Imaging and Biochemical Markers for Osteoarthritis
- Susceptibility/risk biomarker;
- Diagnostic biomarker;
- Monitoring biomarker;
- Prognostic biomarker;
- Predictive biomarker;
- Pharmacodynamic/response biomarker;
- Safety biomarker.
1. Advancement in OA Biomarker Research
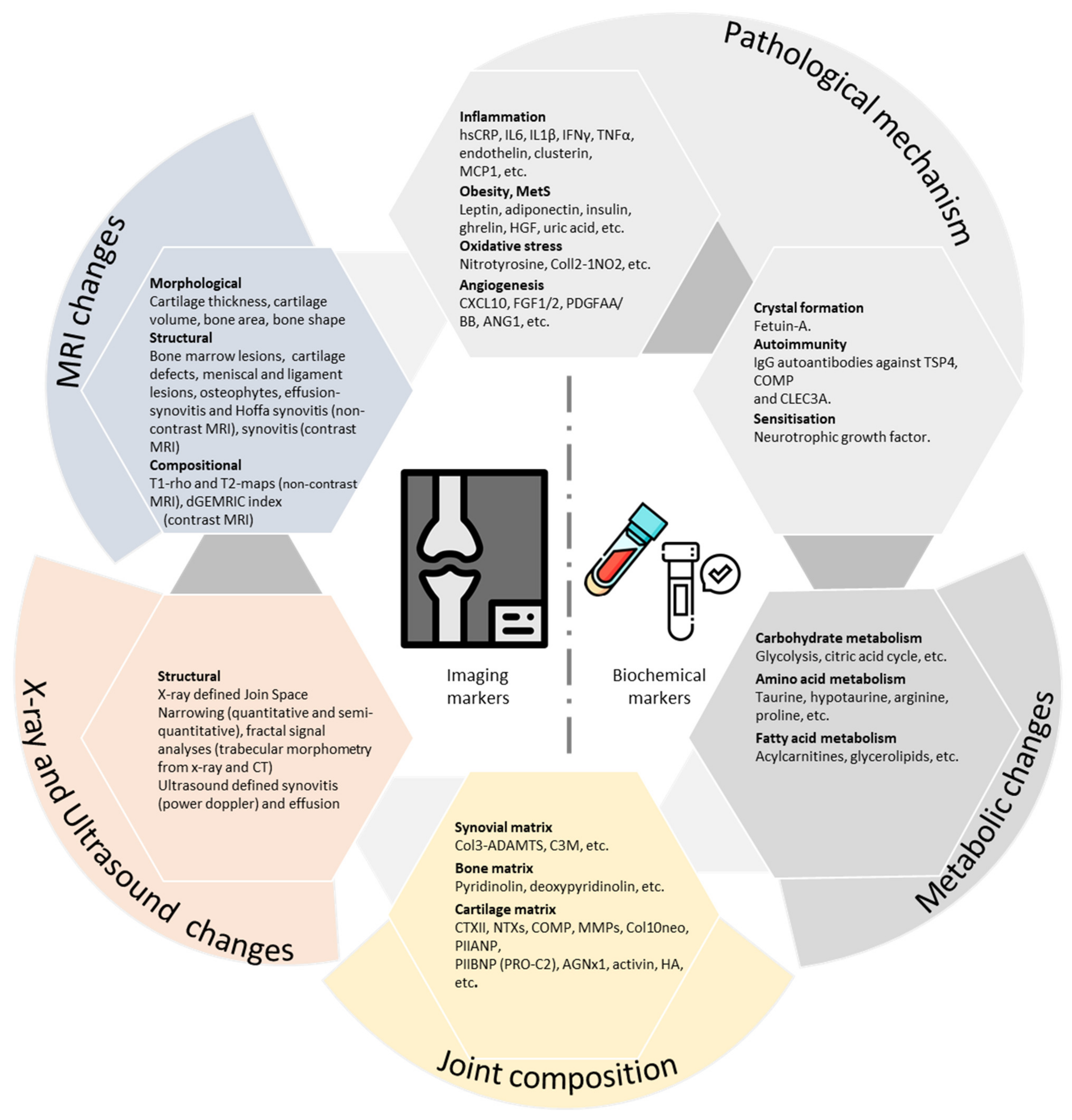
2. Imaging Biomarkers
3. Biochemical Markers
4. OA Biomarkers and Personalised Medicine
5. Conclusions
Funding
Conflicts of Interest
References
- Global Burden of Disease 2019 Disease i, and Impairment Summaries; GBD 2019 Cause and Risk Summaries: Osteoarthritis: Institute for Health Metrics and Evaluation. 2021. Available online: http://www.healthdata.org/results/gbd_summaries/2019/osteoarthritis-level-3-cause (accessed on 24 June 2021).
- Hunter, D.J.; March, L.; Chew, M. Osteoarthritis in 2020 and beyond: A Lancet Commission. Lancet 2020, 396, 1711–1712. [Google Scholar] [CrossRef]
- Antony, B.; Jones, G.; Jin, X.; Ding, C. Do early life factors affect the development of knee osteoarthritis in later life: A narrative review. Arthritis Res. Therapy 2016, 18, 202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- WHO. Decade of Healthy Ageing: Baseline Report; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Singh, A.; Campbell, J.A.; Venn, A.; Jones, G.; Blizzard, L.; Palmer, A.J.; Dwyer, T.; Cicuttini, F.; Ding, C.; Antony, B. Association between knee symptoms, change in knee symptoms over 6–9 years, and SF-6D health state utility among middle-aged Australians. Qual. Life Res. 2021, 1–13. [Google Scholar] [CrossRef]
- Katz, J.N.; Neogi, T.; Callahan, L.F.; Block, J.A.; Conaghan, P.G.; Simon, L.S.; Kraus, V.B.; Hochberg, M.C. Disease modification in osteoarthritis; pathways to drug approval. Osteoarthr. Cartil. Open. 2020, 2, 100059. [Google Scholar] [CrossRef]
- Matthews, G.L.; Hunter, D.J. Emerging drugs for osteoarthritis. Expert Opin. Emerg. Drugs 2011, 16, 479–491. [Google Scholar] [CrossRef] [PubMed]
- Alexandersen, P.; Karsdal, M.A.; Byrjalsen, I.; Christiansen, C. Strontium ranelate effect in postmenopausal women with different clinical levels of osteoarthritis. Climacteric 2011, 14, 236–243. [Google Scholar] [CrossRef]
- Karsdal, M.; Byrjalsen, I.; Henriksen, K.; Riis, B.; Lau, E.; Arnold, M.; Christiansen, C. The effect of oral salmon calcitonin delivered with 5-CNAC on bone and cartilage degradation in osteoarthritic patients: A 14-day randomized study. Osteoarthr. Cartil. 2010, 18, 150–159. [Google Scholar] [CrossRef] [Green Version]
- Reginster, J.Y.; Beaudart, C.; Neuprez, A.; Bruyère, O. Strontium ranelate in the treatment of knee osteoarthritis: New insights and emerging clinical evidence. Adv. Musculoskelet. Dis. 2013, 5, 268–276. [Google Scholar] [CrossRef] [Green Version]
- Reginster, J.Y. Efficacy and safety of strontium ranelate in the treatment of knee osteoarthritis: Results of a double-blind randomised, placebo-controlled trial. Ann. Rheum. Dis. 2014, 73, e8. [Google Scholar] [CrossRef] [Green Version]
- Spector, T.D.; Conaghan, P.G.; Buckland-Wright, J.C.; Garnero, P.; A Cline, G.; Beary, J.F.; Valent, D.J.; Meyer, J.M. Effect of risedronate on joint structure and symptoms of knee osteoarthritis: Results of the BRISK randomized, controlled trial [ISRCTN01928173]. Arthritis Res. Ther. 2005, 7, R625–R633. [Google Scholar] [CrossRef] [Green Version]
- FDA-NIH Biomarker Working Group. BEST (Biomarkers, Endpoints, and Other Tools) Resource [Internet]; National Institutes of Health (US): Bethesda, MD, USA, 2016.
- Bauer, D.; Hunter, D.; Abramson, S.; Attur, M.; Corr, M.; Felson, D.; Heinegård, D.; Jordan, J.; Kepler, T.; Lane, N.; et al. Classification of osteoarthritis biomarkers: A proposed approach. Osteoarthr. Cartil. 2006, 14, 723–727. [Google Scholar] [CrossRef] [Green Version]
- Kraus, V.; Burnett, B.; Coindreau, J.; Cottrell, S.; Eyre, D.; Gendreau, M.; Gardiner, J.; Garnero, P.; Hardin, J.; Henrotin, Y.; et al. Application of biomarkers in the development of drugs intended for the treatment of osteoarthritis. Osteoarthr. Cartil. 2011, 19, 515–542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kraus, V.B.; Nevitt, M.; Sandell, L.J. Summary of the OA biomarkers workshop 2009—Biochemical biomarkers: Biology, validation, and clinical studies. Osteoarthr. Cartil. 2010, 18, 742–745. [Google Scholar] [CrossRef] [Green Version]
- Menetski, J.P.; Hoffmann, S.C.; Cush, S.S.; Kamphaus, T.N.; Austin, C.P.; Herrling, P.L.; Wagner, J.A. The Foundation for the National Institutes of Health Biomarkers Consortium: Past accomplishments and new strategic direction. Clin. Pharmacol. Therapeutics. 2019, 105, 829–843. [Google Scholar] [CrossRef] [PubMed]
- FNIH. Biomarkers Consortium—Osteoarthritis Biomarkers Project: National Institutes of Health. 2021. Available online: https://fnih.org/our-programs/biomarkers-consortium/osteoarthritis-project (accessed on 24 June 2021).
- Hunter, D.J.; Nevitt, M.; Losina, E.; Kraus, V. Biomarkers for osteoarthritis: Current position and steps towards further validation. Best Pract. Res. Clin. Rheumatol. 2014, 28, 61–71. [Google Scholar] [CrossRef] [Green Version]
- US Food and Drug Administration. Guidance for Industry: Expedited Programs for Serious Conditions—Drugs and Biologics; US Food and Drug Administration: Silver Spring, MD, USA, 2014.
- Hochberg, M.C.; Guermazi, A.; Guehring, H.; Aydemir, A.; Wax, S.; Fleuranceau-Morel, P. Effect of intra-articular sprifermin vs placebo on femorotibial joint cartilage thickness in patients with osteoarthritis: The FORWARD randomized clinical trial. JAMA 2019, 322, 1360–1370. [Google Scholar] [CrossRef] [PubMed]
- Eckstein, F.; Hochberg, M.C.; Guehring, H.; Moreau, F.; Ona, V.; Bihlet, A.R.; Byrjalsen, I.; Andersen, J.R.; Daelken, B.; Guenther, O.; et al. Long-term structural and symptomatic effects of intra-articular sprifermin in patients with knee osteoarthritis: 5-year results from the FORWARD study. Ann. Rheum. Dis. 2021, 21, 9181. [Google Scholar]
- Lotz, M.; Martel-Pelletier, J.; Christiansen, C.; Brandi, M.-L.; Bruyere, O.; Chapurlat, R.; Collette, J.; Cooper, C.; Giacovelli, G.; Kanis, J.A.; et al. Value of biomarkers in osteoarthritis: Current status and perspectives. Ann. Rheum. Dis. 2013, 72, 1756–1763. [Google Scholar] [CrossRef]
- Sand, J.M.B.; Knox, A.J.; Lange, P.; Sun, S.; Kristensen, J.H.; Leeming, D.J.; Karsdal, M.A.; Bolton, C.E.; Johnson, S.R. Accelerated extracellular matrix turnover during exacerbations of COPD. Respir. Res. 2015, 16, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kapoor, M.; Mahomed, N.N. Osteoarthritis: Pathogenesis, Diagnosis, Available Treatments, Drug Safety, Regenerative and Precision Medicine; Springer: Cham, Switzerland, 2015. [Google Scholar]
- Kraus, V.B.; Karsdal, M.A. Osteoarthritis: Current molecular biomarkers and the way forward. Calcif. Tissue Int. 2020, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Veillette, C.J.H.; Jurisica, I. Precision Medicine for Osteoarthritis. In Osteoarthritis: Pathogenesis, Diagnosis, Available Treatments, Drug Safety, Regenerative and Precision Medicine; Kapoor, M., Mahomed, N.N., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 257–270. [Google Scholar]
- Karsdal, M.A.; Christiansen, C.; Ladel, C.; Henriksen, K.; Kraus, V.B.; Bay-Jensen, A.C. Osteoarthritis—A case for personalized health care? Osteoarthr. Cartil. 2014, 22, 7–16. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Jones, G.; Winzenberg, T.; Cai, G.; Laslett, L.L.; Aitken, D. Effectiveness of Curcuma longa Extract for the Treatment of Symptoms and Effusion–Synovitis of Knee Osteoarthritis: A Randomized Trial. Ann. Intern. Med. 2020, 173, 861–869. [Google Scholar] [CrossRef]
- Cai, G.; Aitken, D.; Laslett, L.L.; Pelletier, J.-P.; Martel-Pelletier, J.; Hill, C. Effect of Intravenous Zoledronic Acid on Tibiofemoral Cartilage Volume Among Patients With Knee Osteoarthritis With Bone Marrow Lesions: A Randomized Clinical Trial. JAMA 2020, 323, 1456–1466. [Google Scholar] [CrossRef] [PubMed]
- Schieker, M.; Conaghan, P.G.; Mindeholm, L.; Praestgaard, J.; Solomon, D.H.; Scotti, C. Effects of Interleukin-1β Inhibition on Incident Hip and Knee Replacement: Exploratory Analyses From a Randomized, Double-Blind, Placebo-Controlled Trial. Ann. Intern. Med. 2020, 173, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Sawitzke, A.D. Personalized medicine for osteoarthritis: Where are we now? Ther. Adv. Musculoskelet. Dis. 2013, 5, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Antony, B.; Singh, A.; Blizzard, L.; Venn, A.; Jones, G.; Burgess, J.; Parameswaran, V.; Cicuttini, F.; March, L.; Ding, C. Association between osteoarthritis-related serum biochemical markers over 11 years and knee MRI-based imaging biomarkers in middle-aged adults. Ann. Rheum. Dis. 2021, 80 (Suppl S1), 276. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antony, B.; Singh, A. Imaging and Biochemical Markers for Osteoarthritis. Diagnostics 2021, 11, 1205. https://doi.org/10.3390/diagnostics11071205
Antony B, Singh A. Imaging and Biochemical Markers for Osteoarthritis. Diagnostics. 2021; 11(7):1205. https://doi.org/10.3390/diagnostics11071205
Chicago/Turabian StyleAntony, Benny, and Ambrish Singh. 2021. "Imaging and Biochemical Markers for Osteoarthritis" Diagnostics 11, no. 7: 1205. https://doi.org/10.3390/diagnostics11071205
APA StyleAntony, B., & Singh, A. (2021). Imaging and Biochemical Markers for Osteoarthritis. Diagnostics, 11(7), 1205. https://doi.org/10.3390/diagnostics11071205





