MRI of Finger Pulleys at 7T—Direct Characterization of Pulley Ruptures in an Ex Vivo Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of the Specimens
2.2. Magnetic Resonance Imaging
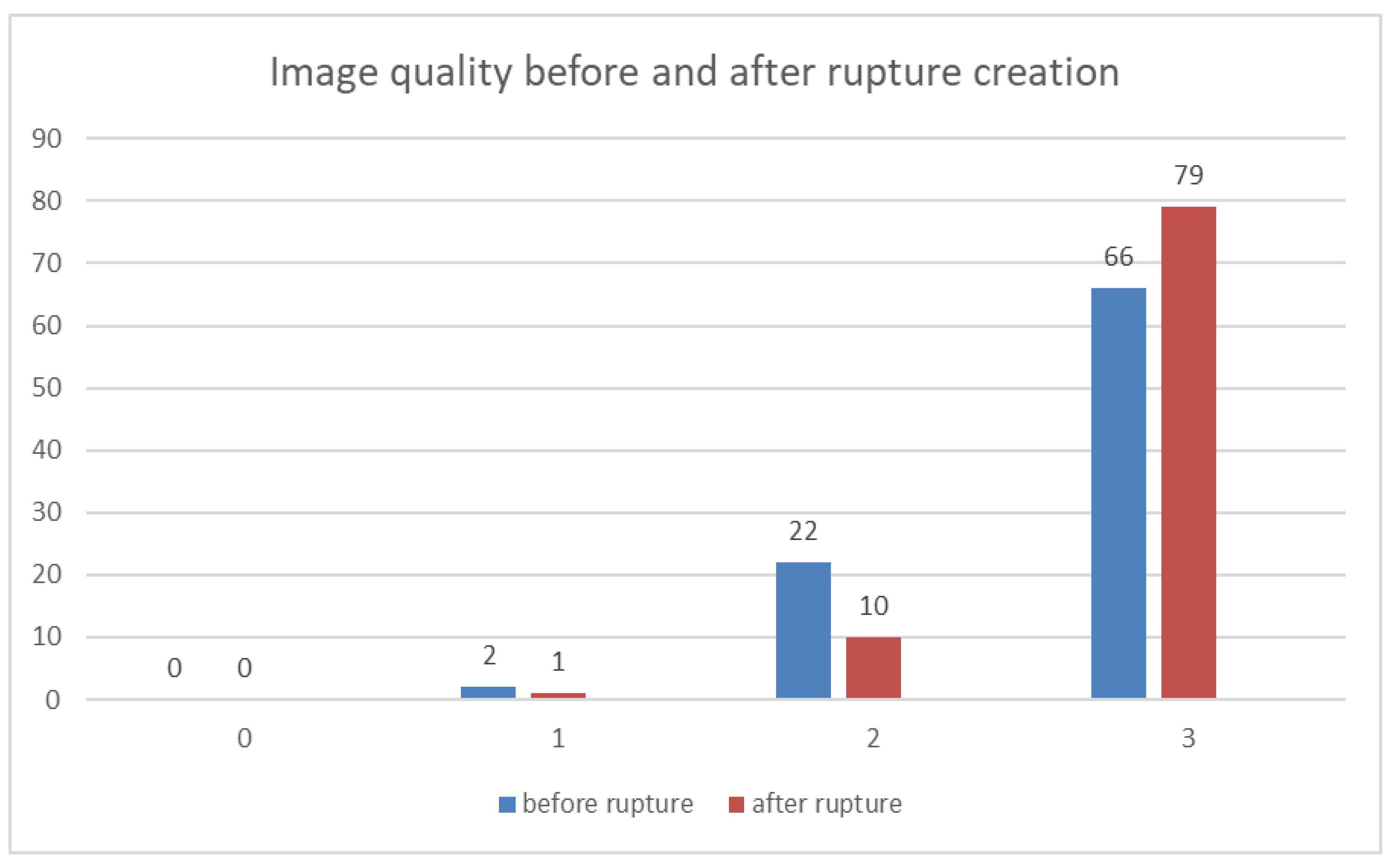
2.3. Image Analysis
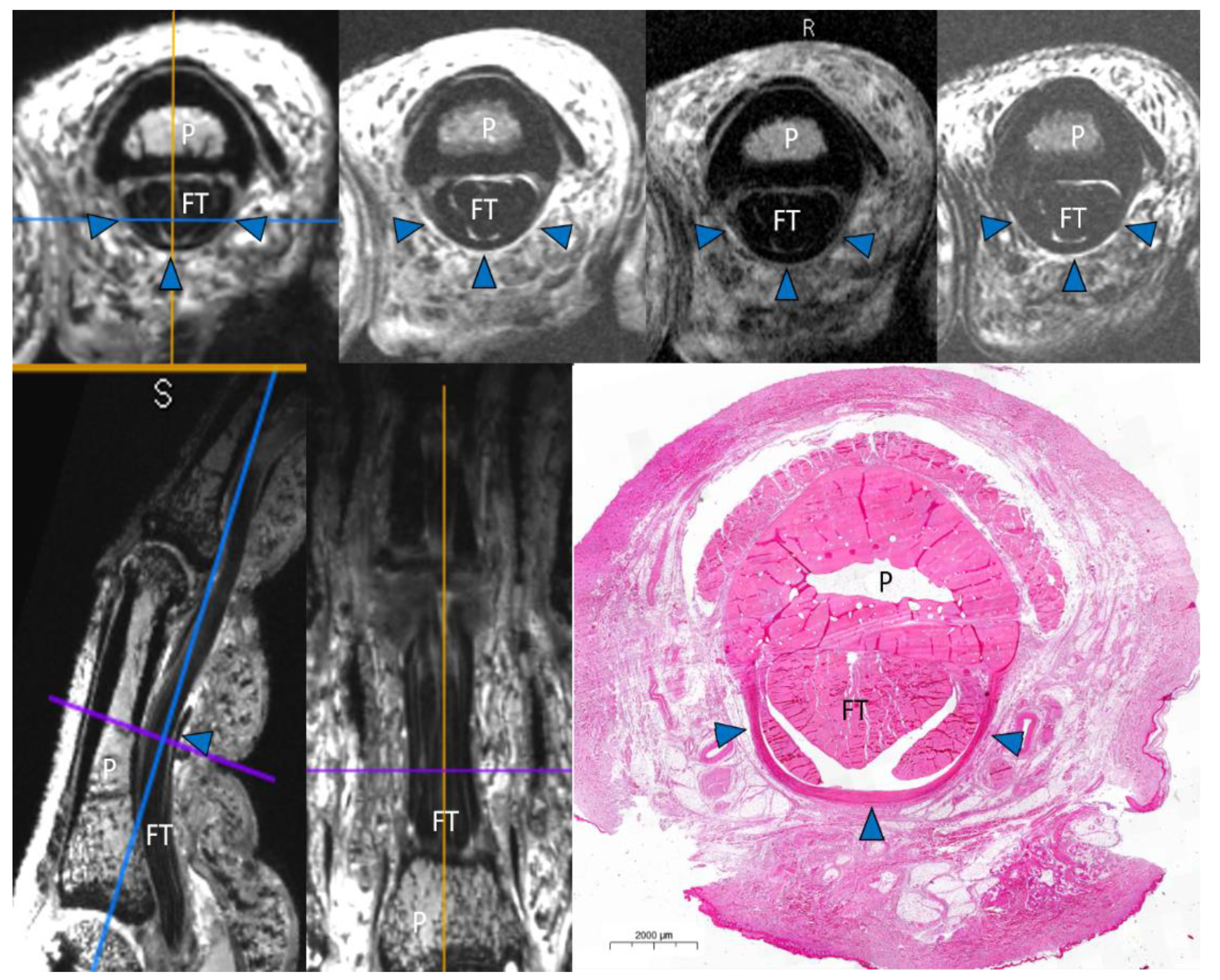
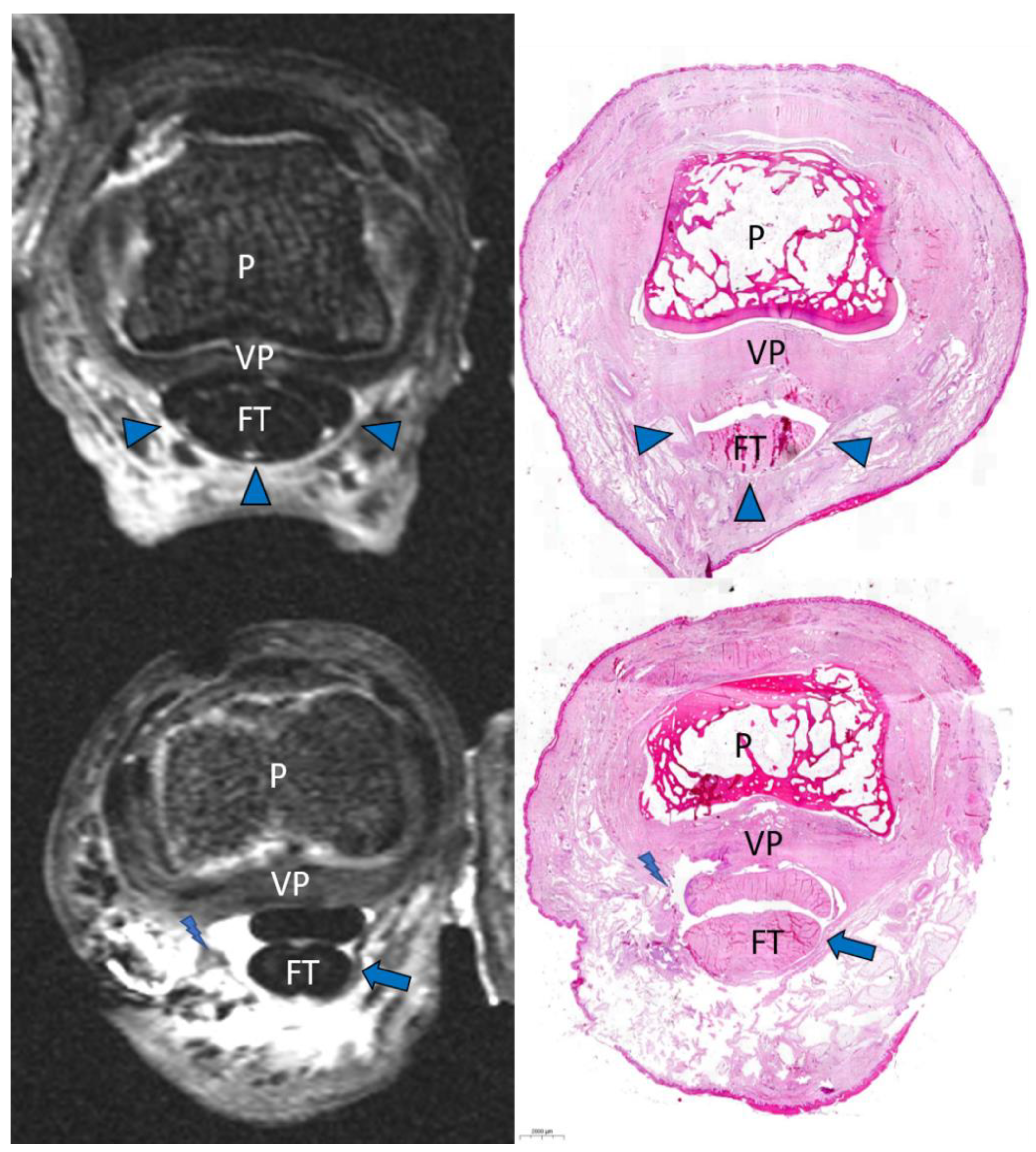
2.4. Macroscopic and Histopathologic Inspection
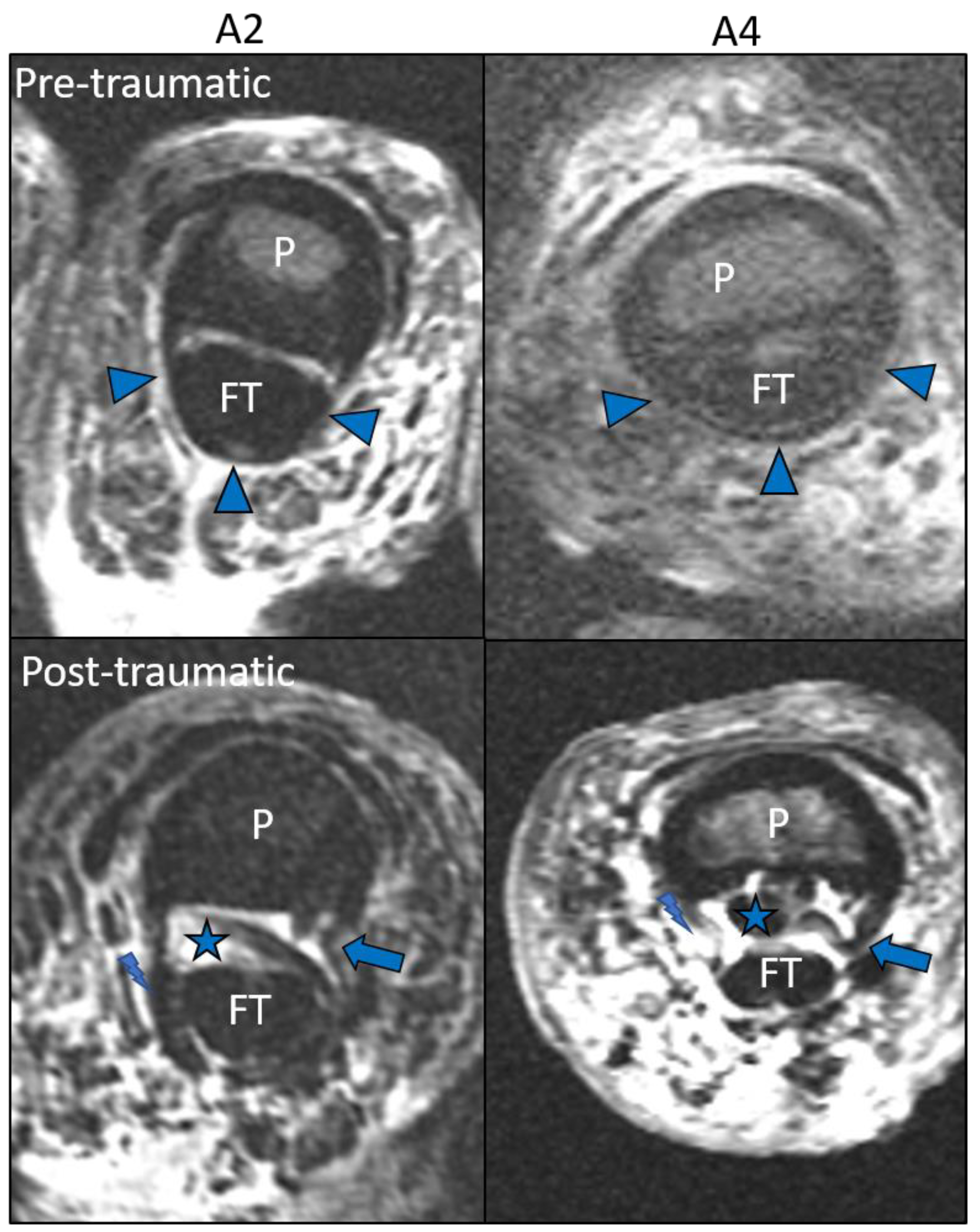
3. Results
Image Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Clavero, J.A.; Alomar, X.; Monill, J.M.; Esplugas, M.; Golano, P.; Mendoza, M.; Salvador, A. MR imaging of ligament and tendon injuries of the fingers. Radiographics 2002, 22, 237–256. [Google Scholar] [CrossRef]
- Lutter, C.; El-Sheikh, Y.; Schoffl, I.; Schoffl, V. Sport climbing: Medical considerations for this new Olympic discipline. Br. J. Sports Med. 2017, 51, 2–3. [Google Scholar] [CrossRef] [PubMed]
- Martinoli, C.; Bianchi, S.; Cotten, A. Imaging of rock climbing injuries. Semin. Musculoskelet. Radiol. 2005, 9, 334–345. [Google Scholar] [CrossRef]
- King, E.A.; Lien, J.R. Flexor Tendon Pulley Injuries in Rock Climbers. Hand Clin. 2017, 33, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Lutter, C.; Tischer, T.; Schoffl, V.R. Olympic competition climbing: The beginning of a new era—A narrative review. Br. J. Sports Med. 2020. [Google Scholar] [CrossRef] [PubMed]
- Goncalves-Matoso, V.; Guntern, D.; Gray, A.; Schnyder, P.; Picht, C.; Theumann, N. Optimal 3-T MRI for depiction of the finger A2 pulley: Comparison between T1-weighted, fat-saturated T2-weighted and gadolinium-enhanced fat-saturated T1-weighted sequences. Skelet. Radiol. 2008, 37, 307–312. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Schoffl, V.; Hochholzer, T.; Winkelmann, H.P.; Strecker, W. Pulley injuries in rock climbers. Wilderness Environ. Med. 2003, 14, 94–100. [Google Scholar] [CrossRef]
- Hauger, O.; Chung, C.B.; Lektrakul, N.; Botte, M.J.; Trudell, D.; Boutin, R.D.; Resnick, D. Pulley system in the fingers: Normal anatomy and simulated lesions in cadavers at MR imaging, CT, and US with and without contrast material distention of the tendon sheath. Radiology 2000, 217, 201–212. [Google Scholar] [CrossRef]
- Bayer, T.; Fries, S.; Schweizer, A.; Schoffl, I.; Janka, R.; Bongartz, G. Stress examination of flexor tendon pulley rupture in the crimp grip position: A 1.5-Tesla MRI cadaver study. Skelet. Radiol. 2015, 44, 77–84. [Google Scholar] [CrossRef]
- Bayer, T.; Adler, W.; Schweizer, A.; Schoffl, I.; Uder, M.; Janka, R. Evaluation of finger A3 pulley rupture in the crimp grip position—A magnetic resonance imaging cadaver study. Skelet. Radiol. 2015, 44, 1279–1285. [Google Scholar] [CrossRef] [PubMed]
- Schoffl, I.; Baier, T.; Schoffl, V. Flap irritation phenomenon (FLIP): Etiology of chronic tenosynovitis after finger pulley rupture. J. Appl. Biomech. 2011, 27, 291–296. [Google Scholar] [CrossRef]
- Ladd, M.E.; Bachert, P.; Meyerspeer, M.; Moser, E.; Nagel, A.M.; Norris, D.G.; Schmitter, S.; Speck, O.; Straub, S.; Zaiss, M. Pros and cons of ultra-high-field MRI/MRS for human application. Prog. Nucl. Magn. Reson. Spectrosc. 2018, 109, 1–50. [Google Scholar] [CrossRef]
- Schoffl, I.; Oppelt, K.; Jungert, J.; Schweizer, A.; Bayer, T.; Neuhuber, W.; Schoffl, V. The influence of concentric and eccentric loading on the finger pulley system. J. Biomech. 2009, 42, 2124–2128. [Google Scholar] [CrossRef] [PubMed]
- Schweizer, A.; Hudek, R. Kinetics of Crimp and Slope Grip in Rock Climbing. J. Appl. Biomech. 2011, 27, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Amca, A.M.; Vigouroux, L.; Aritan, S.; Berton, E. Effect of hold depth and grip technique on maximal finger forces in rock climbing. J. Sport Sci. 2012, 30, 669–677. [Google Scholar] [CrossRef]
- Martin, J.M.; Del Campo, V.L.; Roman, M.L.; Horrillo, J.M.G.V.; Navarrete, J.S.G. Description of the finger mechanical load of climbers of different levels during different hand grips in sport climbing. J. Sport Sci. 2013, 31, 1713–1721. [Google Scholar] [CrossRef]
- Schweizer, A.; Frank, O.; Ochsner, P.E.; Jacob, H.A. Friction between human finger flexor tendons and pulleys at high loads. J. Biomech. 2003, 36, 63–71. [Google Scholar] [CrossRef]
- Klauser, A.; Frauscher, F.; Bodner, G.; Halpern, E.J.; Schocke, M.F.; Springer, P.; Gabl, M.; Judmaier, W.; zur Nedden, D. Finger pulley injuries in extreme rock climbers: Depiction with dynamic US. Radiology 2002, 222, 755–761. [Google Scholar] [CrossRef]
- Leeflang, S.; Coert, J.H. The role of proximal pulleys in preventing tendon bowstringing: Pulley rupture and tendon bowstringing. J. Plast. Reconstr. Aesthetic Surg. JPRAS 2014, 67, 822–827. [Google Scholar] [CrossRef] [PubMed]
- Laistler, E.; Dymerska, B.; Sieg, J.; Goluch, S.; Frass-Kriegl, R.; Kuehne, A.; Moser, E. In vivo MRI of the human finger at 7 T. Magn. Reson. Med. Magn. 2018, 79, 588–592. [Google Scholar] [CrossRef]
- Krug, R.; Stehling, C.; Kelley, D.A.; Majumdar, S.; Link, T.M. Imaging of the musculoskeletal system in vivo using ultra-high field magnetic resonance at 7 T. Investig. Radiol. 2009, 44, 613–618. [Google Scholar] [CrossRef] [PubMed]
- Schoffl, I.; Deeg, J.; Lutter, C.; Bayer, T.; Schoffl, V. Diagnosis of A3 Pulley Injuries Using Ultrasound. Sportverletz Sportschaden 2018, 32, 251–259. [Google Scholar] [CrossRef]
- Bencardino, J.T. MR imaging of tendon lesions of the hand and wrist. Magn. Reson. Imaging Clin. N. Am. 2004, 12, 333–347. [Google Scholar] [CrossRef]
- Zhao, C.F.; Amadio, P.C.; Berglund, L.; An, K.N. The A3 pulley. J. Hand Surg. 2000, 25, 270–276. [Google Scholar] [CrossRef]
- Tang, J.B.; Xie, R.G. Effect of A3 pulley and adjacent sheath integrity on tendon excursion and bowstringing. J. Hand Surg. 2001, 26, 855–861. [Google Scholar] [CrossRef] [PubMed]
- Gabl, M.; Rangger, C.; Lutz, M.; Fink, C.; Rudisch, A.; Pechlaner, S. Disruption of the finger flexor pulley system in elite rock climbers. Am. J. Sports Med. 1998, 26, 651–655. [Google Scholar] [CrossRef] [PubMed]
- Schoffl, V.; Kupper, T.; Hartmann, J.; Schoffl, I. Surgical repair of multiple pulley injuries--evaluation of a new combined pulley repair. J. Hand Surg. Am. 2012, 37, 224–230. [Google Scholar] [CrossRef]
- Schoffl, I.; Meisel, J.; Lutter, C.; Schoffl, V. Feasibility of a New Pulley Repair: A Cadaver Study. J. Hand Surg. Am. 2018, 43, 380 e381–380 e387. [Google Scholar] [CrossRef]
- Voulliaume, D.; Forli, A.; Parzy, O.; Moutet, F. Surgical repair of flexor tendon pulley rupture in high level rock climbing. Chir. Main 2004, 23, 243–248. [Google Scholar] [CrossRef]
- Dy, C.J.; Daluiski, A. Flexor pulley reconstruction. Hand Clin. 2013, 29, 235–242. [Google Scholar] [CrossRef]
| Characterization of Pulley Rupture | ||||
|---|---|---|---|---|
| Specimen | Finger | A2 | A3 | A4 |
| 1 | L2 | 1(SMOOTH.RUPT)r | 0(NO.RUPT) | 0(NO.RUPT) |
| 1 | L3 | 2(DISLOC.RUPT)m | 3(PULLEY.LOST) | 2(DISLOC.RUPT)u |
| 1 | L4 | 2(DISLOC.RUPT)u | 2(DISLOC.RUPT)u | 0(NO.RUPT) |
| 2 | L2 | 2(DISLOC.RUPT)m | 2(DISLOC.RUPT)m | 2(DISLOC.RUPT)u |
| 2 | L3 | 0(NO.RUPT) | 0(NO.RUPT) | 2(DISLOC.RUPT)u |
| 2 | L4 | 0(NO.RUPT) | 0(NO.RUPT) | 2(DISLOC.RUPT)u |
| 2 | R2 | 2(DISLOC.RUPT)r | 2(DISLOC.RUPT)m | 2(DISLOC.RUPT)u |
| 3 | R2 | 2(DISLOC.RUPT)u | 1(SMOOTH.RUPT)m | 2(DISLOC.RUPT)r |
| 3 | R3 | 1(SMOOTH.RUPT)r | 0(NO.RUPT) | 0(NO.RUPT) |
| 4 | R2 | 2(DISLOC.RUPT)r | 2(DISLOC.RUPT)r | 2(DISLOC.RUPT)r |
| 5 | R4 | 2(DISLOC.RUPT)r | 0(NO.RUPT) | 0(NO.RUPT) |
| Total | 9 | 6 | 7 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heiss, R.; Librimir, A.; Lutter, C.; Janka, R.; Kuerten, S.; Roemer, F.W.; Nagel, A.M.; Uder, M.; Bayer, T. MRI of Finger Pulleys at 7T—Direct Characterization of Pulley Ruptures in an Ex Vivo Model. Diagnostics 2021, 11, 1206. https://doi.org/10.3390/diagnostics11071206
Heiss R, Librimir A, Lutter C, Janka R, Kuerten S, Roemer FW, Nagel AM, Uder M, Bayer T. MRI of Finger Pulleys at 7T—Direct Characterization of Pulley Ruptures in an Ex Vivo Model. Diagnostics. 2021; 11(7):1206. https://doi.org/10.3390/diagnostics11071206
Chicago/Turabian StyleHeiss, Rafael, Alexander Librimir, Christoph Lutter, Rolf Janka, Stefanie Kuerten, Frank W. Roemer, Armin M. Nagel, Michael Uder, and Thomas Bayer. 2021. "MRI of Finger Pulleys at 7T—Direct Characterization of Pulley Ruptures in an Ex Vivo Model" Diagnostics 11, no. 7: 1206. https://doi.org/10.3390/diagnostics11071206
APA StyleHeiss, R., Librimir, A., Lutter, C., Janka, R., Kuerten, S., Roemer, F. W., Nagel, A. M., Uder, M., & Bayer, T. (2021). MRI of Finger Pulleys at 7T—Direct Characterization of Pulley Ruptures in an Ex Vivo Model. Diagnostics, 11(7), 1206. https://doi.org/10.3390/diagnostics11071206








