challenges. iScience 2020, 23, 101406. [Google Scholar] [CrossRef]
- Real-Time RT-PCR Assays for the Detection of SARS-CoV-2. Available online: https://www.who.int/docs/default-source/coronaviruse/whoinhouseassays.pdf (accessed on 21 June 2021).
- Vogels, C.B.F.; Brito, A.F.; Wyllie, A.L.; Fauver, J.R.; Ott, I.M.; Kalinich, C.C.; Petrone, M.E.; Casanovas-Massana, A.; Muenker, M.C.; Moore, A.J.; et al. Analytical sensitivity and efficiency comparisons of SARS-CoV-2 RT–qPCR primer–probe sets. Nat. Microbiol. 2020, 5, 1299–1305. [Google Scholar] [CrossRef]
- Corman, V.M.; Landt, O.; Kaiser, M.; Molenkamp, R.; Meijer, A.; Chu, D.K.; Bleicker, T.; Brünink, S.; Schneider, J.; Schmidt, M.L.; et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Eurosurveillance 2020, 25, 2000045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Food and Drug Administration Coronavirus Disease 2019 (COVID-19) EUA Information. Available online: https://www.fda.gov/medical-devices/coronavirus-disease-2019-covid-19-emergency-use-authorizations-medical-devices/vitro-diagnostics-euas (accessed on 30 March 2021).
- Loeffelholz, M.J.; Tang, Y.-W. Laboratory diagnosis of emerging human coronavirus infections–The state of the art. Emerg. Microbes Infect. 2020, 9, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Loeffelholz, M.J.; Alland, D.; Butler-Wu, S.M.; Pandey, U.; Perno, C.F.; Nava, A.; Carroll, K.C.; Mostafa, H.; Davies, E.; McEwan, A.; et al. Multicenter Evaluation of the Cepheid Xpert Xpress SARS-CoV-2 Test. J. Clin. Microbiol. 2020, 58. [Google Scholar] [CrossRef]
- Fung, B.; Gopez, A.; Servellita, V.; Arevalo, S.; Ho, C.; Deucher, A.; Thornborrow, E.; Chiu, C.; Miller, S. Direct Comparison of SARS-CoV-2 Analytical Limits of Detection across Seven Molecular Assays. J. Clin. Microbiol. 2020, 58. [Google Scholar] [CrossRef] [PubMed]
- Cepheid. Xpert Xpress SARS-CoV-2/Flu/RSV: Instructions for Use; Cepheid: Sunnyvale, CA, USA, 2021. Available online: https://www.fda.gov/media/142437/download (accessed on 21 June 2021).
- Hansen, G.; Marino, J.; Wang, Z.-X.; Beavis, K.G.; Rodrigo, J.; Labog, K.; Westblade, L.F.; Jin, R.; Love, N.; Ding, K.; et al. Clinical performance of the point-of-care cobas Liat for detection of SARS-CoV-2 in 20 minutes: A multicenter study. J. Clin. Microbiol. 2020, 59. [Google Scholar] [CrossRef]
- Roche Molecular Systems. cobas SARS-CoV-2 & Influenza A/B Nucleic Acid Test for Use on the cobas Liat System: Instructions for Use; Roche Molecular Systems: South Branchburg, NJ, USA, 2020. Available online: https://www.fda.gov/media/142193/download (accessed on 21 June 2021).
- Craney, A.R.; Velu, P.D.; Satlin, M.J.; Fauntleroy, K.A.; Callan, K.; Robertson, A.; La Spina, M.; Lei, B.; Chen, A.; Alston, T.; et al. Comparison of Two High-Throughput Reverse Transcription-PCR Systems for the Detection of Severe Acute Respiratory Syndrome Coronavirus 2. J. Clin. Microbiol. 2020, 58. [Google Scholar] [CrossRef]
- Roche Molecular Systems. cobas® SARS-CoV-2 Qualitative Assay for Use on the cobas® 6800/8800 Systems; Roche Molecular Systems: South Branchburg, NJ, USA, 2021. Available online: https://www.fda.gov/media/136049/download (accessed on 21 June 2021).
- Roche Molecular Systems. cobas SARS CoV-2 Test: Instructions for Use; Roche Molecular Systems: South Branchburg, NJ, USA, 2021. Available online: https://www.fda.gov/media/136049/download (accessed on 30 June 2021).
- Mesa Biotech. Accula SARS-Cov-2 Test: Instructions for Use; Mesa Biotech: San Diego, CA, USA, 2021; Available online: https://www.mesabiotech.com/wp-content/uploads/2021/02/LBL-60061-Accula-SARS-CoV-2-IFU-03FEB2021).pdf (accessed on 1 July 2021).
- BioFire Diagnostics. BioFire Respiratory Panel 2.1-E.Z. (RP2.1-E.Z.): Instructions for Use; BioFire Diagnostics: Salt Lake City, UT, USA, 2020; Available online: https://docs.biofiredx.com/wp-content/uploads/BFR0001-0044-BioFire-RP2.1-EZ-Instructions-for-Use-EUA-1.pdf (accessed on 21 June 2021).
- Bal, A.; Destras, G.; Gaymard, A.; Stefic, K.; Marlet, J.; Eymieux, S.; Regue, H.; Semanas, Q.; D’Aubarede, C.; Billaud, G.; et al. Two-step strategy for the identification of SARS-CoV-2 variant of concern 202012/01 and other variants with spike deletion H69–V70, France, August to December 2020. Eurosurveillance 2021, 26, 2100008. [Google Scholar] [CrossRef]
- Thermo Fisher Scientific, Inc. TaqPath COVID-19 Combo Kit and TaqPath COVID-19 Combo Kit Advanced Instructions for Use; Thermo Fisher Scientific, Inc.: Waltham, MA, USA, 2021. Available online: https://www.fda.gov/media/136112/download (accessed on 21 June 2021).
- Yu, C.Y.; Chan, K.G.; Yean, C.Y.; Ang, G.Y. Nucleic Acid-Based Diagnostic Tests for the Detection SARS-CoV-2: An Update. Diagnostics 2021, 11, 53. [Google Scholar] [CrossRef]
- Abbott Diagnostics. Abbott RealTime SARS-CoV-2: Instructions for Use; Abbott Molecular, Inc.: Des Plaines, IL, USA, 2020. Available online: https://www.fda.gov/media/136258/download (accessed on 1 July 2021).
- Perkin Elmer Genomics. PerkinElmer SARS-CoV-2 RT-qPCR: Emergency Use Authorization Summary; Perkin Elmer Genomics: Pittsburg, PA, USA, 2020. Available online: https://www.fda.gov/media/147547/download (accessed on 1 July 2021).
- Huang, Z.; Tian, D.; Liu, Y.; Lin, Z.; Lyon, C.J.; Lai, W.; Fusco, D.; Drouin, A.; Yin, X.; Hu, T.; et al. Ultra-sensitive and high-throughput CRISPR-powered COVID-19 diagnosis. Biosens. Bioelectron. 2020, 164, 112316. [Google Scholar] [CrossRef]
- Hologic. Aptima® SARS-CoV-2 Assay (Panther® System): Instructions for Use; Hologic: San Diego, CA, USA, 2021; Available online: https://www.hologic.com/sites/default/files/2021-06/AW-21492-001_007_01.pdf (accessed on 1 July 2021).
- Cue Health, Inc. Cue COVID-19 Test for Home and Over-the-Counter (OTC) Use; Cue Health, Inc.: San Diego, CA, USA, 2021. Available online: https://www.fda.gov/media/146470/download (accessed on 1 July 2021).
- Seasun Biomaterials, Inc. AQ-TOP COVID-19 Rapid Detection Kit PLUS: Instructions for Use; Seasun Biomaterials, Inc.: Seoul, Korea, 2021. Available online: https://www.fda.gov/media/142800/download (accessed on 21 June 2021).
- Pro-Lab Diagnostics. Pro-AmpRT SARS-CoV-2 Test. Available online: https://www.fda.gov/media/141149/download (accessed on 2 November 2020).
- Rhoads, D.D.; Cherian, S.S.; Roman, K.; Stempak, L.M.; Schmotzer, C.L.; Sadri, N. Comparison of Abbott ID Now, DiaSorin Simplexa, and CDC FDA Emergency Use Authorization Methods for the Detection of SARS-CoV-2 from Nasopharyngeal and Nasal Swabs from Individuals Diagnosed with COVID-19. J. Clin. Microbiol. 2020, 58. [Google Scholar] [CrossRef] [Green Version]
- Harrington, A.; Cox, B.; Snowdon, J.; Bakst, J.; Ley, E.; Grajales, P.; Maggiore, J.; Kahn, S. Comparison of Abbott ID Now and Abbott m2000 Methods for the Detection of SARS-CoV-2 from Nasopharyngeal and Nasal Swabs from Symptomatic Patients. J. Clin. Microbiol. 2020, 58. [Google Scholar] [CrossRef] [Green Version]
- Abbott Diagnostics. ID NOW COVID-19: Instructions for Use; Abbott Diagnostics: Scarborough, ME, USA, 2020. Available online: https://www.fda.gov/media/136525/download (accessed on 21 June 2021).
- Broughton, J.P.; Deng, X.; Yu, G.; Fasching, C.L.; Servellita, V.; Singh, J.; Miao, X.; Streithorst, J.A.; Granados, A.; Sotomayor-Gonzalez, A.; et al. CRISPR–Cas12-based detection of SARS-CoV-2. Nat. Biotechnol. 2020, 38, 870–874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sherlock Biosciences. Sherlock CRISPR SARS-CoV-2 kit: Instructions for Use; Sherlock Biosciences: Boston, MA, USA, 2021. Available online: https://www.fda.gov/media/137746/download (accessed on 21 June 2021).
- U.S. Food and Drug Administration. Available online: http://www.fda.gov/ (accessed on 21 June 2021).
- Curti, L.; Primost, I.; Valla, S.; Alegre, D.I.; Perglione, C.O.; Repizo, G.; Lara, J.; Parcerisa, I.; Palacios, A.; Llases, M.; et al. Evaluation of a Lyophilized CRISPR-Cas12 Assay for a Sensitive, Specific, and Rapid Detection of SARS-CoV-2. Viruses 2021, 13, 420. [Google Scholar] [CrossRef]
- Moran, A.; Beavis, K.G.; Matushek, S.M.; Ciaglia, C.; Francois, N.; Tesic, V.; Love, N. Detection of SARS-CoV-2 by Use of the Cepheid Xpert Xpress SARS-CoV-2 and Roche cobas SARS-CoV-2 Assays. J. Clin. Microbiol. 2020, 58. [Google Scholar] [CrossRef] [Green Version]
- Zhen, W.; Manji, R.; Smith, E.; Berry, G.J. Comparison of Four Molecular In Vitro Diagnostic Assays for the Detection of SARS-CoV-2 in Nasopharyngeal Specimens. J. Clin. Microbiol. 2020, 58. [Google Scholar] [CrossRef] [PubMed]
- Zhen, W.; Smith, E.; Manji, R.; Schron, D.; Berry, G.J. Clinical Evaluation of Three Sample-to-Answer Platforms for Detection of SARS-CoV-2. J. Clin. Microbiol. 2020, 58. [Google Scholar] [CrossRef] [Green Version]
- Smith, E.; Zhen, W.; Manji, R.; Schron, D.; Duong, S.; Berry, G.J. Analytical and Clinical Comparison of Three Nucleic Acid Amplification Tests for SARS-CoV-2 Detection. J. Clin. Microbiol. 2020, 58. [Google Scholar] [CrossRef]
- Smithgall, M.C.; Scherberkova, I.; Whittier, S.; Green, D.A. Comparison of Cepheid Xpert Xpress and Abbott ID Now to Roche cobas for the Rapid Detection of SARS-CoV-2. J. Clin. Virol. 2020, 128, 104428. [Google Scholar] [CrossRef]
- Lowe, C.F.; Matic, N.; Ritchie, G.; Lawson, T.; Stefanovic, A.; Champagne, S.; Leung, V.; Romney, M.G. Detection of low levels of SARS-CoV-2 RNA from nasopharyngeal swabs using three commercial molecular assays. J. Clin. Virol. 2020, 128, 104387. [Google Scholar] [CrossRef]
- Pujadas, E.; Ibeh, N.; Hernandez, M.M.; Waluszko, A.; Sidorenko, T.; Flores, V.; Shiffrin, B.; Chiu, N.; Young-Francois, A.; Nowak, M.D.; et al. Comparison of SARS-CoV-2 detection from nasopharyngeal swab samples by the Roche cobas 6800 SARS-CoV-2 test and a laboratory-developed real-time RT-PCR test. J. Med. Virol. 2020, 92, 1695–1698. [Google Scholar] [CrossRef]
- Mannonen, L.; Kallio-Kokko, H.; Loginov, R.; Jääskeläinen, A.; Jokela, P.; Antikainen, J.; Väre, P.; Kekäläinen, E.; Kurkela, S.; Jarva, H.; et al. Comparison of Two Commercial Platforms and a Laboratory-Developed Test for Detection of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) RNA. J. Mol. Diagn. 2021, 23, 407–416. [Google Scholar] [CrossRef]
- Dust, K.; Hedley, A.; Nichol, K.; Stein, D.; Adam, H.; Karlowsky, J.A.; Bullard, J.; Van Caeseele, P.; Alexander, D.C. Comparison of commercial assays and laboratory developed tests for detection of SARS-CoV-2. J. Virol. Methods 2020, 285, 113970. [Google Scholar] [CrossRef]
- Procop, G.W.; Brock, J.E.; Reineks, E.Z.; Shrestha, N.K.; Demkowicz, R.; Cook, E.; Ababneh, E.; Harrington, S.M. A Comparison of Five SARS-CoV-2 Molecular Assays with Clinical Correlations. Am. J. Clin. Pathol. 2021, 155, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Hirschwerk, D.; Foley, M.; Lesser, M.; Farber, B.; Crawford, J.M.; Davidson, K.W.; Berry, G.J.; Smith, E.; Kast, C.; Volel, V.; et al. Estimating the predictive value of negative severe acute respiratory coronavirus virus 2 (SARS-CoV-2) results: A prospective study. Infect. Control. Hosp. Epidemiol. 2020, 10, 1–3. [Google Scholar] [CrossRef]
- Tom, M.; Mina, M.J. To Interpret the SARS-CoV-2 Test, Consider the Cycle Threshold Value. Clin. Infect. Dis. 2020, 71, 2252–2254. [Google Scholar] [CrossRef]
- IDSA and AMP Joint Statement on the Use of SARS-CoV-2 PCR Cycle Threshold (Ct) Values for Clinical Decision-Making. Available online: https://www.idsociety.org/globalassets/idsa/public-health/covid-19/idsa-amp-statement.pdf (accessed on 4 March 2021).
- Lee, S.; Kim, T.; Lee, E.; Lee, C.; Kim, H.; Rhee, H.; Park, S.Y.; Son, H.-J.; Yu, S.; Park, J.W.; et al. Clinical Course and Molecular Viral Shedding among Asymptomatic and Symptomatic Patients with SARS-CoV-2 Infection in a Community Treatment Center in the Republic of Korea. JAMA Intern. Med. 2020, 180. [Google Scholar] [CrossRef]
- Lavezzo, E.; Franchin, E.; Ciavarella, C.; Cuomo-Dannenburg, G.; Barzon, L.; Del Vecchio, C.; Rossi, L.; Manganelli, R.; Loregian, A.; Navarin, N.; et al. Suppression of a SARS-CoV-2 outbreak in the Italian municipality of Vo’. Nature 2020, 584, 425–429. [Google Scholar] [CrossRef]
- Habibzadeh, P.; Mofatteh, M.; Silawi, M.; Ghavami, S.; Faghihi, M.A. Molecular diagnostic assays for COVID-19: An overview. Crit. Rev. Clin. Lab. Sci. 2021, 1–20. [Google Scholar] [CrossRef]
- Antonelli, G.; Stefani, S.; Pistello, M. SARS-CoV-2 diagnostics: Some reflections on current assays. Diagn. Microbiol. Infect. Dis. 2021, 99, 115237. [Google Scholar] [CrossRef]
- He, X.; Lau, E.H.Y.; Wu, P.; Deng, X.; Wang, J.; Hao, X.; Lau, Y.C.; Wong, J.Y.; Guan, Y.; Tan, X.; et al. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat. Med. 2020, 26, 672–675. [Google Scholar] [CrossRef] [Green Version]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Avanzato, V.A.; Matson, M.J.; Seifert, S.N.; Pryce, R.; Williamson, B.N.; Anzick, S.L.; Barbian, K.; Judson, S.D.; Fischer, E.R.; Martens, C.; et al. Case Study: Prolonged Infectious SARS-CoV-2 Shedding from an Asymptomatic Immunocompromised Individual with Cancer. Cell 2020, 183, 1901–1912.e9. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.; Choudhary, M.C.; Regan, J.; Sparks, J.A.; Padera, R.F.; Qiu, X.; Solomon, I.H.; Kuo, H.-H.; Boucau, J.; Bowman, K.; et al. Persistence and Evolution of SARS-CoV-2 in an Immunocompromised Host. N. Engl. J. Med. 2020, 383, 2291–2293. [Google Scholar] [CrossRef] [PubMed]
- Folgueira, M.D.; Luczkowiak, J.; Lasala, F.; Pérez-Rivilla, A.; Delgado, R. Prolonged SARS-CoV-2 cell culture replication in respiratory samples from patients with severe COVID-19. Clin. Microbiol. Infect. 2021, 27, 886–891. [Google Scholar] [CrossRef]
- Carter, L.J.; Garner, L.V.; Smoot, J.W.; Li, Y.; Zhou, Q.; Saveson, C.J.; Sasso, J.M.; Gregg, A.C.; Soares, D.J.; Beskid, T.R.; et al. Assay Techniques and Test Development for COVID-19 Diagnosis. ACS Cent. Sci. 2020, 6, 591–605. [Google Scholar] [CrossRef] [PubMed]
- Gorzalski, A.J.; Tian, H.; Laverdure, C.; Morzunov, S.; Verma, S.C.; VanHooser, S.; Pandori, M.W. High-Throughput Transcription-mediated amplification on the Hologic Panther is a highly sensitive method of detection for SARS-CoV-2. J. Clin. Virol. 2020, 129, 104501. [Google Scholar] [CrossRef] [PubMed]
- James, A.S.; Alawneh, J.I. COVID-19 Infection Diagnosis: Potential Impact of Isothermal Amplification Technology to Reduce Community Transmission of SARS-CoV-2. Diagnostics 2020, 10, 399. [Google Scholar] [CrossRef]
- LumiraDx SARS-CoV-2 RNA STAR Complete Instructions for Use for Emergency Use Authorization (EUA) Only. Available online: https://www.fda.gov/media/143062/download (accessed on 4 April 2021).
- Wandernoth, P.; Kriegsmann, K.; Groh-Mohanu, C.; Daeumer, M.; Gohl, P.; Harzer, O.; Kriegsmann, M.; Kriegsmann, J. Detection of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) by Mass Spectrometry. Viruses 2020, 12, 849. [Google Scholar] [CrossRef] [PubMed]
- Agena Bioscience. MassARRAY® SARS-CoV-2 Panel Instructions for Use Multiplex RT-PCR/MALDI-TOF Test Intended for the Qualitative Detection of Nucleic Acid from SARS-CoV-2; Agena Bioscience: San Diego, CA, USA, 2021. Available online: https://www.fda.gov/media/143334/download (accessed on 4 April 2021).
- Artesi, M.; Bontems, S.; Göbbels, P.; Franckh, M.; Maes, P.; Boreux, R.; Meex, C.; Melin, P.; Hayette, M.-P.; Bours, V.; et al. A Recurrent Mutation at Position 26340 of SARS-CoV-2 Is Associated with Failure of the E Gene Quantitative Reverse Transcription-PCR Utilized in a Commercial Dual-Target Diagnostic Assay. J. Clin. Microbiol. 2020, 58, e01598-20. [Google Scholar] [CrossRef]
- Wang, R.; Hozumi, Y.; Yin, C.; Wei, G.-W. Mutations on COVID-19 diagnostic targets. Genomics 2020, 112, 5204–5213. [Google Scholar] [CrossRef]
- Potential for False Results with Roche Molecular Systems, Inc. cobas SARS-CoV-2 & Influenza Test for use on cobas Liat System-Letter to Clinical Laboratory Staff, Point-of-Care Facility Staff, and Health Care Providers. Available online: https://www.fda.gov/medical-devices/letters-health-care-providers/potential-false-results-roche-molecular-systems-inc-cobas-sars-cov-2-influenza-test-use-cobas-liat (accessed on 30 March 2021).
- Basu, A.; Zinger, T.; Inglima, K.; Woo, K.-M.; Atie, O.; Yurasits, L.; See, B.; Aguero-Rosenfeld, M.E. Performance of Abbott ID Now COVID-19 Rapid Nucleic Acid Amplification Test Using Nasopharyngeal Swabs Transported in Viral Transport Media and Dry Nasal Swabs in a New York City Academic Institution. J. Clin. Microbiol. 2020, 58. [Google Scholar] [CrossRef]
- Cradic, K.; Lockhart, M.; Ozbolt, P.; Fatica, L.; Landon, L.; Lieber, M.; Yang, D.; Swickard, J.; Wongchaowart, N.; Fuhrman, S.; et al. Clinical Evaluation and Utilization of Multiple Molecular In Vitro Diagnostic Assays for the Detection of SARS-CoV-2. Am. J. Clin. Pathol. 2020, 154, 201–207. [Google Scholar] [CrossRef]
- Donato, L.J.; Trivedi, V.A.; Stransky, A.M.; Misra, A.; Pritt, B.S.; Binnicker, M.J.; Karon, B.S. Evaluation of the Cue Health point-of-care COVID-19 (SARS-CoV-2 nucleic acid amplification) test at a community drive through collection center. Diagn. Microbiol. Infect. Dis. 2021, 100, 115307. [Google Scholar] [CrossRef] [PubMed]
- Beck, E.T.; Paar, W.; Fojut, L.; Serwe, J.; Jahnke, R.R. Comparison of the Quidel Sofia SARS FIA Test to the Hologic Aptima SARS-CoV-2 TMA Test for Diagnosis of COVID-19 in Symptomatic Outpatients. J. Clin. Microbiol. 2021, 59. [Google Scholar] [CrossRef] [PubMed]
- Young, S.; Taylor, S.N.; Cammarata, C.L.; Varnado, K.G.; Roger-Dalbert, C.; Montano, A.; Griego-Fullbright, C.; Burgard, C.; Fernandez, C.; Eckert, K.; et al. Clinical Evaluation of BD Veritor SARS-CoV-2 Point-of-Care Test Performance Compared to PCR-Based Testing and versus the Sofia 2 SARS Antigen Point-of-Care Test. J. Clin. Microbiol. 2020, 59. [Google Scholar] [CrossRef]
- Dinnes, J.; Deeks, J.J.; Adriano, A.; Berhane, S.; Davenport, C.; Dittrich, S.; Emperador, D.; Takwoingi, Y.; Cunningham, J.; Beese, S.; et al. Rapid, point-of-care antigen and molecular-based tests for diagnosis of SARS-CoV-2 infection. Cochrane Database Syst. Rev. 2020, 2020, CD013705. [Google Scholar] [CrossRef]
- Pekosz, A.; Parvu, V.; Li, M.; Andrews, J.C.; Manabe, Y.C.; Kodsi, S.; Gary, D.S.; Roger-Dalbert, C.; Leitch, J.; Cooper, C.K. Antigen-Based Testing but Not Real-Time Polymerase Chain Reaction Correlates with Severe Acute Respiratory Syndrome Coronavirus 2 Viral Culture. Clin. Infect. Dis. 2021. [Google Scholar] [CrossRef]
- Interim Guidance for Antigen Testing for SARS-CoV-2. Available online: https://www.cdc.gov/coronavirus/2019-ncov/lab/resources/antigen-tests-guidelines.html (accessed on 13 May 2021).
- Foundation for Innovative New Diagnostics. The Impact of Novel Variants of SARS-CoV2 on Diagnostic Testing. Available online: https://www.finddx.org/covid-19/novel-variants/ (accessed on 13 May 2021).
- Quidel Corporation. Sofia SARS Antigen FIA: Instructions for Use; Quidel Corporation: San Diego, CA, USA, 2020; Available online: https://www.medline.com/media/catalog/Docs/MKT/WP/COVID19/Quidel-Sofia-SARS-Antigen-FIA-Instructions-for-Use-06-20.pdf (accessed on 1 July 2021).
- Veritor System for Rapid Detection of SARS-CoV-2. Available online: https://www.fda.gov/media/139755/download (accessed on 21 June 2021).
- Orasure Technologies, Inc. InteliSwab COVID-19 Rapid Test Pro: Instructions for Use; Orasure Technologies, Inc.: Bethlehem, PA, USA, 2020. Available online: https://www.fda.gov/media/149918/download (accessed on 21 June 2021).
- InBios International, Inc. ScoV-2 Ag Detect Rapid Test: Instructions for Use; InBios International, Inc.: Seattle, WA, USA, 2020. Available online: https://www.fda.gov/media/148353/download (accessed on 21 June 2021).
- Celltrion USA, Inc. Celltrion DiaTrust COVID-19 Ag Rapid Test: Instructions for Use; Celltrion USA, Inc.: Jersey City, NJ, USA, 2020. Available online: https://www.fda.gov/media/147694/download (accessed on 21 June 2021).
- Damaschk, M.; Donicke, T.; Lux, F. Multiclass Text. Classification on Unbalanced, Sparse and Noisy Data; Linköping University Electronic Press: Turku, Finland, 2019; pp. 58–65. Available online: https://www.aclweb.org/anthology/W19-6207 (accessed on 21 June 2021).
- Princeton BioMeditech, Corp. Status COVID-19/Flu: Instructions for Use; Princeton BioMediTech, Corp: Princeton, NJ, USA, 2020. Available online: https://www.fda.gov/media/145697/download (accessed on 21 June 2021).
- Zhang, Y.V.; Wiencek, J.; Meng, Q.H.; Theel, E.S.; Babic, N.; Sepiashvili, L.; Pecora, N.D.; Slev, P.; Cameron, A.; Konforte, D. AACC Practical Recommendations for Implementing and Interpreting SARS-CoV-2 EUA and LDT Serologic Testing in Clinical Laboratories. Clin. Chem. 2021. [Google Scholar] [CrossRef]
- Guo, L.; Ren, L.; Yang, S.; Xiao, M.; Chang, D.; Yang, F.; Cruz, C.S.D.; Wang, Y.; Wu, C.; Xiao, Y.; et al. Profiling Early Humoral Response to Diagnose Novel Coronavirus Disease (COVID-19). Clin. Infect. Dis. 2020, 71, 778–785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lynch, K.L.; Whitman, J.D.; Lacanienta, N.P.; Beckerdite, E.W.; Kastner, S.A.; Shy, B.R.; Goldgof, G.M.; Levine, A.G.; Bapat, S.P.; Stramer, S.L.; et al. Magnitude and Kinetics of Anti–Severe Acute Respiratory Syndrome Coronavirus 2 Antibody Responses and Their Relationship to Disease Severity. Clin. Infect. Dis. 2021, 72, 301–308. [Google Scholar] [CrossRef]
- Rijkers, G.; Murk, J.-L.; Wintermans, B.; Van Looy, B.; Berge, M.V.D.; Veenemans, J.; Stohr, J.; Reusken, C.; Van Der Pol, P.; Reimerink, J. Differences in Antibody Kinetics and Functionality between Severe and Mild Severe Acute Respiratory Syndrome Coronavirus 2 Infections. J. Infect. Dis. 2020, 222, 1265–1269. [Google Scholar] [CrossRef] [PubMed]
- Gudbjartsson, D.F.; Norddahl, G.L.; Melsted, P.; Gunnarsdottir, K.; Holm, H.; Eythorsson, E.; Arnthorsson, A.O.; Helgason, D.; Bjarnadottir, K.; Ingvarsson, R.F.; et al. Humoral Immune Response to SARS-CoV-2 in Iceland. N. Engl. J. Med. 2020, 383, 1724–1734. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Rusling, J.F. COVID-19 Antibody Tests and Their Limitations. ACS Sens. 2021, 6, 593–612. [Google Scholar] [CrossRef]
- Interim Guidelines for COVID-19 Antibody Testing. Available online: https://www.cdc.gov/coronavirus/2019-ncov/lab/resources/antibody-tests-guidelines.html (accessed on 30 March 2021).
- The National SARS-CoV-2 Serology Assay Evaluation Group. Performance characteristics of five immunoassays for SARS-CoV-2: A head-to-head benchmark comparison. Lancet Infect. Dis. 2020, 20, 1390–1400. [Google Scholar] [CrossRef]
- Pérez-García, F.; Pérez-Tanoira, R.; Iglesias, M.E.; Romanyk, J.; Arroyo, T.; Gómez-Herruz, P.; González, R.; García, S.L.; Cuadros-González, J. Comparative evaluation of six immunoassays for the detection of antibodies against SARS-CoV-2. J. Virol. Methods 2021, 289, 114047. [Google Scholar] [CrossRef]
- Nilsson, A.C.; Holm, D.K.; Justesen, U.S.; Gorm-Jensen, T.; Andersen, N.S.; Øvrehus, A.; Johansen, I.S.; Michelsen, J.; Sprogøe, U.; Lillevang, S.T. Comparison of six commercially available SARS-CoV-2 antibody assays—Choice of assay depends on intended use. Int. J. Infect. Dis. 2021, 103, 381–388. [Google Scholar] [CrossRef]
- Padoan, A.; Bonfante, F.; Pagliari, M.; Bortolami, A.; Negrini, D.; Zuin, S.; Bozzato, D.; Cosma, C.; Sciacovelli, L.; Plebani, M. Analytical and clinical performances of five immunoassays for the detection of SARS-CoV-2 antibodies in comparison with neutralization activity. EBioMedicine 2020, 62, 103101. [Google Scholar] [CrossRef]
- Whitman, J.D.; Hiatt, J.; Mowery, C.T.; Shy, B.R.; Yu, R.; Yamamoto, T.N.; Rathore, U.; Goldgof, G.M.; Whitty, C.; Woo, J.M.; et al. Evaluation of SARS-CoV-2 serology assays reveals a range of test performance. Nat. Biotechnol. 2020, 38, 1174–1183. [Google Scholar] [CrossRef] [PubMed]
- Nirimidas Biotech, Inc. MidaSpotTM COVID-19 Antibody Combo Detection Kit: Instructions for Use; Nirimidas Bio-tech, Inc.: Palo Alto, CA, USA, 2020. Available online: https://www.fda.gov/media/144877/download (accessed on 21 June 2021).
- Clarity Diagnostics, LLC. Sienna-Clarity COVIBLOCK COVID-19 IgG/IgM Rapid Test Cassette: Instructions for Use; Clarity Diagnostics, LLC.: Boca Raton, FL, USA, 2020. Available online: https://www.fda.gov/media/140082/download (accessed on 21 June 2021).
- Healgen Scientific, LLC. COVID-19 IgG/IgM Rapid Test Cassette: Instructions for Use; Healgen Scientific, LLC: Houston, TX, USA, 2020. Available online: https://www.fda.gov/media/138438/download (accessed on 21 June 2021).
- Sugentech, Inc. SGTI-flux COVID-19 IgG Test: Instructions for Use; Sugentech, Inc.: Daejeon, Korea, 2020. Available online: https://www.fda.gov/media/141891/download (accessed on 21 June 2021).
- Symbiotica, Inc. COVID-19 Antibody Combo Detection Kit: Emergency Use Authorization Summary; Symbiotica, Inc.: Vacaville, CA, USA, 2020. Available online: https://www.fda.gov/media/147365/download (accessed on 21 June 2021).
- Kantaro Biosciences, LLC. COVID-SeroKlir, Kantaro Semi-Quantitative SARS-CoV-2 IgG Antibody Kit: Emergency Use Authorization Summary; Kantaro Biosciences, LLC.: New York, NY, USA, 2020. Available online: https://www.fda.gov/media/144010/download (accessed on 21 June 2021).
- GenScript USA, Inc. cPASS SARS-CoV-2 Neutralization Antibody Detection Kit: Instructions for Use; GenScript USA, Inc.: Piscataway, NJ, USA, 2020. Available online: https://www.fda.gov/media/143583/download (accessed on 21 June 2021).
- ZEUS Scientific, Inc. ZEUS ELISA SARS-CoV-2 IgG Test System: Instructions for Use; ZEUS Scientific, Inc.: Branchburg, NJ, USA, 2020. Available online: https://www.fda.gov/media/142809/download (accessed on 21 June 2021).
- Mount Sinai Laboratories. COVID-19 ELISA IgG Antibody Test: Emergency Use Authorization Summary; Mount Sinai Lab: New York, NY, USA, 2020. Available online: https://www.fda.gov/media/137029/download (accessed on 21 June 2021).
- Siemens Healthcare Diagnostics, Inc. Dimension EXL SARS-CoV-2 IgG Test: Instructions for Use; Siemens Healthcare Diagnostics: Newark, DE, USA, 2020. Available online: https://www.fda.gov/media/138757/download (accessed on 21 June 2021).
- Ortho-Clinical Diagnostics. VITROS Immunodiagnostic Products Anti-SARS-CoV-2 Total Reagent Pack: Instructions for Use; Ortho-Clinical Diagnostics: Raritan, NJ, USA, 2020. Available online: https://www.fda.gov/media/137363/download (accessed on 21 June 2021).
- Roche Molecular Systems. Elecsys Anti-SARS-CoV-2: Instructions for Use; Roche Diagnostics: Indianapolis, IN, USA, 2020. Available online: https://www.fda.gov/media/144037/download (accessed on 21 June 2021).
- Beckman Coulter, Inc. Access SARS-CoV-2 IgM Test: Instructions for Use; Beckman Coulter, Inc.: Brea, CA, USA, 2020. Available online: https://www.fda.gov/media/142911/download (accessed on 21 June 2021).
- DiaSorin, Inc. LIAISON SARS-CoV-2 S1/S2 IgG Test: Instructions for Use; DiaSorin, Inc.: Stillwater, MN, USA, 2020. Available online: https://www.fda.gov/media/137359/download (accessed on 21 June 2021).
- BioCheck, Inc. BioCheck SARS-CoV-2 IgG Antibody Test Kit-Instructions for Use; BioCheck, Inc.: South San Francisco, CA, USA, 2020. Available online: https://www.fda.gov/media/142006/download (accessed on 21 June 2021).
- Vibrant America Clinical Labs. Vibrant America Labs Vibrant COVID-19 Ab Kit: Emergency Use Authorization Summary; Vibrant America Clinical Labs: San Carlos, CA, USA, 2020. Available online: https://www.fda.gov/media/138629/download (accessed on 21 June 2021).
| Nucleic Acid Amplification Tests (NAAT) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Name of Test | Developer | Nature of Specimen | Target Gene | Sensitivity (PPA) | Specificity (NPA) | Limit of Detection (LoD) | Time to Result | Status | References |
| Non-Isothermal: RT-PCR | |||||||||
| Xpert ® Xpress SARS-CoV-2/Flu/RSV | Cepheid | NS, NPS, OPS, MTS, NA, NW | E, N2 | 97.9% | 100% | 131 GCE/mL | 45 min run | EUA | [33,34,35] |
| Cobas ® SARS-CoV-2 & Influenza A/B: cobas Liat System | Roche Molecular Systems | NPS, NS | Orf1ab, N | 100% | 97.4% | 0.012 TCID50/mL | 20 min run | EUA | [36,37] |
| Cobas ® SARS-CoV-2 Test | Roche Molecular Systems | NS, NPS, OPS | Orf1ab, E | 100% | 95.5% | 0.007 TCID50/mL | 3.5 h run | EUA; CE-IVD | [33,38,39,40] |
| Accula ™ SARS-CoV-2 Test | Mesa Biotech | NS, MTS | N | 95.8% | 100% | 150 copies/mL | 30 min run | EUA | [41] |
| BioFire ® Respiratory Panel 2.1-EZ | BioFire Diagnostics | NPS | S, M | 98% | 100% | 6000 copies/mL | 45 min run | EUA | [42] |
| TaqPath ™ COVID-19 Pooling Kit | ThermoFisher Scientific, Inc. | NPS, NA, BAL | Orf1ab, S, N | 100% | 100% | 10 GCE/reaction | 4 h run | EUA; CE-IVD | [43,44,45] |
| Abbott RealTime ™ SARS-CoV-2 | Abbott Molecular | NS, NPS, OPS, BAL | RdRp, N | 100% | 100% | 100 copies/mL | 6.8 h run | EUA | [34,46] |
| Non-Isothermal: RT-qSTAR amplification | |||||||||
| PerkinElmer ® SARS-CoV-2 RT-qPCR Reagent Kit | Perkin Elmer Genomics | NPS, OPS, NA | Orf1ab, N | 100% | 100% | 120 copies/mL | 1 h run | CE-IVD; WHO-EUL | [47] |
| LumiraDx ™ SARS-CoV-2 RNA STAR complete | LumiraDx UK, Ltd. | NPS, OPS, NA, MTS | Orf1ab | 95% | 100% | 1875 copies/mL | 20 min run | EUA | [48] |
| Isothermal: Transcription mediated amplification | |||||||||
| Aptima ® SARS-CoV-2 Assay | Hologic, Inc. | NPS, OPS, NS, NA | Orf1ab | 100% | 99.7% | 0.026 TCID50/mL | 2.5 h run | EUA; CE-IVD | [49] |
| ISOTHERMAL: RT-LAMP/NEAR | |||||||||
| Cue ™ COVID-19 Test for Home and OTC Use | Cue Health, Inc. | NS | N | 97.4% | 99.1% | 2700 copies/mL | 20 min run | EUA | [50] |
| AQ-TOP ™ COVID-19 Rapid Detection Test PLUS | Seasun Biomaterials, Inc. | NPS, OPS, NS, NA, MTS | Orf1ab, N | 100% | 100% | 1 copy/µL | 2 h run | EUA | [51] |
| Pro-AmpRT SARS-CoV-2 Test | Pro-Lab Diagnostics | NPS, OPS, NS, NW, MTS | Orf1ab | 96.6% | 100% | 125 copies/swab | 30 min run | EUA | [52] |
| ID Now ™ COVID-19 (NEAR) | Abbott Diagnostics | NPS, OPS, NS | RdRp | 100% | 100% | 125 GCE/mL | 13 min run | EUA; CE-IVD | [53,54,55] |
| isothermal: RT-PCR/CRISPR | |||||||||
| Sherlock ™ CRISPR SARS-CoV-2 Kit | Sherlock Biosciences Inc. | NPS, OPS, NS, NPW, NA, BAL | Orf1ab, N | 100% | 100% | 6750 copies/mL | 1h run | EUA | [56,57] |
| SARS-CoV-2 DETECTR ™ Reagent Kit | Mammoth Biosciences, Inc. | NPS, OPS, MTS, NPA, NA | N | 95% | 100% | 20,000 copies/mL | 15 min run | EUA | [58] |
| Caspr Lyo-CRISPR SARS-CoV-2 Kit (FAM) | Caspr Biotech | NS, NPS, OPS | Orf1ab, N | 99% | 99% | 25,000 copies/mL | 1 h run | EUA | [59] |
| Rapid Antigen Tests (RATs) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Name of Test | Developer | Nature of Specimen | Target Antigen | Sensitivity (PPA) | Specificity (NPA) | Limit of Detection (LoD) | Time to Result | Status | References |
| Sofia ® SARS Antigen FIA | Quidel Corporation | NPS, NS, ANS | Nucleocapsid Protein | 100% | 100% | 113 TCID50/mL | 15 min run | EUA | [94,95,100] |
| BD Veritor ™ System for RAPID Detection of SARS-CoV-2 & Flu A+B | Becton, Dickinson and Company | NS | Nucleocapsid Protein | 84% | 100% | 140 TCID50/mL | 15 min run | EUA | [95,101] |
| InteliSwab ™ COVID-19 Rapid Test Pro | OraSure Technologies, Inc. | NS | Nucleocapsid Protein | 84% | 98% | 2500 TCID50/mL | 35 min run | EUA: CE-IVD | [102] |
| SCoV-2 Ag Detect ™ Rapid Test | InBios International, Inc. | ANS, NS | Nucleocapsid Protein | 86.6% | 100% | 6300 TCID50/mL | 25 min run | EUA; CE-IVD | [103] |
| Celltrion DiaTrust ™ COVID-19 Ag Rapid Test | Celltrion USA, Inc. | NPS | Nucleocapsid Protein, Spike RBD | 93.3% | 99% | 32 TCID50/mL | 15 min run | EUA | [104] |
| BinaxNOW ™ COVID-19 Ag Card2 Home Tool | Abbott Diagnostics Scarborough, Inc. | NS | Nucleocapsid Protein | 84.6% | 98.5% | 140.6 TCID50/mL | 15 min run | EUA | [105] |
| Status ™ COVID-19 Antigen Test | Princeton BioMeditech Corporation. | NPS | Nucleocapsid Protein | 93.9% | 100% | 2700 TCID50/mL | 15 min run | EUA | [106] |
| Serology Tests | ||||||||
|---|---|---|---|---|---|---|---|---|
| Name of Test | Developer | Nature of Specimen | Target Antibody | Sensitivity (PPA) | Specificity (NPA) | Time to Result | Status | References |
| Rapid Serology Test (RST) | ||||||||
| MidaSpot ™ COVID-19 Antibody Combo Detection Test | Nirmidas Biotech, Inc. | WB, EDTA P, LHP, S | IgM, IgG | IgM (100%); IgG (96.7%) | IgM (98.8%); IgG (97.5%) | ~22 min run | EUA; CE-IVD | [119] |
| Sienna ™-Clarity COVIBLOCK ™ COVID-19 IgG/IgM Rapid Test Cassette | Clarity Diagnostics, LLC. | WB, EDTA P, SCP, SHP, S | IgM, IgG | IgM (90%); IgG (93.3%) | IgM (100%); IgG (98.8%) | 10 min run | EUA; CE-IVD | [120] |
| Helagen ® COVID-19 IgG/IgM Rapid Test Cassette | Healgen Scientific, LLC. | WB, EDTA P LHP, CSP, S | IgM, IgG | IgM (87.9%); IgG (97.2%) | IgM & IgG (100%) | 10 min run | EUA | [121] |
| SGTI-flux © COVID-19 IgG Test | Sugentech, Inc. | WB, EDTA P, LHP, SCP, SHP, S | IgG | IgG (93.3%) | IgG (100%) | 10 min run | EUA; CE-IVD | [122] |
| ENZYME-LINKED IMMUNOSORBENT ASSAY (ELISA) | ||||||||
| COVID-19 Antibody Combo Detection Kit | Symbiotica, Inc. | DBS | IgG | IgG (100%) | IgG (98%) | N/A | EUA; CE-IVD | [123] |
| COVID-SeroKlir ®, Kantaro semi-quantitative SARS-CoV-2 IgG Antibody Kit | Kantaro Biosciences, LLC. | LHP, S | IgG | IgG (98.87%) | IgG (99.6%) | 30 min run | EUA | [124] |
| cPASS ™ SARS-CoV-2 Neutralization Antibody Detection Kit | GenScript USA, Inc. | EDTA P, S | IgG | IgG (100%) | IgG (100%) | 15 min run | EUA; CE-IVD | [125] |
| ZEUS ELISA ™ SARS-CoV-2 IgG Test System | ZEUS Scientific, Inc. | EDTA P, LHP, SCP, S | IgG | 93.3% | 100% | 30 min run | EUA | [126] |
| COVID-19 ELISA IgG Antibody Test | Mount Sinai Laboratories | EDTA P, S | IgG | 92.5% | 100% | N/A | EUA | [127] |
| CHEMOLUMINESCENT IMMUNOASSY (ChLIA) | ||||||||
| Dimension EXL SARS-CoV-2 IgG Test | Siemens Healthcare Diagnostics, Inc. | EDTA P, LHP, S | IgG | 92% | 99.9% | 25 min run | EUA | [128] |
| VITROS ® Immunodiagnostic Products Anti-SARS-CoV-2 Total Reagent Pack | Ortho-Clinical Diagnostics | EDTA P, S | IgM, IgG, IgA | 100% | 100% | 48 min run | EUA | [129] |
| Elecsys ® Anti-SARS-CoV-2 | Roche Molecular Systems | EDTA P, LHP, S | IgM, IgG | 88.1% | 99.81% | 18 min run | EUA | [114,130] |
| Access ™ SARS-CoV-2 IgM Test | Beckman Coutler | EDTA P, LHP, SCP, S | IgM | 95.3% | IgG 100% | 30 min run | EUA; CE-IVD | [131] |
| LIAISON ® SARS-CoV-2 S1/S2 IgG Test | DiaSorin, Inc. | EDTA P, LHP, S | IgG | 91.3% | 99.8% | 30 min run | EUA | [115,132] |
| BioCheck SARS-CoV-2 IgG Antibody Test Kit | BioCheck, Inc. | S | IgG | 100% | 100% | 30 min run | EUA | [133] |
| Vibrant COVID-19 Antibody Assay | Vibrant America Clinical Labs | DBS | IgM, IgG | 98.1% | 98.6% | Home collection: (45 min run in lab) | EUA: CE-IVD | [134] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gitman, M.R.; Shaban, M.V.; Paniz-Mondolfi, A.E.; Sordillo, E.M. Laboratory Diagnosis of SARS-CoV-2 Pneumonia. Diagnostics 2021, 11, 1270. https://doi.org/10.3390/diagnostics11071270
Gitman MR, Shaban MV, Paniz-Mondolfi AE, Sordillo EM. Laboratory Diagnosis of SARS-CoV-2 Pneumonia. Diagnostics. 2021; 11(7):1270. https://doi.org/10.3390/diagnostics11071270
Chicago/Turabian StyleGitman, Melissa R., Maryia V. Shaban, Alberto E. Paniz-Mondolfi, and Emilia M. Sordillo. 2021. "Laboratory Diagnosis of SARS-CoV-2 Pneumonia" Diagnostics 11, no. 7: 1270. https://doi.org/10.3390/diagnostics11071270
APA StyleGitman, M. R., Shaban, M. V., Paniz-Mondolfi, A. E., & Sordillo, E. M. (2021). Laboratory Diagnosis of SARS-CoV-2 Pneumonia. Diagnostics, 11(7), 1270. https://doi.org/10.3390/diagnostics11071270






