Nuclear Imaging for the Diagnosis of Cardiac Amyloidosis in 2021
Abstract
1. Introduction
2. Imaging Techniques and Radiotracers
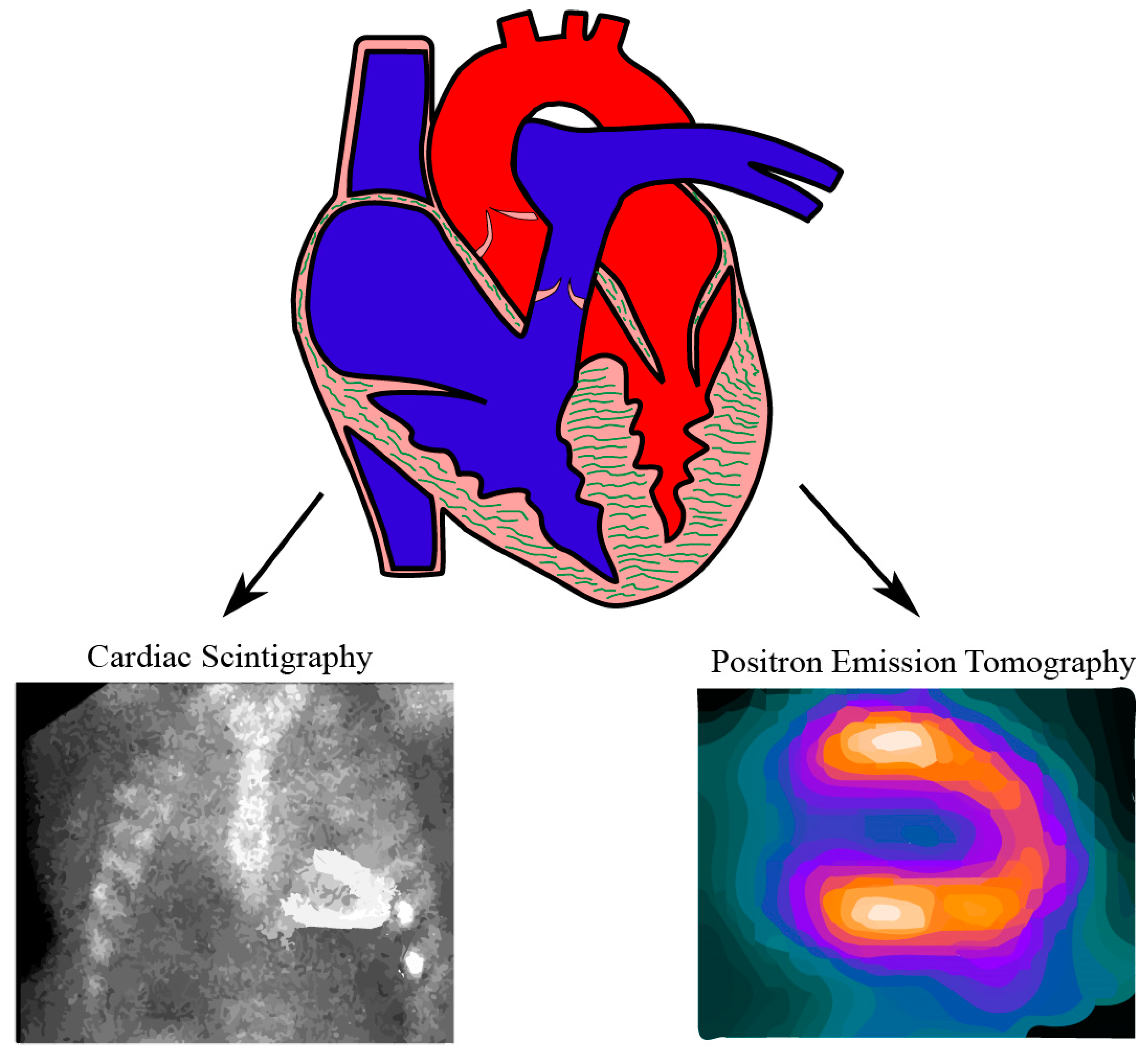
3. Cardiac Scintigraphy
4. 99mTc-3,3-diphosphono-1,2-propanodicarboxylic Acid (99mTc-DPD) Scintigraphy
5. 99mTc-Pyrophosphate (99mTc-PYP) Scintigraphy
6. Positron Emission Tomography (PET)
7. 11C-Pittsburgh Compound B PET Imaging
8. 18F-Labelled Agents PET Imaging
9. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alexander, K.M.; Orav, J.; Singh, A.; Jacob, S.A.; Menon, A.; Padera, R.F.; Kijewski, M.F.; Liao, R.; Di Carli, M.F.; Laubach, J.P.; et al. Geographic Disparities in Reported US Amyloidosis Mortality From 1979 to 2015: Potential Underdetection of Cardiac Amyloidosis. JAMA Cardiol. 2018, 3, 865–870. [Google Scholar] [CrossRef] [PubMed]
- Blancas-Mejía, L.M.; Ramirez-Alvarado, M. Systemic Amyloidoses. Annu. Rev. Biochem. 2013, 82, 745–774. [Google Scholar] [CrossRef] [PubMed]
- Ravichandran, S.; Lachmann, H.J.; Wechalekar, A.D. Epidemiologic and Survival Trends in Amyloidosis, 1987–2019. N. Engl. J. Med. 2020, 382, 1567–1568. [Google Scholar] [CrossRef] [PubMed]
- Pinney, J.H.; Smith, C.J.; Taube, J.B.; Lachmann, H.J.; Venner, C.P.; Gibbs, S.D.J.; Dungu, J.; Banypersad, S.M.; Wechalekar, A.D.; Whelan, C.J.; et al. Systemic Amyloidosis in England: An epidemiological study. Br. J. Haematol. 2013, 161, 525–532. [Google Scholar] [CrossRef]
- Seo, S.R.; Jang, S.Y.; Lee, G.Y.; Choi, B.; Chun, H.; Cho, E.J.; Cho, S.-I. Prevalence of Amyloidosis in Korea. Orphanet J. Rare Dis. 2017, 12, 1–5. [Google Scholar] [CrossRef]
- Nienhuis, H.L.; Bijzet, J.; Hazenberg, B.P. The Prevalence and Management of Systemic Amyloidosis in Western Countries. Kidney Dis. 2016, 2, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Tanskanen, M.; Peuralinna, T.; Polvikoski, T.; Notkola, I.; Sulkava, R.; Hardy, J.; Singleton, A.; Kiuru-Enari, S.; Paetau, A.; Tienari, P.J.; et al. Senile systemic amyloidosis affects 25% of the very aged and associates with genetic variation in alpha2-macroglobulin and tau: A population-based autopsy study. Ann. Med. 2008, 40, 232–239. [Google Scholar] [CrossRef]
- Cornwell, G.G., III; Sletten, K.; Johansson, B.; Westermark, P. Evidence that the amyloid fibril protein in senile systemic amyloidosis is derived from normal prealbumin. Biochem. Biophys. Res. Commun. 1988, 154, 648–653. [Google Scholar] [CrossRef]
- Ueda, M.; Horibata, Y.; Shono, M.; Misumi, Y.; Oshima, T.; Su, Y.; Tasaki, M.; Shinriki, S.; Kawahara, S.; Jono, H.; et al. Clinicopathological features of senile systemic amyloidosis: An ante- and post-mortem study. Mod. Pathol. 2011, 24, 1533–1544. [Google Scholar] [CrossRef]
- Shah, K.B.; Inoue, Y.; Mehra, M.R. Amyloidosis and the heart: A comprehensive review. Arch. Intern. Med. 2006, 166, 1805–1813. [Google Scholar] [CrossRef]
- Gilstrap, L.G.; Dominici, F.; Wang, Y.; El-Sady, M.S.; Singh, A.; Di Carli, M.F.; Falk, R.H.; Dorbala, S. Epidemiology of Cardiac Amyloidosis–Associated Heart Failure Hospitalizations Among Fee-for-Service Medicare Beneficiaries in the United States. Circ. Heart Fail. 2019, 12, e005407. [Google Scholar] [CrossRef]
- Cuscaden, C.; Ramsay, S.C.; Prasad, S.; Goodwin, B.; Smith, J. Estimation of prevalence of transthyretin (ATTR) cardiac amyloidosis in an Australian subpopulation using bone scans with echocardiography and clinical correlation. J. Nucl. Cardiol. 2020, 1–12. [Google Scholar] [CrossRef]
- Wechalekar, K.; Wechalekar, A.D. Wild-type transthyretin cardiac amyloidosis: When is a rare disease no longer a rare disease? J. Nucl. Cardiol. 2020, 1–3. [Google Scholar] [CrossRef]
- Sanchorawala, V. Light-Chain (AL) Amyloidosis: Diagnosis and Treatment. Clin. J. Am. Soc. Nephrol. 2006, 1, 1331–1341. [Google Scholar] [CrossRef]
- Yamamoto, H.; Yokochi, T. Transthyretin cardiac amyloidosis: An update on diagnosis and treatment. ESC Heart Fail. 2019, 6, 1128–1139. [Google Scholar] [CrossRef] [PubMed]
- Halatchev, I.G.; Zheng, J.; Ou, J. Wild-type transthyretin cardiac amyloidosis (ATTRwt-CA), previously known as senile cardiac amyloidosis: Clinical presentation, diagnosis, management and emerging therapies. J. Thorac. Dis. 2018, 10, 2034–2045. [Google Scholar] [CrossRef] [PubMed]
- Puig-Carrion, G.D.; Reyentovich, A.; Katz, S.D. Diagnosis and treatment of heart failure in hereditary transthyretin amyloidosis. Clin. Auton. Res. 2019, 29 (Suppl. 1), 45–53. [Google Scholar] [CrossRef]
- Macedo, A.V.S.; Schwartzmann, P.V.; De Gusmão, B.M.; De Melo, M.D.T.; Coelho-Filho, O.R. Advances in the Treatment of Cardiac Amyloidosis. Curr. Treat. Options Oncol. 2020, 21, 1–18. [Google Scholar] [CrossRef]
- Palladini, G.; Milani, P.; Merlini, G. Management of AL amyloidosis in 2020. Blood 2020, 136, 2620–2627. [Google Scholar] [CrossRef]
- Maurer, M.S.; Schwartz, J.H.; Gundapaneni, B.; Elliott, P.M.; Merlini, G.; Waddington-Cruz, M.; Kristen, A.V.; Grogan, M.; Witteles, R.; Damy, T.; et al. Tafamidis Treatment for Patients with Transthyretin Amyloid Cardiomyopathy. N. Engl. J. Med. 2018, 379, 1007–1016. [Google Scholar] [CrossRef] [PubMed]
- Pellikka, P.A.; Holmes, D.R.; Edwards, W.D.; Nishimura, R.A.; Tajik, A.J.; Kyle, R.A. Endomyocardial biopsy in 30 patients with primary amyloidosis and suspected cardiac involvement. Arch. Intern. Med. 1988, 148, 662–666. [Google Scholar] [CrossRef]
- Wisniowski, B.; Wechalekar, A. Confirming the Diagnosis of Amyloidosis. Acta Haematol. 2020, 143, 312–321. [Google Scholar] [CrossRef]
- Yilmaz, A.; Kindermann, I.; Kindermann, M.; Mahfoud, F.; Ukena, C.; Athanasiadis, A.; Hill, S.; Mahrholdt, H.; Voehringer, M.; Schieber, M.; et al. Comparative evaluation of left and right ventricular endomyocardial biopsy: Differences in complication rate and diagnostic performance. Circulation 2010, 122, 900–909. [Google Scholar] [CrossRef]
- From, A.M.; Maleszewski, J.J.; Rihal, C.S. Current Status of Endomyocardial Biopsy. Mayo Clin. Proc. 2011, 86, 1095–1102. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.K.M.; Hassan, O.K.A.; Jaber, W.; Xu, B. Multi-modality imaging of cardiac amyloidosis: Contemporary update. World J. Radiol. 2020, 12, 87–100. [Google Scholar] [CrossRef]
- Dorbala, S.; Ando, Y.; Bokhari, S.; Dispenzieri, A.; Falk, R.H.; Ferrari, V.; Fontana, M.; Gheysens, O.; Gillmore, J.D.; Glaudemans, A.W.; et al. ASNC/AHA/ASE/EANM/HFSA/ISA/SCMR/SNMMI Expert Consensus Recommendations for Multimodality Imaging in Cardiac Amyloidosis: Part 1 of 2—Evidence Base and Standardized Methods of Imaging. J. Card. Fail. 2019, 25, e1–e39. [Google Scholar] [CrossRef] [PubMed]
- Dorbala, S.; Ando, Y.; Bokhari, S.; Dispenzieri, A.; Falk, R.H.; Ferrari, V.; Fontana, M.; Gheysens, O.; Gillmore, J.D.; Glaudemans, A.W.; et al. ASNC/AHA/ASE/EANM/HFSA/ISA/SCMR/SNMMI Expert Consensus Recommendations for Multimodality Imaging in Cardiac Amyloidosis: Part 2 of 2—Diagnostic Criteria and Appropriate Utilization. J. Card. Fail. 2019, 25, 854–865. [Google Scholar] [CrossRef] [PubMed]
- Kinahan, P.E.; Fletcher, J.W. Positron Emission Tomography-Computed Tomography Standardized Uptake Values in Clinical Practice and Assessing Response to Therapy. Semin. Ultrasound CT MRI 2010, 31, 496–505. [Google Scholar] [CrossRef]
- Antoni, G.; Lubberink, M.; Estrada, S.; Axelsson, J.; Carlson, K.; Lindsjö, L.; Kero, T.; Långström, B.R.; Granstam, S.-O.; Rosengren, S.; et al. In Vivo Visualization of Amyloid Deposits in the Heart with 11C-PIB and PET. J. Nucl. Med. 2013, 54, 213–220. [Google Scholar] [CrossRef]
- Cuddy, S.A.M.; Bravo, P.E.; Falk, R.H.; El-Sady, S.; Kijewski, M.F.; Park, M.; Ruberg, F.L.; Sanchorawala, V.; Landau, H.; Yee, A.J.; et al. Improved Quantification of Cardiac Amyloid Burden in Systemic Light Chain Amyloidosis: Redefining Early Disease? JACC Cardiovasc. Imaging 2020, 13, 1325–1336. [Google Scholar] [CrossRef] [PubMed]
- Kero, T.; Sörensen, J.; Antoni, G.; Wilking, H.; Carlson, K.; Vedin, O.; Rosengren, S.; Wikström, G.; Lubberink, M. Quantification of 11C-PIB kinetics in cardiac amyloidosis. J. Nucl. Cardiol. 2020, 27, 774–784. [Google Scholar] [CrossRef]
- Kula, R.; Engel, W.; Line, B. Scanning for soft-tissue amyloid. Lancet 1977, 309, 92–93. [Google Scholar] [CrossRef]
- Gillmore, J.D.; Maurer, M.S.; Falk, R.H.; Merlini, G.; Damy, T.; Dispenzieri, A.; Wechalekar, A.D.; Berk, J.L.; Quarta, C.C.; Grogan, M.; et al. Nonbiopsy Diagnosis of Cardiac Transthyretin Amyloidosis. Circulation 2016, 133, 2404–2412. [Google Scholar] [CrossRef]
- Treglia, G.; Glaudemans, A.W.J.M.; Bertagna, F.; Hazenberg, B.P.C.; Erba, P.A.; Giubbini, R.; Ceriani, L.; Prior, J.O.; Giovanella, L.; Slart, R.H.J.A. Diagnostic accuracy of bone scintigraphy in the assessment of cardiac transthyretin-related amyloidosis: A bivariate meta-analysis. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 1945–1955. [Google Scholar] [CrossRef] [PubMed]
- Bokhari, S.; Castaño, A.; Pozniakoff, T.; Deslisle, S.; Latif, F.; Maurer, M.S. 99m Tc-Pyrophosphate Scintigraphy for Differentiating Light-Chain Cardiac Amyloidosis From the Transthyretin-Related Familial and Senile Cardiac Amyloidoses. Circ. Cardiovasc. Imaging 2013, 6, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Pavia, P.; Rapezzi, C.; Adler, Y.; Arad, M.; Basso, C.; Brucato, A.; Burazor, I.; Caforio, A.L.P.; Damy, T.; Eriksson, U.; et al. Diagnosis and treatment of cardiac amyloidosis: A position statement of the ESC Working Group on Myocardial and Pericardial Diseases. Eur. Heart J. 2021, 42, 1554–1568. [Google Scholar] [CrossRef] [PubMed]
- Grigoratos, C.; Aimo, A.; Rapezzi, C.; Genovesi, D.; Barison, A.; Aquaro, G.D.; Vergaro, G.; Pucci, A.; Passino, C.; Marzullo, P.; et al. Diphosphonate single-photon emission computed tomography in cardiac transthyretin amyloidosis. Int. J. Cardiol. 2020, 307, 187–192. [Google Scholar] [CrossRef]
- Perugini, E.; Guidalotti, P.L.; Salvi, F.; Cooke, R.M.T.; Pettinato, C.; Riva, L.; Leone, O.; Farsad, M.; Ciliberti, P.; Bacchi-Reggiani, L.; et al. Noninvasive etiologic diagnosis of cardiac amyloidosis using 99mTc-3,3-diphosphono-1,2-propanodicarboxylic acid scintigraphy. J. Am. Coll. Cardiol. 2005, 46, 1076–1084. [Google Scholar] [CrossRef]
- Caobelli, F.; Braun, M.; Haaf, P.; Wild, D.; Zellweger, M.J. Quantitative 99mTc-DPD SPECT/CT in patients with suspected ATTR cardiac amyloidosis: Feasibility and correlation with visual scores. J. Nucl. Cardiol. 2020, 27, 1456–1463. [Google Scholar] [CrossRef]
- Hutt, D.F.; Fontana, M.; Burniston, M.; Quigley, A.; Petrie, A.; Ross, J.C.; Page, J.; Martinez-Naharro, A.; Wechalekar, A.D.; Lachmann, H.J.; et al. Prognostic utility of the Perugini grading of 99mTc-DPD scintigraphy in transthyretin (ATTR) amyloidosis and its relationship with skeletal muscle and soft tissue amyloid. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 1344–1350. [Google Scholar] [CrossRef] [PubMed]
- Gallini, C.; Tutino, F.; Martone, R.; Ciaccio, A.; Costanzo, E.N.; Taborchi, G.; Morini, S.; Bartolini, S.; Farsetti, S.; di Mario, C.; et al. Semi-quantitative indices of cardiac uptake in patients with suspected cardiac amyloidosis undergoing 99mTc-HMDP scintigraphy. J. Nucl. Cardiol. 2019. [Google Scholar] [CrossRef]
- Moore, P.T.; Burrage, M.K.; MacKenzie, E.; Law, W.P.; Korczyk, D.; Mollee, P. The Utility of 99m Tc-DPD Scintigraphy in the Diagnosis of Cardiac Amyloidosis: An Australian Experience. Heart Lung Circ. 2017, 26, 1183–1190. [Google Scholar] [CrossRef] [PubMed]
- Rapezzi, C.; Quarta, C.C.; Guidalotti, P.L.; Pettinato, C.; Fanti, S.; Leone, O.; Ferlini, A.; Longhi, S.; Lorenzini, M.; Reggiani, L.B.; et al. Role of 99mTc-DPD Scintigraphy in Diagnosis and Prognosis of Hereditary Transthyretin-Related Cardiac Amyloidosis. JACC Cardiovasc. Imaging 2011, 4, 659–670. [Google Scholar] [CrossRef]
- Sachchithanantham, S.; Hutt, D.F.; Hawkins, P.; Wechalekar, A.D.; Quigley, A.-M. Role of 99m Tc-DPD scintigraphy in imaging extra-cardiac light chain (AL) amyloidosis. Br. J. Haematol. 2018, 183, 506–509. [Google Scholar] [CrossRef]
- Manrique, A.; Dudoignon, D.; Brun, S.; N’Ganoa, C.; Cassol, E.; Legallois, D.; Lavie-Badie, Y.; Agostini, D.; Lairez, O. Quantification of myocardial 99mTc-labeled bisphosphonate uptake with cadmium zinc telluride camera in patients with transthyretin-related cardiac amyloidosis. EJNMMI Res. 2019, 9, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Scully, P.R.; Morris, E.; Patel, K.P.; Treibel, T.A.; Burniston, M.; Klotz, E.; Newton, J.D.; Sabharwal, N.; Kelion, A.; Manisty, C.; et al. DPD Quantification in Cardiac Amyloidosis: A Novel Imaging Biomarker. JACC Cardiovasc. Imaging 2020, 13, 1353–1363. [Google Scholar] [CrossRef] [PubMed]
- Wollenweber, T.; Rettl, R.; Kretschmer-Chott, E.; Rasul, S.; Kulterer, O.; Rainer, E.; Raidl, M.; Schaffarich, M.P.; Matschitsch, S.; Stadler, M.; et al. In Vivo Quantification of Myocardial Amyloid Deposits in Patients with Suspected Transthyretin-Related Amyloidosis (ATTR). J. Clin. Med. 2020, 9, 3446. [Google Scholar] [CrossRef]
- Löfbacka, V.; Axelsson, J.; Pilebro, B.; Suhr, O.B.; Lindqvist, P.; Sundström, T. Cardiac transthyretin amyloidosis 99mTc-DPD SPECT correlates with strain echocardiography and biomarkers. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 1822–1832. [Google Scholar] [CrossRef]
- Ruberg, F.L.; Miller, E.J. Nuclear tracers for transthyretin cardiac amyloidosis: Time to bone up? Circ. Cardiovasc. Imaging 2013, 6, 162–164. [Google Scholar] [CrossRef]
- Castano, A.; Bokhari, S.; Brannagan, T.H.; Wynn, J.; Maurer, M.S. Technetium pyrophosphate myocardial uptake and peripheral neuropathy in a rare variant of familial transthyretin (TTR) amyloidosis (Ser23Asn): A case report and literature review. Amyloid 2012, 19, 41–46. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bokhari, S.; Shahzad, R.; Castaño, A.; Maurer, M.S. Nuclear imaging modalities for cardiac amyloidosis. J. Nucl. Cardiol. 2014, 21, 175–184. [Google Scholar] [CrossRef]
- Masri, A.; Bukhari, S.; Ahmad, S.; Nieves, R.; Eisele, Y.S.; Follansbee, W.; Brownell, A.; Wong, T.C.; Schelbert, E.; Soman, P. Efficient 1-Hour Technetium-99 m Pyrophosphate Imaging Protocol for the Diagnosis of Transthyretin Cardiac Amyloidosis. Circ. Cardiovasc. Imaging 2020, 13, e010249. [Google Scholar] [CrossRef]
- Castano, A.; Haq, M.; Narotsky, D.L.; Goldsmith, J.; Weinberg, R.L.; Morgenstern, R.; Pozniakoff, T.; Ruberg, F.L.; Miller, E.J.; Berk, J.L.; et al. Multicenter Study of Planar Technetium 99m Pyrophosphate Cardiac Imaging: Predicting Survival for Patients with ATTR Cardiac Amyloidosis. JAMA Cardiol. 2016, 1, 880–889. [Google Scholar] [CrossRef] [PubMed]
- Asif, T.; Gomez, J.; Singh, V.; Doukky, R.; Nedeltcheva, A.; Malhotra, S. Comparison of planar with tomographic pyrophosphate scintigraphy for transthyretin cardiac amyloidosis: Perils and pitfalls. J. Nucl. Cardiol. 2021, 28, 104–111. [Google Scholar] [CrossRef]
- Tamarappoo, B.; Otaki, Y.; Manabe, O.; Hyun, M.; Cantu, S.; Arnson, Y.; Gransar, H.; Hayes, S.W.; Friedman, J.D.; Thomson, L.; et al. Simultaneous Tc-99m PYP/Tl-201 dual-isotope SPECT myocardial imaging in patients with suspected cardiac amyloidosis. J. Nucl. Cardiol. 2019, 27, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Ochi, Y.; Kubo, T.; Nakashima, Y.; Baba, Y.; Hirota, T.; Yamasaki, N.; Yamashita, T.; Ueda, M.; Ando, Y.; Kitaoka, H. Integrated diagnostic approach to wild-type transthyretin cardiac amyloidosis with the use of high-sensitivity cardiac troponin T measurement and 99mTc-pyrophosphate scintigraphy. J. Cardiol. 2020, 75, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Takasone, K.; Katoh, N.; Takahashi, Y.; Abe, R.; Ezawa, N.; Yoshinaga, T.; Yanagisawa, S.; Yazaki, M.; Oguchi, K.; Koyama, J.; et al. Non-invasive detection and differentiation of cardiac amyloidosis using 99mTc-pyrophosphate scintigraphy and 11C-Pittsburgh compound B PET imaging. Amyloid 2020, 27, 266–274. [Google Scholar] [CrossRef]
- Rosengren, S.; Clemmensen, T.S.; Tolbod, L.P.; Granstam, S.-O.; Eiskjær, H.; Wikström, G.; Vedin, O.; Kero, T.; Lubberink, M.; Harms, H.J.; et al. Diagnostic Accuracy of [11C]PIB Positron Emission Tomography for Detection of Cardiac Amyloidosis. JACC Cardiovasc. Imaging 2020, 13, 1337–1347. [Google Scholar] [CrossRef]
- Lee, S.-P.; Suh, H.-Y.; Park, S.; Oh, S.; Kwak, S.-G.; Kim, H.-M.; Koh, Y.; Park, J.-B.; Kim, H.-K.; Cho, H.-J.; et al. Pittsburgh B Compound Positron Emission Tomography in Patients With AL Cardiac Amyloidosis. J. Am. Coll. Cardiol. 2020, 75, 380–390. [Google Scholar] [CrossRef] [PubMed]
- Genovesi, D.; Vergaro, G.; Giorgetti, A.; Marzullo, P.; Scipioni, M.; Santarelli, M.F.; Pucci, A.; Buda, G.; Volpi, E.; Emdin, M. [18F]-Florbetaben PET/CT for Differential Diagnosis Among Cardiac Immunoglobulin Light Chain, Transthyretin Amyloidosis, and Mimicking Conditions. JACC Cardiovasc. Imaging 2021, 14, 246–255. [Google Scholar] [CrossRef]
- Andrews, J.P.M.; Trivieri, M.G.; Everett, R.; Spath, N.; MacNaught, G.; Moss, A.J.; Doris, M.K.; Pawade, T.; Van Beek, E.J.R.; Lucatelli, C.; et al. 18F-fluoride PET/MR in cardiac amyloid: A comparison study with aortic stenosis and age- and sex-matched controls. J. Nucl. Cardiol. 2020, 1–9. [Google Scholar] [CrossRef]
- Zhang, L.X.; Martineau, P.; Finnerty, V.; Giraldeau, G.; Parent, M.; Harel, F.; Pelletier-Galarneau, M. Comparison of 18F-sodium fluoride positron emission tomography imaging and 99mTc-pyrophosphate in cardiac amyloidosis. J. Nucl. Cardiol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Shukla, A.K.; Kumar, U. Positron emission tomography: An overview. J. Med. Phys. 2006, 31, 13–21. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, G.Y.; Kim, S.J.; Kim, K.H.; Jeon, E.-S.; Lee, K.-H.; Kim, B.-T.; Choi, J.Y. Imaging Findings and Literature Review of 18F-FDG PET/CT in Primary Systemic AL Amyloidosis. Nucl. Med. Mol. Imaging 2015, 49, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Wolk, D.A.; Zhang, Z.; Boudhar, S.; Clark, C.M.; Pontecorvo, M.J.; Arnold, S.E. Amyloid imaging in Alzheimer’s disease: Comparison of florbetapir and Pittsburgh compound-B positron emission tomography. J. Neurol. Neurosurg. Psychiatry 2012, 83, 923–926. [Google Scholar] [CrossRef] [PubMed]
- Lhommel, R.; Sempoux, C.; Ivanoiu, A.; Michaux, L.; Gerber, B. Is 18F-Flutemetamol PET/CT Able to Reveal Cardiac Amyloidosis? Clin. Nucl. Med. 2014, 39, 747–749. [Google Scholar] [CrossRef]
- Lee, S.-P.; Lee, E.S.; Choi, H.; Im, H.-J.; Koh, Y.; Lee, M.-H.; Kwon, J.-H.; Paeng, J.C.; Kim, H.-K.; Cheon, G.J.; et al. 11C-Pittsburgh B PET Imaging in Cardiac Amyloidosis. JACC Cardiovasc. Imaging 2015, 8, 50–59. [Google Scholar] [CrossRef]
- Dorbala, S.; Vangala, D.; Semer, J.; Strader, C.; Bruyere, J.R.; Di Carli, M.F.; Moore, S.C.; Falk, R.H. Imaging cardiac amyloidosis: A pilot study using 18F-florbetapir positron emission tomography. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 1652–1662. [Google Scholar] [CrossRef]
- Law, W.P.; Wang, W.Y.S.; Moore, P.T.; Mollee, P.N.; Ng, A.C.T. Cardiac Amyloid Imaging with 18F-Florbetaben PET: A Pilot Study. J. Nucl. Med. 2016, 57, 1733–1739. [Google Scholar] [CrossRef]
- Dietemann, S.; Nkoulou, R. Amyloid PET imaging in cardiac amyloidosis: A pilot study using 18F-flutemetamol positron emission tomography. Ann. Nucl. Med. 2019, 33, 624–628. [Google Scholar] [CrossRef]
- Kim, Y.J.; Ha, S. Cardiac amyloidosis imaging with amyloid positron emission tomography: A systematic review and meta-analysis. J. Nucl. Cardiol. 2020, 27, 123–132. [Google Scholar] [CrossRef]
- Cohen, A.D.; Rabinovici, G.D.; Mathis, C.A.; Jagust, W.J.; Klunk, W.; Ikonomovic, M.D. Using Pittsburgh Compound B for In Vivo PET Imaging of Fibrillar Amyloid-Beta. Charact. Porous Solids III 2012, 64, 27–81. [Google Scholar] [CrossRef]
- Levine, H., III. Soluble multimeric Alzheimer beta(1-40) pre-amyloid complexes in dilute solution. Neurobiol. Aging 1995, 16, 755–764. [Google Scholar] [CrossRef]
- Dorbala, S.; Cuddy, S.; Falk, R.H. How to Image Cardiac Amyloidosis: A Practical Approach. JACC Cardiovasc. Imaging 2020, 13, 1368–1383. [Google Scholar] [CrossRef] [PubMed]
- Martínez, G.; Vernooij, R.W.; Padilla, P.F.; Zamora, J.; Cosp, X.B.; Flicker, L. 18F PET with florbetapir for the early diagnosis of Alzheimer’s disease dementia and other dementias in people with mild cognitive impairment (MCI). Cochrane Database Syst. Rev. 2017, 11, CD012216. [Google Scholar] [CrossRef] [PubMed]
- Martínez, G.; Vernooij, R.W.M.; Padilla, P.F.; Zamora, J.; Flicker, L.; Cosp, X.B. 18F PET with florbetaben for the early diagnosis of Alzheimer’s disease dementia and other dementias in people with mild cognitive impairment (MCI). Cochrane Database Syst. Rev. 2017, 11, CD012883. [Google Scholar] [CrossRef]
- Manwani, R.; Page, J.; Lane, T.; Burniston, M.; Skillen, A.; Lachmann, H.J.; Gillmore, J.D.; Fontana, M.; Whelan, C.; Hawkins, P.N.; et al. A pilot study demonstrating cardiac uptake with 18F-florbetapir PET in AL amyloidosis patients with cardiac involvement. Amyloid 2018, 25, 247–252. [Google Scholar] [CrossRef]
- Park, M.-A.; Padera, R.F.; Belanger, A.; Dubey, S.; Hwang, D.H.; Veeranna, V.; Falk, R.H.; di Carli, M.F.; Dorbala, S. 18F-Florbetapir Binds Specifically to Myocardial Light Chain and Transthyretin Amyloid Deposits: Autoradiography Study. Circ. Cardiovasc. Imaging 2015, 8. [Google Scholar] [CrossRef]
- Kircher, M.; Ihne, S.; Brumberg, J.; Morbach, C.; Knop, S.; Kortüm, K.M.; Störk, S.; Buck, A.K.; Reiter, T.; Bauer, W.R.; et al. Detection of cardiac amyloidosis with 18F-Florbetaben-PET/CT in comparison to echocardiography, cardiac MRI and DPD-scintigraphy. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 1407–1416. [Google Scholar] [CrossRef]
- Martineau, P.; Finnerty, V.; Giraldeau, G.; Authier, S.; Harel, F.; Pelletier-Galarneau, M. Examining the sensitivity of 18F-NaF PET for the imaging of cardiac amyloidosis. J. Nucl. Cardiol. 2019. [Google Scholar] [CrossRef]
| First Author | Publication Year | Radiotracer | Method | Results |
|---|---|---|---|---|
| Caobelli et al. [39] | 2020 | 99mTc-DPD | Retrospective single-center study including 13 patients with 8 ATTR cardiac amyloidosis and 5 not. | Myocardial SUVmax and SUVpeak showed strong correlation with Perugini score but a great degree of overlap between patients in Perugini score 2 and 3. |
| Scully et al. [46] | 2020 | 99mTc-DPD | Single-center, retrospective study of 100 DPD scan (40 were Perugini grade 0, 12 were grade 1, 41 were grade 2, and 7 were grade 3). | SUV retention index which is calculated as: ((Cardiac SUVpeak/Vertebral SUVpeak) × paraspinal muscle SUVpeak) increased across all Perugini grades. Cardiac SUVpeak and SUV retention index had excellent diagnostic accuracy with the area under the curve being 0.999. |
| Wollenweber et al. [47] | 2020 | 99mTc-DPD | 32 patients with bioptically-proven or suspected cardiac ATTR amyloidosis received a DPD total body bone scan with additional SPECT/CT. | Patients with Perugini grade 2 and 3 can be clearly separated from those with Perugini grade 0 and 1 with a SUVpeak cut-off of 3.1. |
| Löfbacka et al. [48] | 2020 | 99mTc-DPD | 48 patients with genetically-verified hereditary ATTR cardiac amyloidosis and positive 99mTc-DPD SPECT/CT were assessed manually for amyloid burden. | Statistically significant correlation between DPD uptake and all echocardiographic strain parameters in all regions, as well as the biomarkers of troponin and logarithmic NT-proBNP. |
| Masri et al. [52] | 2020 | 99mTc-PYP | 233 patients with suspected ATTR cardiac amyloidosis underwent planar and SPECT imaging at 1 and 3 hours with a positive scan considered as visual grades ≥ 2 and heart to contralateral ratios ≥ 1.5 | 1-hour and 3-hour protocols have identical SPECT results. Planar imaging at 1 hour had 98% sensitivity and 96% specificity. |
| Asif et al. [54] | 2020 | 99mTc-PYP | 99mTc-PYP scintigraphy was performed including 1-hour planar imaging assessing visual score as well as H/CL ratio and SPECT | Visual score had a diagnostic accuracy of 98% for ATTR cardiac amyloidosis but addition of H/CL ratio reduced the accuracy. SPECT is necessary to perform to prevent misdiagnoses. |
| Tamarappoo et al. [55] | 2020 | 99mTc-PYP/Tl-201 | Dual isotope of 99mTc-PYP/Tl-201 SPECT was performed in 112 patients suspicious of cardiac amyloidosis (39 ATTR, 26 AL, 47 no amyloidosis) and compared with single isotope. H/CL ratio was calculated. | Interobserver agreement of visual assessment was better with dual-isotope SPECT. Area under the curve for ATTR cardiac amyloidosis by visual assessment and H/CL ratio were higher with dual-isotope SPECT than single-isotope SPECT. |
| Ochi et al. [56] | 2020 | 99mTc-PYP | 39 patients with wild-type ATTR cardiac amyloidosis with 8 patients in group A who were diagnosed before the introduction of hs-cTnT and 99mTc-PYP scintigraphy and 31 patients in group B who were diagnosed after the introduction of the two tools. | Increased diagnostic yield in patients who used the combined approach using hs-cTnT and 99mTc-PYP scintigraphy. |
| Takasone et al. [57] | 2020 | 99mTc-PYP, 11C-PiB | 17 patients with AL cardiac amyloidosis, 22 patients with hereditary ATTR cardiac amyloidosis, and 8 patients with wild-type ATTR cardiac amyloidosis underwent both 11C-PiB PET imaging and 99mTc-PYP scintigraphy. | All patients with cardiac amyloidosis are detectable by 99mTc-PYP or 11C-PiB PET imaging. The combination of positive 11C-PiB PET and negative 99mTc-PYP was observed in all AL cardiac amyloidosis and early onset V30M hereditary ATTR cardiac amyloidosis, while the combination of positive 99mTc-PYP and negative 11C-PiB PET was consistent in all wild-type ATTR cardiac amyloidosis, as well as the late-onset V30M and non-V30M hereditary ATTR cardiac amyloidosis. |
| Rosengren et al. [58] | 2020 | 11C-PiB | A dual-center study included 51 subjects with 36 patients with known cardiac amyloidosis and increased wall thickness (15 AL, 21 ATTR) and 15 control patients. All the subjects underwent 11C-PiB PET imaging and echocardiography. | High diagnostic accuracy of both visual inspection and semi-quantitative methods of 11C-PiB PET imaging to distinguish cardiac amyloidosis from controls. The uptake of 11C-PiB was significantly higher in AL cardiac amyloidosis than ATTR cardiac amyloidosis. |
| Lee et al. [59] | 2020 | 11C-PiB | 41 chemotherapy-naïve AL cardiac amyloidosis patients were enrolled. Myocardial uptake of 11C-PiB on PET was compared with endomyocardial biopsy for quantification of amyloid deposit. | The degree of myocardial 11C-PiB uptake is significantly higher in patients with cardiac amyloidosis and higher degrees of uptake was associated with lowest survival from death, heart transplantation and acute decompensated heart failure. |
| Genovesi et al. [60] | 2021 | 18F-florbetaben | 40 patients with biopsy-proven diagnoses of cardiac amyloidosis (20 AL amyloidosis, 20 ATTR amyloidosis) and 20 patients with non-cardiac amyloidosis pathology. | Patients with AL amyloidosis have higher mean SUV, heart-to-background uptake ratio, and molecular volume than ATTR amyloidosis and patients with non-cardiac amyloidosis. |
| Andrews et al. [61] | 2020 | 18F-fluoride | A prospective multicenter study included 53 patients (10 ATTR and 8 AL cardiac amyloidosis, 13 controls and 22 with aortic stenosis). All patients were scanned by 18F-fluoride PET/MRI. SUV and tissue-to-background ratio (TBRmean) were obtained in the septum and areas of late gadolinium enhancement. | TBRmean values are higher in ATTR amyloidosis than controls and those with AL amyloidosis. A TBRmean threshold >1.14 in areas of late gadolinium enhancement has 100% sensitivity and 100% specificity for ATTR amyloidosis compared to AL amyloidosis. |
| Zhang et al. [62] | 2020 | 18F-sodium fluoride and 99mTc-PYP | 12 subjects with ATTR cardiac amyloidosis and 5 controls underwent 18F-sodium fluoride and 99mTc-PYP-SPECT/CT. | Visual assessment of 18F-sodium fluoride PET/CT had a sensitivity of 25% for ATTR cardiac amyloidosis when compared with 100% sensitivity in 99mTc-PYP-SPECT/CT. |
| Imaging Technique | Radiotracer Component | Radiotracer Analog | Radiotracers Original Application | Amyloidosis Type | Advantage | Disadvantage |
|---|---|---|---|---|---|---|
| 99mTc-DPD Scintigraphy | 99mTc-3,3-diphosphono-1,2-propanodicarboxylic acid | Phosphate | Bone scintigraphy | ATTR amyloidosis >> AL amyloidosis. | High diagnostic accuracy for ATTR when combined with SPECT and the absence of a monoclonal protein in serum or urine. | Limited on accurate quantification of amyloid burden. |
| 99mTc-PYP Scintigraphy | 99mTc-Pyrophosphate | |||||
| 11C-PiB PET imaging | N-methyl-[11C]2-(4′-methylaminophenyl)-6-hydroxybenzothiazole | Thioflavin-T | Brain imaging in Alzheimer dementia. | AL amyloidosis > ATTR amyloidosis. | Detect both AL and ATTR amyloidosis, ability to detect early disease, short study session. Can complement 99mTc-PYP Scintigraphy. | Requirement of onsite cyclotron for generation; high synthesis cost with 20-min half-life. |
| 18F-labelled agents PET imaging | 18F-florbetapir, 18F-florbetaben18F-NaF | Stilbene | Can diagnose both AL amyloidosis and ATTR amyloidosis. Allows for early detection of cardiac amyloidosis, aid in therapy response assessment. | Lack of large-sized studies to confirm its efficacy. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; Uppal, D.; Wang, Y.C.; Xu, X.; Kokkinidis, D.G.; Travin, M.I.; Tauras, J.M. Nuclear Imaging for the Diagnosis of Cardiac Amyloidosis in 2021. Diagnostics 2021, 11, 996. https://doi.org/10.3390/diagnostics11060996
Li W, Uppal D, Wang YC, Xu X, Kokkinidis DG, Travin MI, Tauras JM. Nuclear Imaging for the Diagnosis of Cardiac Amyloidosis in 2021. Diagnostics. 2021; 11(6):996. https://doi.org/10.3390/diagnostics11060996
Chicago/Turabian StyleLi, Weijia, Dipan Uppal, Yu Chiang Wang, Xiaobo Xu, Damianos G. Kokkinidis, Mark I. Travin, and James M. Tauras. 2021. "Nuclear Imaging for the Diagnosis of Cardiac Amyloidosis in 2021" Diagnostics 11, no. 6: 996. https://doi.org/10.3390/diagnostics11060996
APA StyleLi, W., Uppal, D., Wang, Y. C., Xu, X., Kokkinidis, D. G., Travin, M. I., & Tauras, J. M. (2021). Nuclear Imaging for the Diagnosis of Cardiac Amyloidosis in 2021. Diagnostics, 11(6), 996. https://doi.org/10.3390/diagnostics11060996






