18F-FDG PET/CT versus Diagnostic Contrast-Enhanced CT for Follow-Up of Stage IV Melanoma Patients Treated by Immune Checkpoint Inhibitors: Frequency and Management of Discordances over a 3-Year Period in a University Hospital
Abstract
:1. Introduction
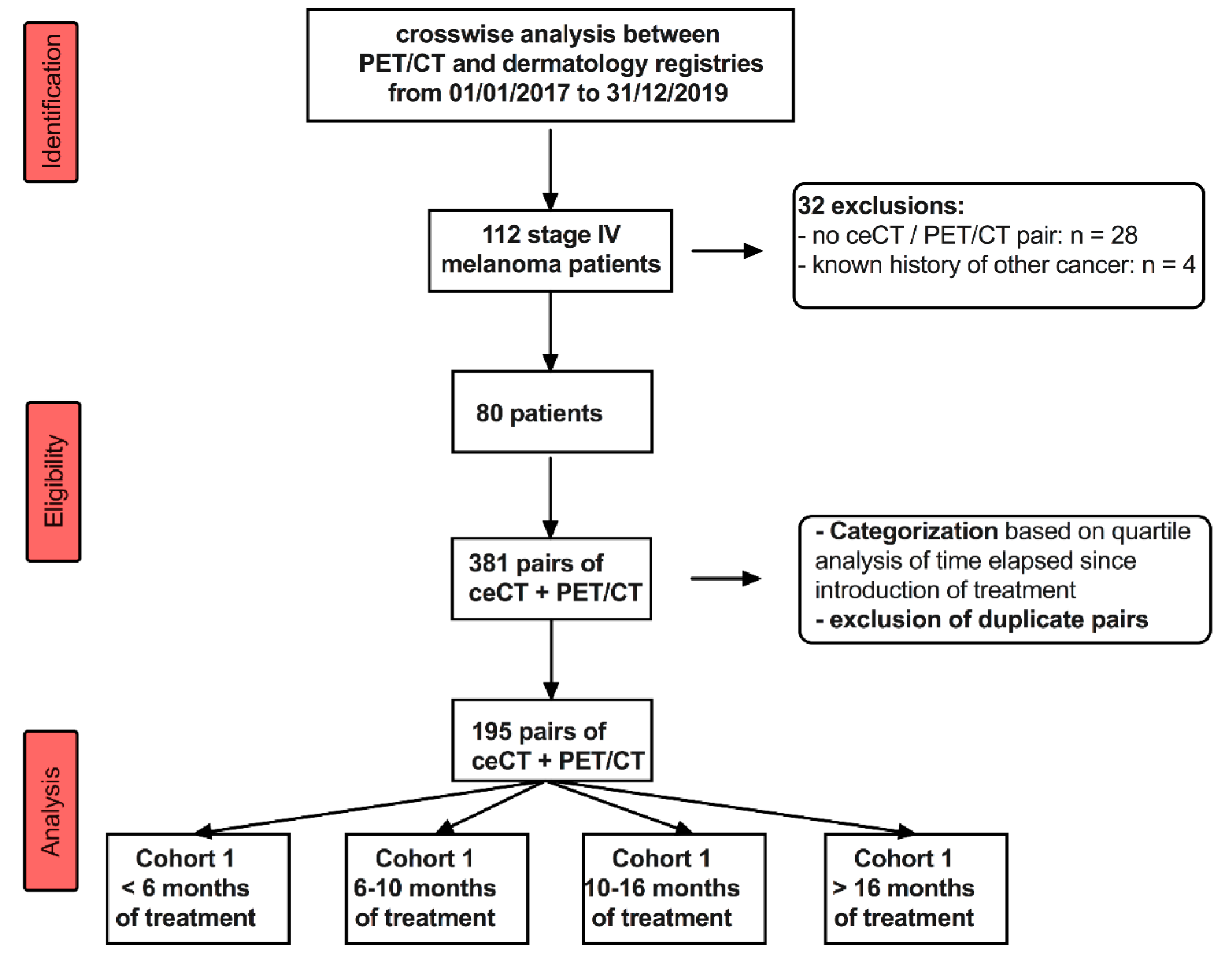
2. Materials and Methods
2.1. Study Design
2.2. 18F-FDG PET/CT Protocol
2.3. Diagnostic CT Scan
2.4. Extraction and Quotation of 18F-FDG PET/CT and ceCT Reports
- with a clinical benefit: complete response, partial response, stable disease.
- with no clinical benefit: progressive disease.
- inconclusive
2.5. Analysis of Discordant Findings between 18F-FDG PET/CT and ceCT Scans
- Biopsy of one of the anatomical sites/surgery;
- Complementary radiological examination (such as MRI or echography);
- No change, follow-up;
- Switch from one line of treatment to another;
- Radiotherapy.
2.6. Statistical Analysis
- (i).
- early assessment: <6 months,
- (ii).
- interim assessments: 6–10 months and 10–16 months, and
- (iii).
- late assessments, >16 months.
- 0.0–0.20: poor agreement;
- 0.21–0.40: fair agreement;
- 0.41–0.60: moderate agreement;
- 0.61–0.80: good agreement;
- 0.81–1.00: very good agreement.
3. Results
3.1. Patients’ Demographics
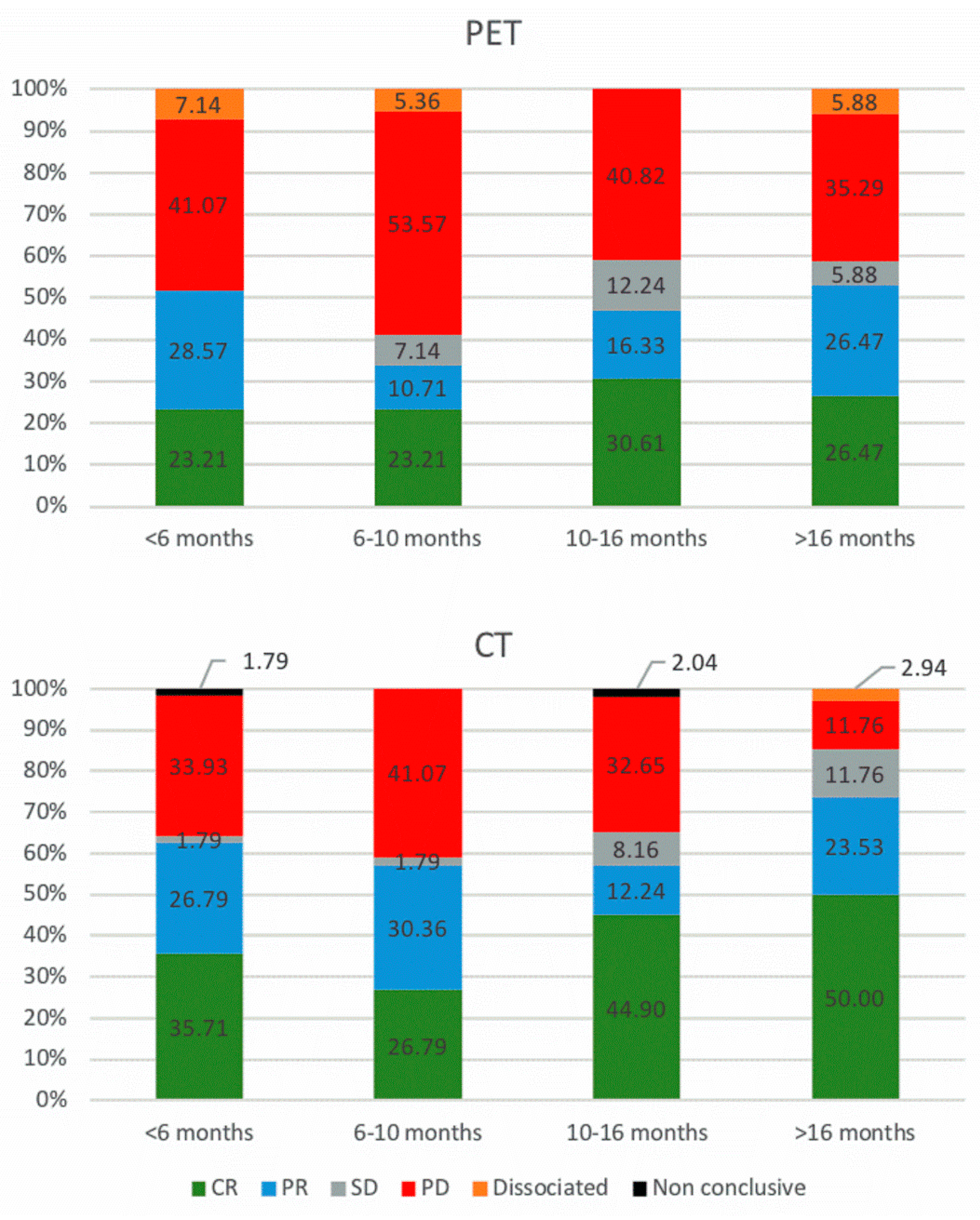
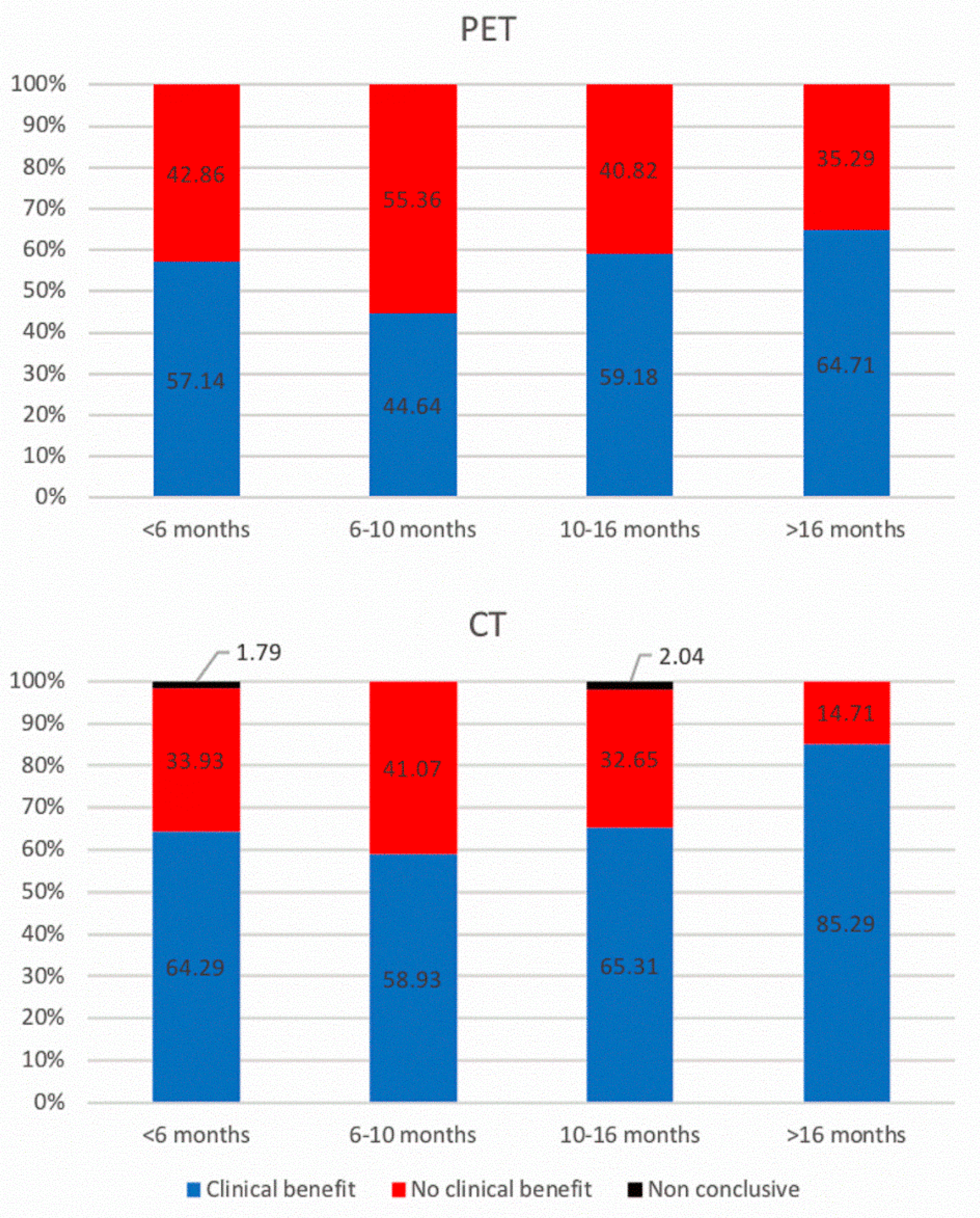
3.2. 18F-FDG PET/CT and ceCT Scans
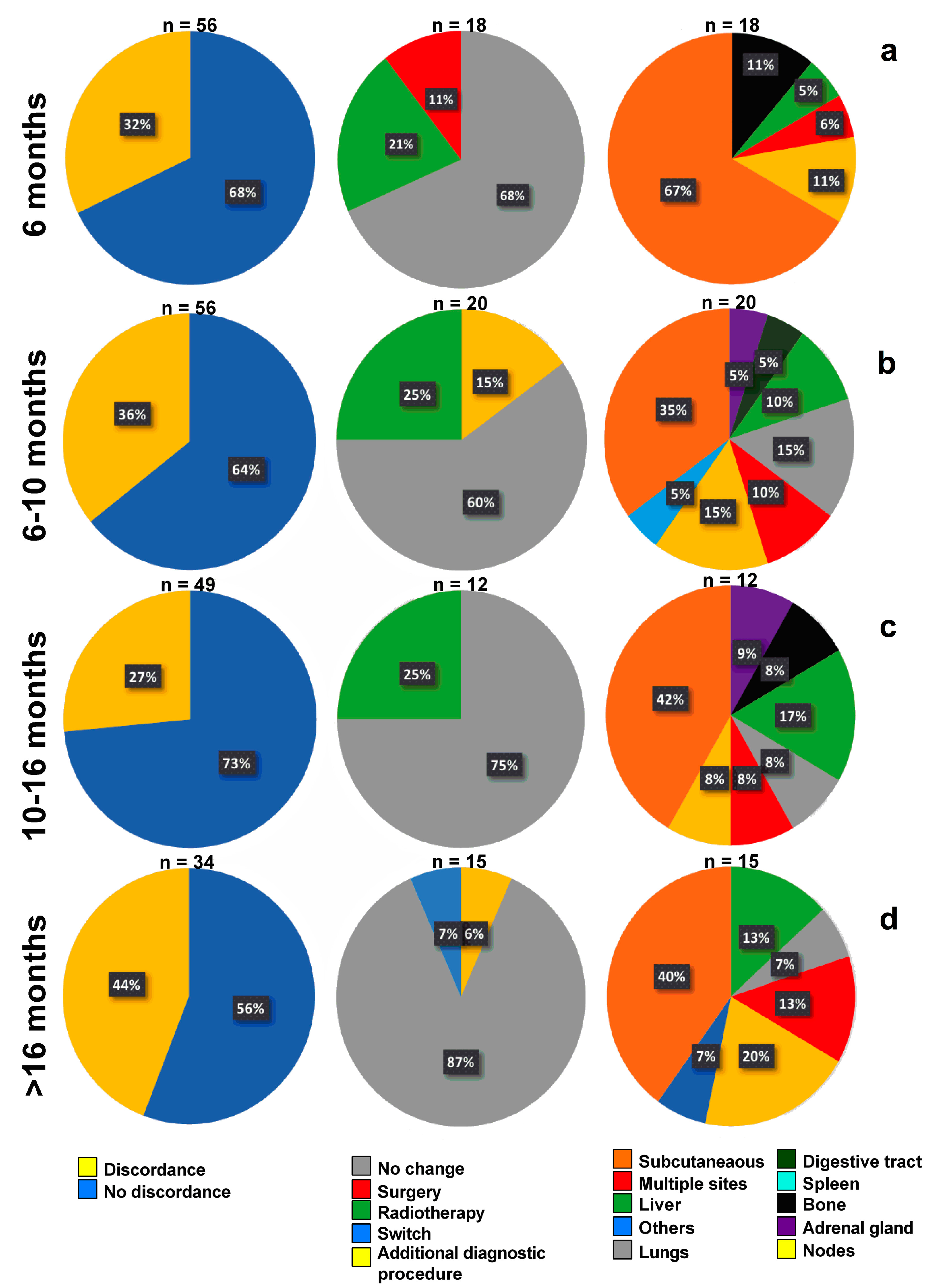
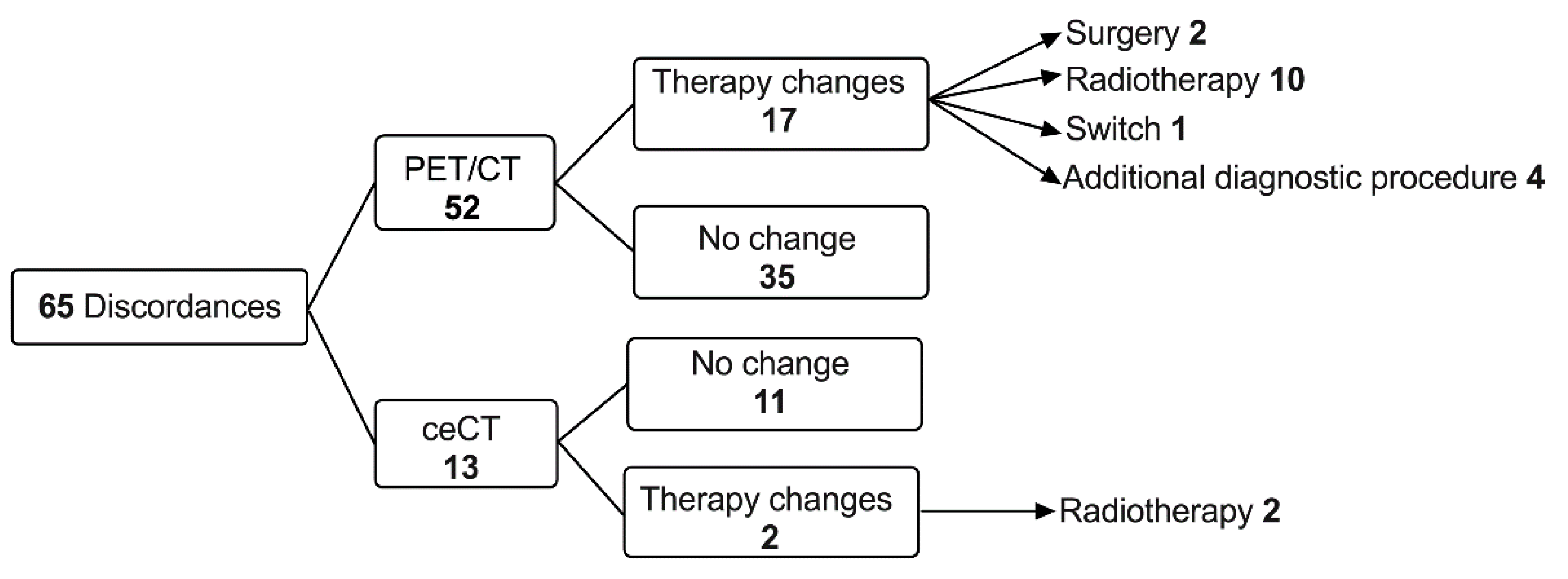
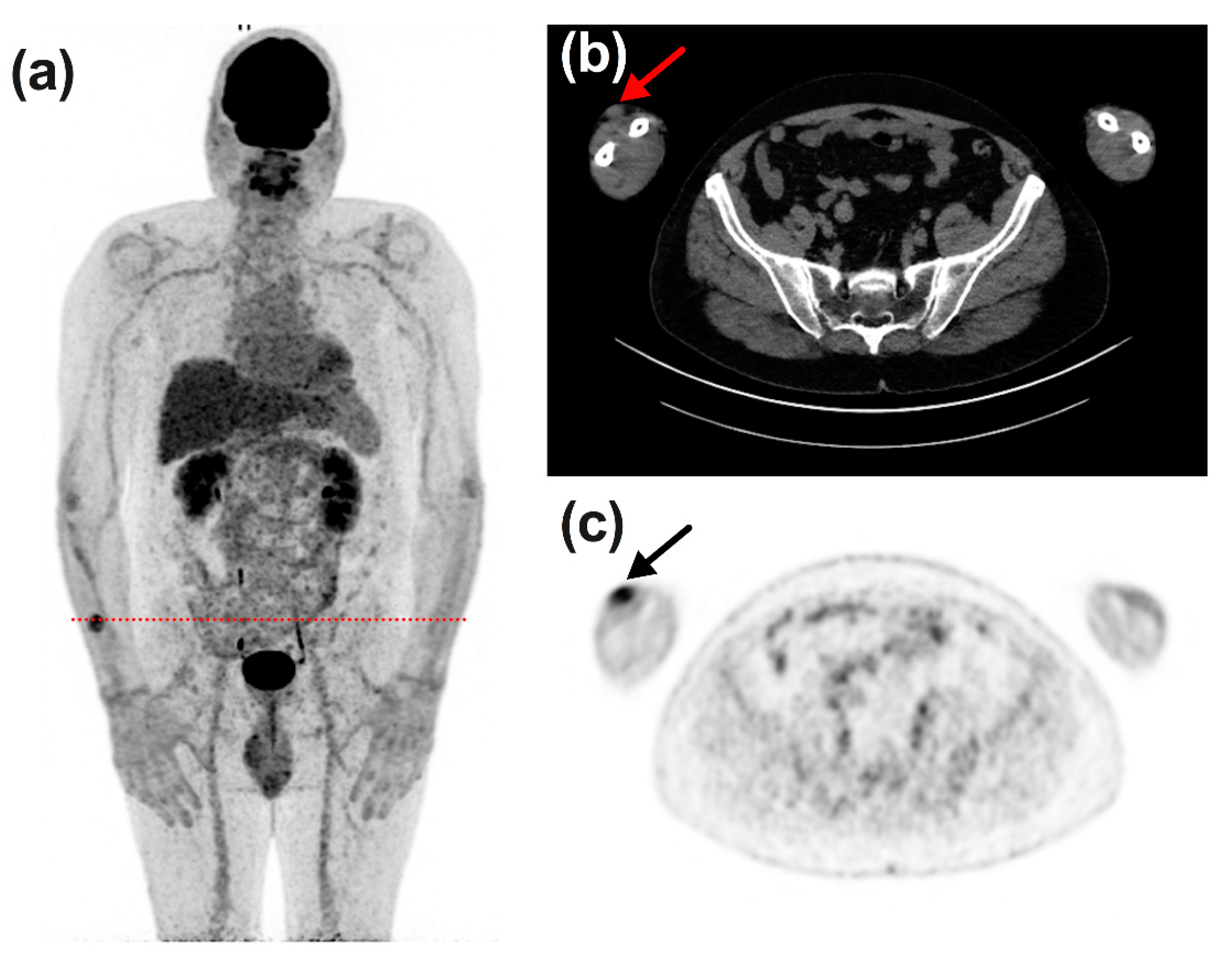
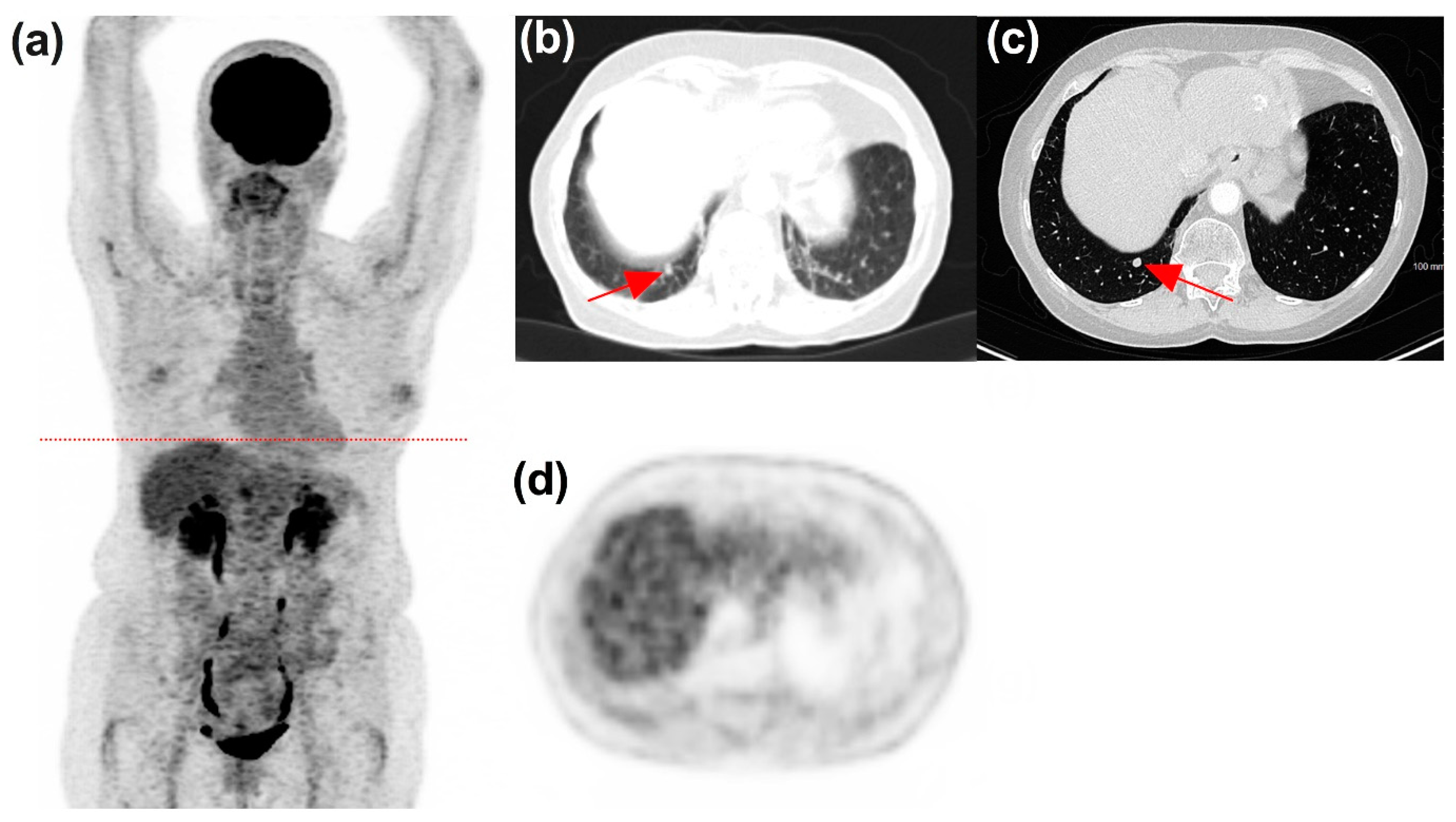
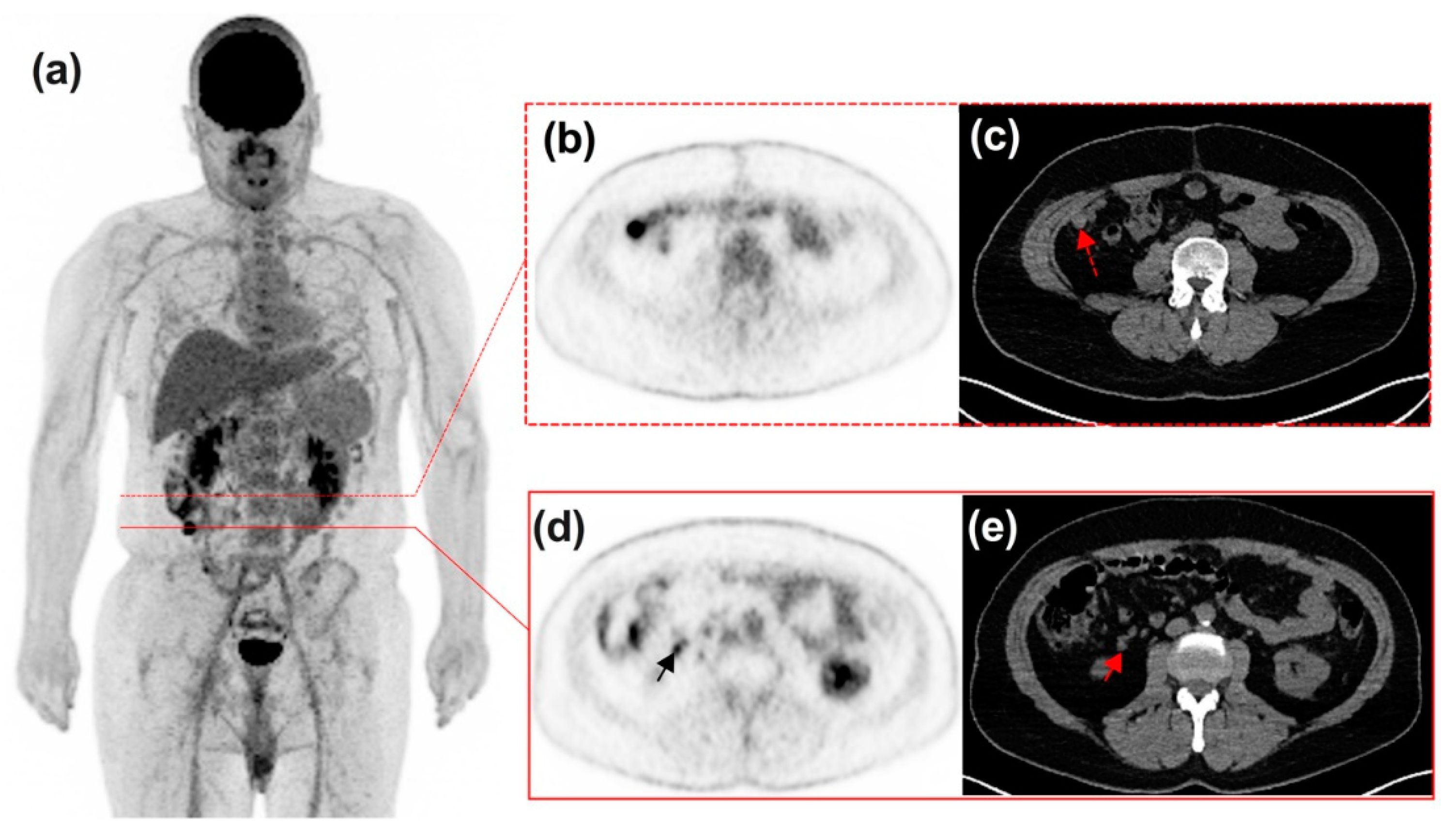
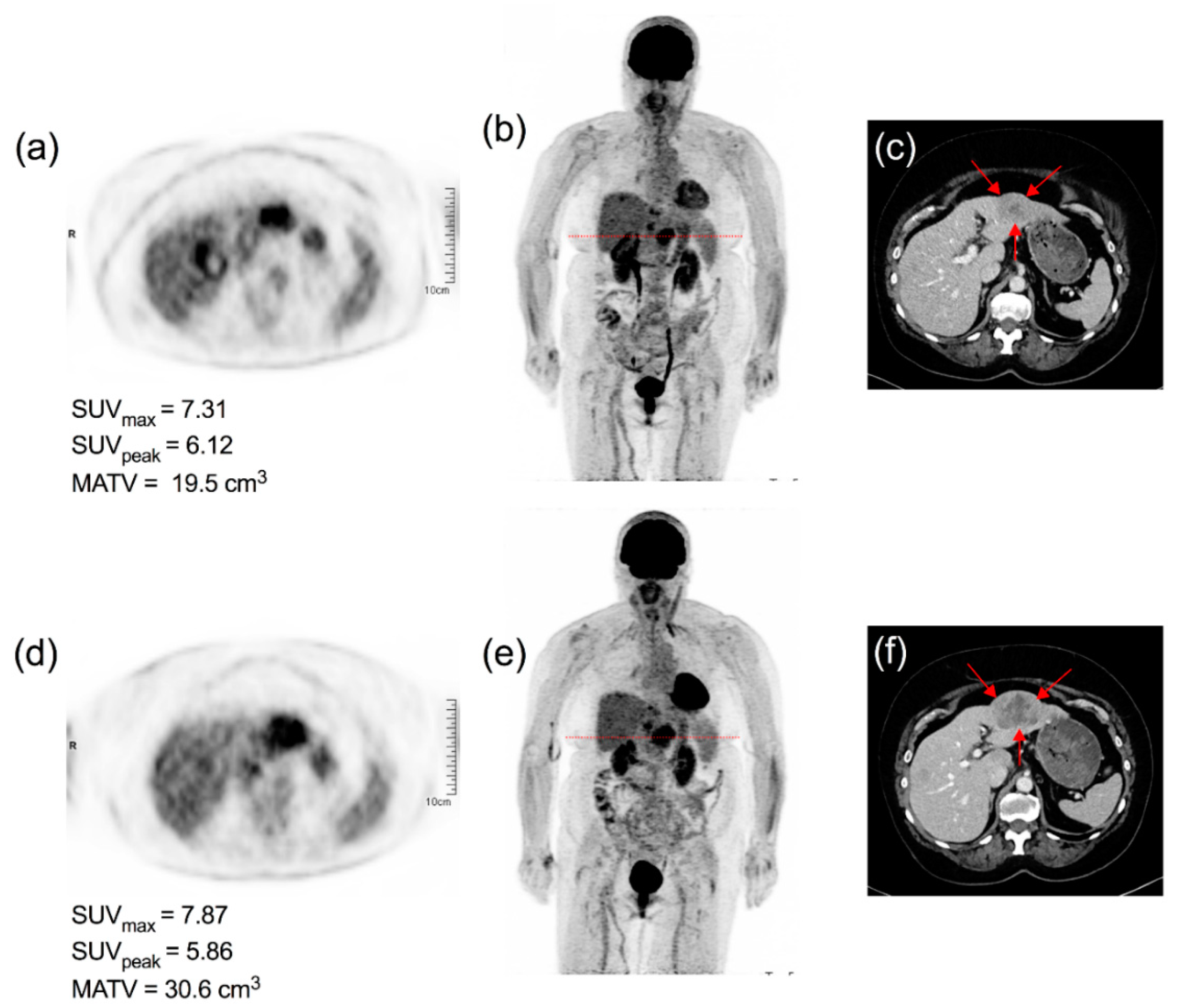
3.3. Timeline, Causes, and Consequences of Discordances
3.4. Impact of Discordances on Patient’s Management
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA A Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [Green Version]
- Whiteman, D.C.; Green, A.C.; Olsen, C.M. The Growing Burden of Invasive Melanoma: Projections of Incidence Rates and Numbers of New Cases in Six Susceptible Populations through 2031. J. Investig. Dermatol. 2016, 136, 1161–1171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grob, J.J.; Amonkar, M.M.; Karaszewska, B.; Schachter, J.; Dummer, R.; Mackiewicz, A.; Stroyakovskiy, D.; Drucis, K.; Grange, F.; Chiarion-Sileni, V.; et al. Comparison of dabrafenib and trametinib combination therapy with vemurafenib monotherapy on health-related quality of life in patients with unresectable or metastatic cutaneous BRAF Val600-mutation-positive melanoma (COMBI-v): Results of a phase 3, open-label, randomised trial. Lancet Oncol. 2015, 16, 1389–1398. [Google Scholar]
- Larkin, J.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.J.; Cowey, C.L.; Lao, C.D.; Schadendorf, D.; Dummer, R.; Smylie, M.; Rutkowski, P.; et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N. Engl. J. Med. 2015, 373, 23–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ribas, A.; Puzanov, I.; Dummer, R.; Schadendorf, D.; Hamid, O.; Robert, C.; Hodi, F.S.; Schachter, J.; Pavlick, A.C.; Lewis, K.D.; et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): A randomised, controlled, phase 2 trial. Lancet Oncol. 2015, 16, 908–918. [Google Scholar] [CrossRef] [Green Version]
- Seth, R.; Messersmith, H.; Kaur, V.; Kirkwood, J.M.; Kudchadkar, R.; McQuade, J.L.; Provenzano, A.; Swami, U.; Weber, J.; Alluri, K.C.; et al. Systemic Therapy for Melanoma: ASCO Guideline. J. Clin. Oncol. 2020, 38, 3947–3970. [Google Scholar] [CrossRef]
- Michielin, O.; van Akkooi, A.C.; Ascierto, P.A.; Dummer, R.; Keilholz, U. Cutaneous melanoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2019, 30, 1884–1901. [Google Scholar] [CrossRef] [Green Version]
- Reinhardt, M.J.; Joe, A.Y.; Jaeger, U.; Huber, A.; Matthies, A.; Bucerius, J.; Roedel, R.; Strunk, H.; Bieber, T.; Biersack, H.J.; et al. Diagnostic performance of whole body dual modality 18F-FDG PET/CT imaging for N- and M-staging of malignant melanoma: Experience with 250 consecutive patients. J. Clin. Oncol. 2006, 24, 1178–1187. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.N.; McArthur, G.A.; Hofman, M.S.; Hicks, R.J. The Advantages and Challenges of Using FDG PET/CT for Response Assessment in Melanoma in the Era of Targeted Agents and Immunotherapy. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 67–77. [Google Scholar] [CrossRef]
- McArthur, G.A.; Puzanov, I.; Amaravadi, R.; Ribas, A.; Chapman, P.; Kim, K.B.; Sosman, J.A.; Lee, R.J.; Nolop, K.; Flaherty, K.T.; et al. Marked, homogeneous, and early [18F]fluorodeoxyglucose-positron emission tomography responses to vemurafenib in BRAF-mutant advanced melanoma. J. Clin. Oncol. 2012, 30, 1628–1634. [Google Scholar] [CrossRef] [Green Version]
- Aide, N.; Hicks, R.J.; Le Tourneau, C.; Lheureux, S.; Fanti, S.; Lopci, E. FDG PET/CT for assessing tumour response to immunotherapy: Report on the EANM symposium on immune modulation and recent review of the literature. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 238–250. [Google Scholar] [CrossRef] [Green Version]
- Iravani, A.; Hicks, R.J. Imaging the Cancer Immune Environment and Its Response to Pharmacologic Intervention, Part 1: The Role of (18)F-FDG PET/CT. J. Nucl. Med. 2020, 61, 943–950. [Google Scholar] [CrossRef] [PubMed]
- Iravani, A.; Osman, M.M.; Weppler, A.M.; Wallace, R.; Galligan, A.; Lasocki, A.; Hunter, M.O.; Akhurst, T.; Hofman, M.S.; Lau, P.K.; et al. FDG PET/CT for tumoral and systemic immune response monitoring of advanced melanoma during first-line combination ipilimumab and nivolumab treatment. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 2776–2786. [Google Scholar] [CrossRef] [PubMed]
- Marandino, L.; Capozza, A.; Bandini, M.; Raggi, D.; Fare, E.; Pederzoli, F.; Gallina, A.; Capitanio, U.; Bianchi, M.; Gandaglia, G.; et al. Incidence and Clinical Impact of Inflammatory Fluorodeoxyglucose Positron Emission Tomography Uptake After Neoadjuvant Pembrolizumab in Patients with Organ-confined Bladder Cancer Undergoing Radical Cystectomy. Eur. Urol. Focus 2020. [Google Scholar] [CrossRef]
- Prigent, K.; Aide, N. (18)F-Fludeoxyglucose PET/Computed Tomography for Assessing Tumor Response to Immunotherapy and Detecting Immune-Related Side Effects: A Checklist for the PET Reader. PET Clin. 2020, 15, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Prigent, K.; Lasnon, C.; Ezine, E.; Janson, M.; Coudrais, N.; Joly, E.; Cesaire, L.; Stefan, A.; Depontville, M.; Aide, N. Assessing immune organs on (18)F-FDG PET/CT imaging for therapy monitoring of immune checkpoint inhibitors: Inter-observer variability, prognostic value and evolution during the treatment course of melanoma patients. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 2573–2585. [Google Scholar] [CrossRef]
- Seban, R.D.; Moya-Plana, A.; Antonios, L.; Yeh, R.; Marabelle, A.; Deutsch, E.; Schwartz, L.H.; Gomez, R.G.; Saenger, Y.; Robert, C.; et al. Prognostic 18F-FDG PET biomarkers in metastatic mucosal and cutaneous melanoma treated with immune checkpoint inhibitors targeting PD-1 and CTLA-4. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 2301–2312. [Google Scholar] [CrossRef]
- Boellaard, R.; Delgado-Bolton, R.; Oyen, W.J.; Giammarile, F.; Tatsch, K.; Eschner, W.; Verzijlbergen, F.J.; Barrington, S.F.; Pike, L.C.; Weber, W.A.; et al. FDG PET/CT: EANM procedure guidelines for tumour imaging: Version 2.0. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 328–354. [Google Scholar] [CrossRef]
- Aide, N.; Lasnon, C.; Veit-Haibach, P.; Sera, T.; Sattler, B.; Boellaard, R. EANM/EARL harmonization strategies in PET quantification: From daily practice to multicentre oncological studies. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 17–31. [Google Scholar] [CrossRef]
- Weisman, A.J.; Bradshaw, T.J.; Namías, M.; Jeraj, R. Impact of scanner harmonization on PET-based treatment response assessment in metastatic melanoma. Phys. Med. Biol. 2020, 65, 225003. [Google Scholar] [CrossRef]
- Lasnon, C.; Coudrais, N.; Houdu, B.; Nganoa, C.; Salomon, T.; Enilorac, B.; Aide, N. How fast can we scan patients with modern (digital) PET/CT systems? Eur. J. Radiol. 2020, 129, 109144. [Google Scholar] [CrossRef] [PubMed]
- Wahl, R.L.; Jacene, H.; Kasamon, Y.; Lodge, M.A. From RECIST to PERCIST: Evolving Considerations for PET response criteria in solid tumors. J. Nucl. Med. 2009, 50 (Suppl 1), 122S–150S. [Google Scholar] [CrossRef] [Green Version]
- Holtkamp, L.H.; Chakera, A.H.; Fung, S.; Stretch, J.R.; Saw, R.P.; Lee, K.; Ch’ng, S.; Gonzalez, M.; Thompson, J.F.; Emmett, L.; et al. Staging 18F-FDG PET/CT influences the treatment plan in melanoma patients with satellite or in-transit metastases. Melanoma Res. 2020, 30, 358–363. [Google Scholar] [CrossRef]
- Singnurkar, A.; Wang, J.; Joshua, A.M.; Langer, D.L.; Metser, U. 18F-FDG-PET/CT in the Staging and Management of Melanoma: A Prospective Multicenter Ontario PET Registry Study. Clin. Nucl. Med. 2016, 41, 189–193. [Google Scholar] [CrossRef]
- Ito, K.; Teng, R.; Schoder, H.; Humm, J.L.; Ni, A.; Michaud, L.; Nakajima, R.; Yamashita, R.; Wolchok, J.D.; Weber, W.A. (18)F-FDG PET/CT for Monitoring of Ipilimumab Therapy in Patients with Metastatic Melanoma. J. Nucl. Med. 2019, 60, 335–341. [Google Scholar] [CrossRef] [Green Version]
- Matthews, N.H.; Li, W.Q.; Qureshi, A.A.; Weinstock, M.A.; Cho, E. Epidemiology of Melanoma. In Cutaneous Melanoma: Etiology and Therapy; Ward, W.H., Farma, J.M., Eds.; Codon Publications: Brisbane, Australia, 2017. [Google Scholar]
- Franken, M.G.; Leeneman, B.; Jochems, A.; Schouwenburg, M.G.; Aarts, M.J.; van Akkooi, A.C.; van den Berkmortel, F.; van den Eertwegh, A.J.; de Groot, J.W.; van der Hoeven, K.J.; et al. Real-world healthcare costs of ipilimumab in patients with advanced cutaneous melanoma in The Netherlands. Anti-Cancer Drugs 2018, 29, 579–588. [Google Scholar] [CrossRef]
- Kandel, M.; Allayous, C.; Dalle, S.; Mortier, L.; Dalac, S.; Dutriaux, C.; Leccia, M.T.; Guillot, B.; Saiag, P.; Lacour, J.P.; et al. Update of survival and cost of metastatic melanoma with new drugs: Estimations from the MelBase cohort. Eur. J. Cancer 2018, 105, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Noels, E.; Hollestein, L.; Luijkx, K.; Louwman, M.; de Uyl-de Groot, C.; van den Bos, R.; van der Veldt, A.; Grunhagen, D.; Wakkee, M. Increasing Costs of Skin Cancer due to Increasing Incidence and Introduction of Pharmaceuticals, 2007–2017. Acta Derm. Venereol. 2020, 100, adv00147. [Google Scholar] [CrossRef] [PubMed]
- Stamell, E.F.; Wolchok, J.D.; Gnjatic, S.; Lee, N.Y.; Brownell, I. The abscopal effect associated with a systemic anti-melanoma immune response. Int. J. Radiat. Oncol. Biol. Phys. 2013, 85, 293–295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grahek, D.; Montravers, F.; Kerrou, K.; Aide, N.; Lotz, J.P.; Talbot, J.N. [18F]FDG in recurrent breast cancer: Diagnostic performances, clinical impact and relevance of induced changes in management. Eur. J. Nucl. Med. Mol. Imaging 2004, 31, 179–188. [Google Scholar] [CrossRef]
- Yap, C.S.; Seltzer, M.A.; Schiepers, C.; Gambhir, S.S.; Rao, J.; Phelps, M.E.; Valk, P.E.; Czernin, J. Impact of whole-body 18F-FDG PET on staging and managing patients with breast cancer: The referring physician’s perspective. J. Nucl. Med. 2001, 42, 1334–1337. [Google Scholar] [PubMed]
- Fuentes-Ocampo, F.; Lopez-Mora, D.A.; Flotats, A.; Camacho, V.; Sizova, M.; Abouzian, S.; Duch, J.; Fernandez, A.; Estorch, M.; Carrio, I. Digital versus analog PET/CT in patients with known or suspected liver metastases. Nucl. Med. Commun. 2020, 42, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Pfluger, T.; Melzer, H.I.; Schneider, V.; La Fougere, C.; Coppenrath, E.; Berking, C.; Bartenstein, P.; Weiss, M. PET/CT in malignant melanoma: Contrast-enhanced CT versus plain low-dose CT. Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 822–831. [Google Scholar] [CrossRef] [PubMed]
| Variable\Statistic | Categories | Frequency | Relative Frequency (%) |
|---|---|---|---|
| Gender | Female | 48 | 60.0 |
| Male | 32 | 40.0 | |
| Location | Lower limb | 20 | 25 |
| Upper limb | 16 | 20 | |
| Trunk | 15 | 18.8 | |
| Head and neck | 11 | 13.7 | |
| No primary lesion | 11 | 13.7 | |
| Others | 7 | 8.8 | |
| Stage at diagnostic | IA | 4 | 5.0 |
| IB | 7 | 8.8 | |
| IIA | 6 | 7.5 | |
| IIB | 16 | 20.0 | |
| IIC | 6 | 7.5 | |
| IIIB | 2 | 2.5 | |
| IIIC | 1 | 1.3 | |
| IIID | 4 | 5.0 | |
| IV | 15 | 18.8 | |
| na | 7 | 8.8 | |
| Missing | 12 | 15.0 | |
| Histology | Acral lentiginous melanoma | 2 | 2.5 |
| Lentigo malignant melanoma | 1 | 1.3 | |
| Nodular melanoma | 16 | 20.0 | |
| Superficial spreading melanoma | 30 | 37.5 | |
| Others | 9 | 11.3 | |
| No primary lesion | 11 | 13.8 | |
| Missing | 11 | 13.8 | |
| Breslow | in situ | 1 | 1.3 |
| 0.1–1 | 7 | 8.8 | |
| 1.01–2 | 13 | 16.3 | |
| >2 | 35 | 43.8 | |
| na | 18 | 22.5 | |
| Missing | 6 | 7.5 | |
| Ulceration | No | 23 | 28.8 |
| Yes | 26 | 32.5 | |
| na | 18 | 22.5 | |
| Missing | 13 | 16.3 | |
| Regression | No | 40 | 50.0 |
| Yes | 4 | 5.0 | |
| na | 18 | 22.5 | |
| Missing | 18 | 22.5 | |
| Mitotic index | High | 16 | 20.0 |
| Low | 8 | 10.0 | |
| na | 18 | 22.5 | |
| Missing | 38 | 47.5 | |
| BRAF mutation | Yes | 34 | 42.5 |
| No | 46 | 57.5 |
| Concordance (Cohen’s Kappa) | ||||
|---|---|---|---|---|
| <6 Months | 6–10 Months | 10–16 Months | >16 Months | |
| Clinical benefit * vs. no clinical benefit ** | 0.71 | 0.51 | 0.67 | 0.39 |
| CR vs. SD vs. PR Vs. PD vs. dissociated response | 0.32 | 0.28 | 0.51 | 0.57 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Le Goubey, J.-B.; Lasnon, C.; Nakouri, I.; Césaire, L.; de Pontville, M.; Nganoa, C.; Kottler, D.; Aide, N. 18F-FDG PET/CT versus Diagnostic Contrast-Enhanced CT for Follow-Up of Stage IV Melanoma Patients Treated by Immune Checkpoint Inhibitors: Frequency and Management of Discordances over a 3-Year Period in a University Hospital. Diagnostics 2021, 11, 1198. https://doi.org/10.3390/diagnostics11071198
Le Goubey J-B, Lasnon C, Nakouri I, Césaire L, de Pontville M, Nganoa C, Kottler D, Aide N. 18F-FDG PET/CT versus Diagnostic Contrast-Enhanced CT for Follow-Up of Stage IV Melanoma Patients Treated by Immune Checkpoint Inhibitors: Frequency and Management of Discordances over a 3-Year Period in a University Hospital. Diagnostics. 2021; 11(7):1198. https://doi.org/10.3390/diagnostics11071198
Chicago/Turabian StyleLe Goubey, Jean-Baptiste, Charline Lasnon, Ines Nakouri, Laure Césaire, Michel de Pontville, Catherine Nganoa, Diane Kottler, and Nicolas Aide. 2021. "18F-FDG PET/CT versus Diagnostic Contrast-Enhanced CT for Follow-Up of Stage IV Melanoma Patients Treated by Immune Checkpoint Inhibitors: Frequency and Management of Discordances over a 3-Year Period in a University Hospital" Diagnostics 11, no. 7: 1198. https://doi.org/10.3390/diagnostics11071198
APA StyleLe Goubey, J.-B., Lasnon, C., Nakouri, I., Césaire, L., de Pontville, M., Nganoa, C., Kottler, D., & Aide, N. (2021). 18F-FDG PET/CT versus Diagnostic Contrast-Enhanced CT for Follow-Up of Stage IV Melanoma Patients Treated by Immune Checkpoint Inhibitors: Frequency and Management of Discordances over a 3-Year Period in a University Hospital. Diagnostics, 11(7), 1198. https://doi.org/10.3390/diagnostics11071198






