Effects of Anti-Inflammatory Treatment and Surgical Intervention on Endothelial Glycocalyx, Peripheral and Coronary Microcirculatory Function and Myocardial Deformation in Inflammatory Bowel Disease Patients: A Two-Arms Two-Stage Clinical Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Protocol
- at baseline and
- 4 months after intervention, whereby intervention is herein specifically defined as surgical intervention (35 patients, designated as group A) or pharmaceutical treatment (systemic inflammatory inhibitor (anti-TNFa) for 25 patients, designated as group B).
- Endoscopic and histologic disease confirmation at least 6 months before admission.
- Uncontrolled inflammatory status, clinically (elevated Mayo—HBI scores) and biochemically (white blood cells (WBC)–c-reactive protein (CRP) values), with frequent recurrences in their classic treatment (salicylates, antibiotics, corticosteroids) or immunomodulatory treatment (methotrexate, azathioprine). Subjects in a stable or improving clinical state were excluded.
- Patients in both groups who had not received, for at least 6 months before admission, any anti-TNFa (tumour necrosis factor alpha inhibitor) or anti-IL (anti-interleukin) agent, with clinical worsening. For this reason, they needed to undergo a new systemic anti-inflammatory medical treatment (group B) in order to eliminate the disease burden or a local surgical approach (group A) because of major intestinal complications such as bowel obstruction, abscesses or fistulas.
- No history of established or first diagnosed—during the baseline visit—cardiovascular disease (CVD) or CV risk factors (diabetes mellitus, dyslipidaemia, arterial hypertension, smoking, family history).
2.2. Measurements
2.2.1. Vascular Endothelium Assessment
2.2.2. Echocardiography Measurements
2.2.3. Laboratory Assays
2.3. Primary and Secondary Endpoints
2.4. Statistical Methodology
3. Results
3.1. Study Population
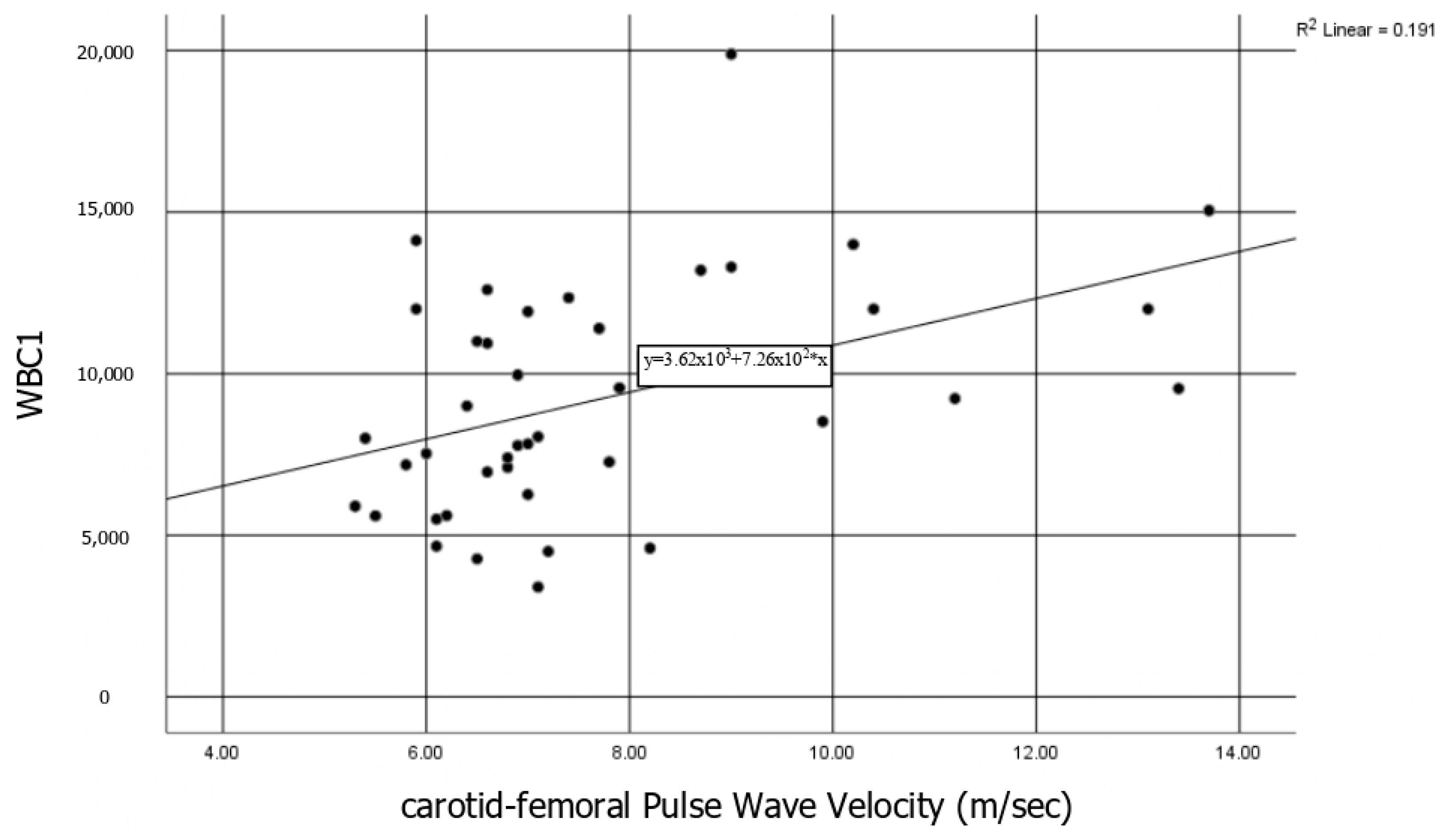
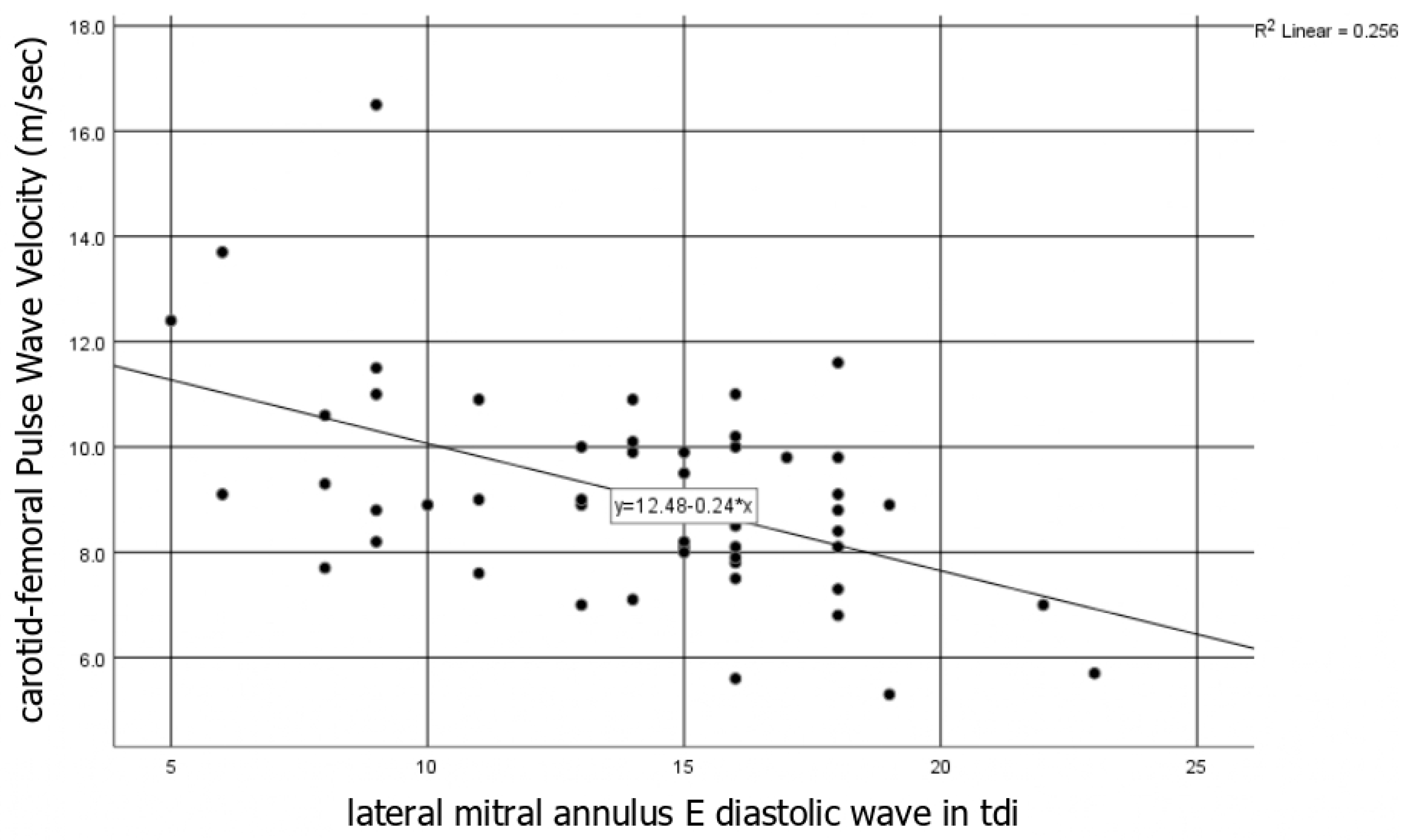
3.1.1. Vascular Markers and Association with the Disease Activity and the Biomarkers
3.1.2. Cardiac Markers and Association with the Disease Activity and the Biomarkers
3.1.3. Interrelation between Vascular and Cardiac Markers
3.2. Effects of Surgical Intervention (Group A) and Pharmaceutical Treatment (Group B)
3.2.1. Vascular Markers and Association with the Disease Activity and the Biomarkers
3.2.2. Cardiac Markers and Association with the Disease Activity and the Biomarkers
4. Discussion
4.1. Baseline Characteristics
4.1.1. Baseline Vascular Markers Analysis
4.1.2. Baseline Cardiac Markers Analysis
4.1.3. Analysis of Overall Baseline Results
4.2. Post-Intervention Analysis
4.2.1. Post-Intervention Vascular Markers Analysis
4.2.2. Post-Intervention Cardiac Markers Analysis
4.2.3. Group Analysis
4.2.4. Analysis of Post-Intervention Results
4.3. Additional Remarks for Potential Consideration in Future Research
4.4. Summary
4.5. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABTS | 2, 2′-Azino-Bis-3-Ethylbenzothiazoline-6-Sulfonic Acid |
| AI | augmentation index |
| anti-IL | anti-interleukin |
| anti-IL1 | anti-interleukin-1 |
| anti-IL12 | anti-interleukin-12 |
| anti-IL6 | anti-interleukin-6 |
| anti-TNFa | tumour necrosis factor alpha inhibitor |
| CD | Crohn’s disease |
| cfPWV | carotid-femoral pulse wave velocity |
| CFR | coronary flow reserve |
| CFRv | coronary flow reserve velocity |
| CFRvti | coronary flow reserve velocity-time integral |
| cIMT | carotid intima-media thickness |
| CIs | confidence intervals |
| CRP | C-reactive protein |
| cSBP | central systolic blood pressure |
| CV | cardiovascular |
| CVD | cardiovascular disease |
| CRP | c-reactive protein |
| Ea | arterial elastance |
| ECG | electrocardiogram |
| Ees | end-systolic elastance |
| eNOS | endothelial nitric oxide synthase |
| FMD | flow-mediated vasodilatation |
| GcircS | global circumferential strain |
| GLS | global longitudinal strain |
| GLSR | global longitudinal strain rate |
| HBI | Harvey-Bradshaw Index |
| IBD | inflammatory bowel diseases |
| IFN-γ | gamma-interferon |
| IgG | Immunoglobulin G |
| IL-12 | interleukin-12 |
| IL-18 | interleukin-18 |
| IL-1β | interleukin-1β |
| IL-23 | interleukin-23 |
| L4chS | longitudinal four chambers strain |
| LAD | left anterior descending |
| LV | left ventricle |
| LVEF | left ventricular ejection fraction |
| MAdCAM-1 | Mucosal vascular addressin cell adhesion molecule 1 |
| MDA | malondialdehyde |
| MI | myocardial infarction |
| NO | nitric oxide |
| P1 | early forward systolic wave |
| P2 | late backward systolic wave |
| PBR | perfused boundary region |
| PP | pulse pressure |
| pTw | peak twisting |
| pTwVel | peak twisting velocity |
| pUtwVel | peak untwisting velocity |
| PWV | pulse wave velocity |
| RP | reducing power |
| RV | right ventricle |
| SCBP | systolic central blood pressure |
| SD | standard deviation |
| TAC | total antioxidant capacity |
| tdi | tissue doppler imaging |
| TBARS | thiobarbituric acid reactive substances |
| TNFa | tumour necrosis factor alpha |
| TNF | tumour necrosis factor |
| UC | ulcerative colitis |
| UtwMVO | untwisting velocity at the time of mitral valve opening |
| UtwPEF | untwisting velocity at the time of peak mitral E wave |
| VTI | velocity-time integral |
| WBC | white blood cells |
References
- Cromer, W.E.; Mathis, J.M.; Granger, D.N.; Chaitanya, G.V.; Alexander, J.S. Role of the endothelium in inflammatory bowel diseases. World J. Gastroenterol. 2011, 17, 578–593. [Google Scholar] [CrossRef]
- Zanoli, L.; Rastelli, S.; Inserra, G.; Castellino, P. Arterial structure and function in inflammatory bowel disease. World J. Gastroenterol. 2015, 21, 11304–11311. [Google Scholar] [CrossRef] [PubMed]
- Aslan, A.N.; Sarı, C.; Özer Sarı, S.; Tayfur Yürekli, Ö.; Baştuğ, S.; Sivri, S.; Ersoy, O.; Bozkurt, E. Association between aortic stiffness and left ventricular function in inflammatory bowel disease. Cardiol. J. 2016, 23, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Caliskan, Z.; Keles, N.; Gokturk, H.S.; Ozdil, K.; Aksu, F.; Ozturk, O.; Kahraman, R.; Kostek, O.; Tekin, A.S.; Ozgur, G.T.; et al. Is activation in inflammatory bowel diseases associated with further impairment of coronary microcirculation? Int. J. Cardiol. 2016, 223, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Cibor, D.; Domagala-Rodacka, R.; Rodacki, T.; Jurczyszyn, A.; Mach, T.; Owczarek, D. Endothelial dysfunction in inflammatory bowel diseases: Pathogenesis, assessment and implications. World J. Gastroenterol. 2016, 22, 1067–1077. [Google Scholar] [CrossRef]
- Kivrak, T.; Sunbul, M.; Cincin, A.; Kani, T.; Durmus, E.; Banzragch, M.; Bozbay, M.; Aydin, Y.; Imeryuz, N.; Sari, I.; et al. Two-dimensional speckle tracking echocardiography is useful in early detection of left ventricular impairment in patients with Crohn’s disease. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 3249–3254. Available online: https://www.europeanreview.org/article/11241 (accessed on 22 April 2021). [PubMed]
- Triantafyllou, C.; Nikolaou, M.; Ikonomidis, I.; Bamias, G.; Papaconstantinou, I. Endothelial and Cardiac Dysfunction in Inflammatory Bowel Diseases: Does Treatment Modify the Inflammatory Load on Arterial and Cardiac Structure and Function? Curr. Vasc. Pharmacol. 2020, 18, 27–37. [Google Scholar] [CrossRef]
- Akdoğan, R.A.; Durakoğlugil, M.E.; Kocaman, S.A.; Çiçek, Y.; Durakoğlugil, T.; Ergül, E.; Rakıcı, H. Increased pulse wave velocity and carotid intima-media thickness in patients with ulcerative colitis. Dig. Dis. Sci. 2013, 58, 2293–2300. [Google Scholar] [CrossRef]
- Aytaç, E.; Büyüktaş, D.; Baysal, B.; Atar, M.; Yıldız, M.; Baca, B.; Karahasanoğlu, T.; Çelik, A.; Seymen, H.O.; Hamzaoğlu, İ.; et al. Visual evoked potentials and pulse wave velocity in inflammatory bowel disease. Turk. J. Gastroenterol. Off. J. Turk. Soc. Gastroenterol. 2015, 26, 15–19. [Google Scholar] [CrossRef]
- Ozturk, K.; Guler, A.K.; Cakir, M.; Ozen, A.; Demirci, H.; Turker, T.; Demirbas, S.; Uygun, A.; Gulsen, M.; Bagci, S.; et al. Pulse Wave Velocity, Intima Media Thickness, and Flow-mediated Dilatation in Patients with Normotensive Normoglycemic Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2015, 21, 1314–1320. [Google Scholar] [CrossRef]
- Papa, A.; Santoliquido, A.; Danese, S.; Covino, M.; Di Campli, C.; Urgesi, R.; Grillo, A.; Guglielmo, S.; Tondi, P.; Guidi, L.; et al. Increased carotid intima-media thickness in patients with inflammatory bowel disease. Aliment. Pharmacol. Ther. 2005, 22, 839–846. [Google Scholar] [CrossRef] [PubMed]
- Principi, M.; Mastrolonardo, M.; Scicchitano, P.; Gesualdo, M.; Sassara, M.; Guida, P.; Bucci, A.; Zito, A.; Caputo, P.; Albano, F.; et al. Endothelial function and cardiovascular risk in active inflammatory bowel diseases. J. Crohn’s Colitis 2013, 7, e427–e433. [Google Scholar] [CrossRef] [PubMed]
- Principi, M.; Montenegro, L.; Losurdo, G.; Zito, A.; Devito, F.; Bulzis, G.; Carbonara, R.; Ierardi, E.; Di Leo, A.; Ciccone, M.M.; et al. Endothelial function and cardiovascular risk in patients with inflammatory bowel disease in remission phase. Scand. J. Gastroenterol. 2016, 51, 253–255. [Google Scholar] [CrossRef]
- Theocharidou, E.; Gossios, T.D.; Griva, T.; Giouleme, O.; Douma, S.; Athyros, V.G.; Karagiannis, A. Is there an association between inflammatory bowel diseases and carotid intima-media thickness? Prelim. Data. Angiol. 2014, 65, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Altorjay, I.; Veréb, Z.; Serfozo, Z.; Bacskai, I.; Bátori, R.; Erdodi, F.; Udvardy, M.; Sipka, S.; Lányi, Á.; Rajnavölgyi, É.; et al. Anti-TNF-alpha antibody (infliximab) therapy supports the recovery of eNOS and VEGFR2 protein expression in endothelial cells. Int. J. Immunopathol. Pharmacol. 2011, 24, 323–335. [Google Scholar] [CrossRef]
- Schinzari, F.; Armuzzi, A.; De Pascalis, B.; Mores, N.; Tesauro, M.; Melina, D.; Cardillo, C. Tumor necrosis factor-alpha antagonism improves endothelial dysfunction in patients with Crohn’s disease. Clin. Pharmacol. Ther. 2008, 83, 70–76. [Google Scholar] [CrossRef]
- Zanoli, L.; Rastelli, S.; Inserra, G.; Lentini, P.; Valvo, E.; Calcagno, E.; Boutouyrie, P.; Laurent, S.; Castellino, P. Increased arterial stiffness in inflammatory bowel diseases is dependent upon inflammation and reduced by immunomodulatory drugs. Atherosclerosis 2014, 234, 346–351. [Google Scholar] [CrossRef]
- Rutella, S.; Fiorino, G.; Vetrano, S.; Correale, C.; Spinelli, A.; Pagano, N.; Arena, V.; Maggiano, N.; Repici, A.; Malesci, A.; et al. Infliximab therapy inhibits inflammation-induced angiogenesis in the mucosa of patients with Crohn’s disease. Am. J. Gastroenterol. 2011, 106, 762–770. [Google Scholar] [CrossRef] [PubMed]
- Harvey, R.F.; Bradshaw, J.M. A simple index of Crohn’s-disease activity. Lancet 1980, 1, 514. [Google Scholar] [CrossRef]
- Lewis, J.D.; Chuai, S.; Nessel, L.; Lichtenstein, G.R.; Aberra, F.N.; Ellenberg, J.H. Use of the noninvasive components of the Mayo score to assess clinical response in ulcerative colitis. Inflamm. Bowel Dis. 2008, 14, 1660–1666. [Google Scholar] [CrossRef]
- Peyrin-Biroulet, L.; Panés, J.; Sandborn, W.J.; Vermeire, S.; Danese, S.; Feagan, B.G.; Colombel, J.F.; Hanauer, S.B.; Rycroft, B. Defining Disease Severity in Inflammatory Bowel Diseases: Current and Future Directions. Clin. Gastroenterol. Hepatol. 2016, 14, 348–354.e17. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, K.W.; Tremaine, W.J.; Ilstrup, D.M. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N. Engl. J. Med. 1987, 317, 1625–1629. [Google Scholar] [CrossRef] [PubMed]
- Loftus, E.V.; Reinisch, W.; Panaccione, R.; Berg, S.; Alperovich, G.; Bereswill, M.; Kalabic, J.; Petersson, J.; Thakkar, R.; Robinson, A.M.; et al. Adalimumab Effectiveness Up to Six Years in Adalimumab-naïve Patients with Crohn’s Disease: Results of the PYRAMID Registry. Inflamm. Bowel Dis. 2019, 25, 1522–1531. [Google Scholar] [CrossRef]
- Papamichael, K.; Lin, S.; Moore, M.; Papaioannou, G.; Sattler, L.; Cheifetz, A.S. Infliximab in inflammatory bowel disease. Ther. Adv. Chronic Dis. 2019. [Google Scholar] [CrossRef]
- Tursi, A.; Elisei, W.; Brandimarte, G.; Giorgetti, G.; Penna, A.; Castrignano, V. Safety and effectiveness of infliximab for inflammatory bowel diseases in clinical practice. Eur. Rev. Med Pharmacol. Sci. 2010, 14, 47–55. Available online: https://pubmed.ncbi.nlm.nih.gov/20184089/ (accessed on 22 April 2021).
- Wasan, S.K.; Kane, S.V. Adalimumab for the treatment of inflammatory bowel disease. Expert Rev. Gastroenterol. Hepatol. 2011, 5, 679–684. [Google Scholar] [CrossRef] [PubMed]
- Mease, P.J. Adalimumab in the treatment of arthritis. Ther. Clin. Risk Manag. 2007, 3, 133–148. [Google Scholar] [CrossRef]
- Kirman, I.; Whelan, R.L.; Nielsen, O.H. Infliximab: Mechanism of action beyond TNF-alpha neutralization in inflammatory bowel disease. Eur. J. Gastroenterol. Hepatol. 2004, 16, 639–641. [Google Scholar] [CrossRef]
- Danese, S. Mechanisms of action of infliximab in inflammatory bowel disease: An anti-inflammatory multitasker. Dig. Liver Dis. 2008, 40 (Suppl. 2), S225–S2258. [Google Scholar] [CrossRef]
- Alkan, E.; Karakaş, M.S.; Yıldırım, B. Evaluation of increased subclinical atherosclerosis risk with carotid intima-media thickness and pulse wave velocity in inflamatory bowel disease. Off. J. Turk. Soc. Gastroenterol. 2014, 25 (Suppl. 1), 20–25. [Google Scholar] [CrossRef]
- Lekakis, J.; Abraham, P.; Balbarini, A.; Blann, A.; Boulanger, C.M.; Cockcroft, J.; Cosentino, F.; Deanfield, J.; Gallino, A.; Ikonomidis, I.; et al. Methods for evaluating endothelial function: A position statement from the European Society of Cardiology Working Group on Peripheral Circulation. Eur. J. Cardiovasc. Prev. Rehabil. 2011, 18, 775–789. [Google Scholar] [CrossRef]
- Steyers, C.M., 3rd; Miller, F.J., Jr. Endothelial dysfunction in chronic inflammatory diseases. Int. J. Mol. Sci. 2014, 15, 11324–11349. [Google Scholar] [CrossRef]
- Vlachopoulos, C.; Xaplanteris, P.; Aboyans, V.; Brodmann, M.; Cífková, R.; Cosentino, F.; De Carlo, M.; Gallino, A.; Landmesser, U.; Laurent, S.; et al. The role of vascular biomarkers for primary and secondary prevention. A position paper from the European Society of Cardiology Working Group on peripheral circulation: Endorsed by the Association for Research into Arterial Structure and Physiology (ARTERY) Society. Atherosclerosis 2015, 241, 507–532. [Google Scholar] [CrossRef]
- Zanoli, L.; Boutouyrie, P.; Fatuzzo, P.; Granata, A.; Lentini, P.; Oztürk, K.; Cappello, M.; Theocharidou, E.; Tuttolomondo, A.; Pinto, A.; et al. Inflammation and Aortic Stiffness: An Individual Participant Data Meta-Analysis in Patients with Inflammatory Bowel Disease. J. Am. Heart Assoc. 2017, 6. [Google Scholar] [CrossRef]
- Zanoli, L.; Cannavò, M.; Rastelli, S.; Di Pino, L.; Monte, I.; Di Gangi, M.; Boutouyrie, P.; Inserra, G.; Laurent, S.; Castellino, P.; et al. Arterial stiffness is increased in patients with inflammatory bowel disease. J. Hypertens. 2012, 30, 1775–1781. [Google Scholar] [CrossRef]
- Corretti, M.C.; Anderson, T.J.; Benjamin, E.J.; Celermajer, D.; Charbonneau, F.; Creager, M.A.; Deanfield, J.; Drexler, H.; Gerhard-Herman, M.; Herrington, D.; et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: A report of the International Brachial Artery Reactivity Task Force. J. Am. Coll. Cardiol. 2002, 39, 257–265. [Google Scholar] [CrossRef]
- Deanfield, J.; Donald, A.; Ferri, C.; Giannattasio, C.; Halcox, J.; Halligan, S.; Lerman, A.; Mancia, G.; Oliver, J.J.; Pessina, A.C.; et al. Endothelial function and dysfunction. Part I: Methodological issues for assessment in the different vascular beds: A statement by the Working Group on Endothelin and Endothelial Factors of the European Society of Hypertension. J. Hypertens. 2005, 23, 7–17. [Google Scholar] [CrossRef]
- Ikonomidis, I.; Tzortzis, S.; Andreadou, I.; Paraskevaidis, I.; Katseli, C.; Katsimbri, P.; Pavlidis, G.; Parissis, J.; Kremastinos, D.; Anastasiou-Nana, M.; et al. Increased benefit of interleukin-1 inhibition on vascular function, myocardial deformation, and twisting in patients with coronary artery disease and coexisting rheumatoid arthritis. Circ. Cardiovasc. Imaging 2014, 7, 619–628. [Google Scholar] [CrossRef]
- Becker, B.F.; Chappell, D.; Bruegger, D.; Annecke, T.; Jacob, M. Therapeutic strategies targeting the endothelial glycocalyx: Acute deficits, but great potential. Cardiovasc. Res. 2010, 87, 300–310. [Google Scholar] [CrossRef]
- Becker, B.F.; Chappell, D.; Jacob, M. Endothelial glycocalyx and coronary vascular permeability: The fringe benefit. Basic Res. Cardiol. 2010, 105, 687–701. [Google Scholar] [CrossRef]
- Broekhuizen, L.N.; Mooij, H.L.; Kastelein, J.J.; Stroes, E.S.; Vink, H.; Nieuwdorp, M. Endothelial glycocalyx as potential diagnostic and therapeutic target in cardiovascular disease. Curr. Opin. Lipidol. 2009, 20, 57–62. [Google Scholar] [CrossRef]
- Chappell, D.; Westphal, M.; Jacob, M. The impact of the glycocalyx on microcirculatory oxygen distribution in critical illness. Curr. Opin. Anaesthesiol. 2009, 22, 155–162. [Google Scholar] [CrossRef]
- Ikonomidis, I.; Pavlidis, G.; Lambadiari, V.; Kousathana, F.; Varoudi, M.; Spanoudi, F.; Maratou, E.; Parissis, J.; Triantafyllidi, H.; Dimitriadis, G.; et al. Early detection of left ventricular dysfunction in first-degree relatives of diabetic patients by myocardial deformation imaging: The role of endothelial glycocalyx damage. Int. J. Cardiol. 2017, 233, 105–112. [Google Scholar] [CrossRef]
- Ikonomidis, I.; Voumvourakis, A.; Makavos, G.; Triantafyllidi, H.; Pavlidis, G.; Katogiannis, K.; Benas, D.; Vlastos, D.; Trivilou, P.; Varoudi, M.; et al. Association of impaired endothelial glycocalyx with arterial stiffness, coronary microcirculatory dysfunction, and abnormal myocardial deformation in untreated hypertensives. J. Clin. Hypertens. 2018, 20, 672–679. [Google Scholar] [CrossRef]
- Kolářová, H.; Ambrůzová, B.; Svihálková Šindlerová, L.; Klinke, A.; Kubala, L. Modulation of endothelial glycocalyx structure under inflammatory conditions. Mediat. Inflamm. 2014, 2014, 694312. [Google Scholar] [CrossRef]
- Lipowsky, H.H. Protease Activity and the Role of the Endothelial Glycocalyx in Inflammation. Drug Discov. Today Dis. Models 2011, 8, 57–62. [Google Scholar] [CrossRef]
- Mulivor, A.W.; Lipowsky, H.H. Inflammation- and ischemia-induced shedding of venular glycocalyx. Am. J. Physiol. Heart Circ. Physiol. 2004, 286, H1672–H1680. [Google Scholar] [CrossRef]
- van Golen, R.F.; van Gulik, T.M.; Heger, M. Mechanistic overview of reactive species-induced degradation of the endothelial glycocalyx during hepatic ischemia/reperfusion injury. Free Radic. Biol. Med. 2012, 52, 1382–1402. [Google Scholar] [CrossRef] [PubMed]
- Ikonomidis, I.; Aboyans, V.; Blacher, J.; Brodmann, M.; Brutsaert, D.L.; Chirinos, J.A.; De Carlo, M.; Delgado, V.; Lancellotti, P.; Lekakis, J.; et al. The role of ventricular-arterial coupling in cardiac disease and heart failure: Assessment, clinical implications and therapeutic interventions. A consensus document of the European Society of Cardiology Working Group on Aorta & Peripheral Vascular Diseases, European Association of Cardiovascular Imaging, and Heart Failure Association. Eur. J. Heart Fail. 2019, 21, 402–424. [Google Scholar] [CrossRef] [PubMed]
- Ikonomidis, I.; Katsanos, S.; Triantafyllidi, H.; Parissis, J.; Tzortzis, S.; Pavlidis, G.; Trivilou, P.; Makavos, G.; Varoudi, M.; Frogoudaki, A.; et al. Pulse wave velocity to global longitudinal strain ratio in hypertension. Eur. J. Clin. Investig. 2019, 49, e13049. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, T.; Dulgheru, R.; Bernard, A.; Ilardi, F.; Contu, L.; Addetia, K.; Caballero, L.; Akhaladze, N.; Athanassopoulos, G.D.; Barone, D.; et al. Echocardiographic reference ranges for normal left ventricular 2D strain: Results from the EACVI NORRE study. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 833–840. [Google Scholar] [CrossRef] [PubMed]
- Cortigiani, L.; Rigo, F.; Gherardi, S.; Bovenzi, F.; Picano, E.; Sicari, R. Implication of the continuous prognostic spectrum of Doppler echocardiographic derived coronary flow reserve on left anterior descending artery. Am. J. Cardiol. 2010, 105, 158–162. [Google Scholar] [CrossRef]
- Ikonomidis, I.; Lambadiari, V.; Pavlidis, G.; Koukoulis, C.; Kousathana, F.; Varoudi, M.; Spanoudi, F.; Maratou, E.; Parissis, J.; Triantafyllidi, H.; et al. Insulin resistance and acute glucose changes determine arterial elastic properties and coronary flow reserve in dysglycaemic and first-degree relatives of diabetic patients. Atherosclerosis 2015, 241, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Rigo, F. Coronary flow reserve in stress-echo lab. From pathophysiologic toy to diagnostic tool. Cardiovasc. Ultrasound 2005, 3, 8. [Google Scholar] [CrossRef]
- Tzortzis, S.; Ikonomidis, I.; Lekakis, J.; Papadopoulos, C.; Triantafyllidi, H.; Parissis, J.; Trivilou, P.; Paraskevaidis, I.; Anastasiou-Nana, M.; Kremastinos, D.T.; et al. Incremental predictive value of carotid intima-media thickness to arterial stiffness for impaired coronary flow reserve in untreated hypertensives. Hypertens. Res. Off. J. Jpn. Soc. Hypertens. 2010, 33, 367–373. [Google Scholar] [CrossRef][Green Version]
- Bouzid, D.; Gargouri, B.; Mansour, R.B.; Amouri, A.; Tahri, N.; Lassoued, S.; Masmoudi, H. Oxidative stress markers in intestinal mucosa of Tunisian inflammatory bowel disease patients. Saudi J. Gastroenterol. Off. J. Saudi Gastroenterol. Assoc. 2013, 19, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Choghakhori, R.; Abbasnezhad, A.; Hasanvand, A.; Amani, R. Inflammatory cytokines and oxidative stress biomarkers in irritable bowel syndrome: Association with digestive symptoms and quality of life. Cytokine 2017, 93, 34–43. [Google Scholar] [CrossRef]
- Ikonomidis, I.; Michalakeas, C.A.; Lekakis, J.; Paraskevaidis, I.; Kremastinos, D.T. Multimarker approach in cardiovascular risk prediction. Dis. Markers 2009, 26, 273–285. [Google Scholar] [CrossRef]
- Sengul Samanci, N.; Poturoglu, S.; Samanci, C.; Ustabasioglu, F.E.; Koldas, M.; Duman, A.E.; Ormeci, A.C. The Relationship between Ocular Vascular Changes and the Levels of Malondialdehyde and Vascular Endothelial Growth Factor in Patients with Inflammatory Bowel Disease. Ocul. Immunol. Inflamm. 2020, 1–5. [Google Scholar] [CrossRef]
- Tüzün, A.; Erdil, A.; Inal, V.; Aydin, A.; Bağci, S.; Yeşilova, Z.; Sayal, A.; Karaeren, N.; Dağalp, K. Oxidative stress and antioxidant capacity in patients with inflammatory bowel disease. Clin. Biochem. 2002, 35, 569–572. [Google Scholar] [CrossRef]
- Rezaie, A.; Parker, R.D.; Abdollahi, M. Oxidative stress and pathogenesis of inflammatory bowel disease: An epiphenomenon or the cause? Dig. Dis. Sci. 2007, 52, 2015–2021. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, C.N.; Wajda, A.; Blanchard, J.F. The incidence of arterial thromboembolic diseases in inflammatory bowel disease: A population-based study. Clin. Gastroenterol. Hepatol. 2008, 6, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Caliskan, Z.; Keles, N.; Kahraman, R.; Özdil, K.; Karagoz, V.; Aksu, F.; Aciksari, G.; Yilmaz, Y.; Kul, S.; Caliskan, M.; et al. Imparied retrobulbar blood flow and increased carotid IMT in patients with Crohn’s disease. Int. J. Cardiovasc. Imaging 2016, 32, 1617–1623. [Google Scholar] [CrossRef]
- Cappello, M.; Licata, A.; Calvaruso, V.; Bravatà, I.; Aiello, A.; Torres, D.; Della Corte, V.; Tuttolomondo, A.; Perticone, M.; Licata, G.; et al. Increased expression of markers of early atherosclerosis in patients with inflammatory bowel disease. Eur. J. Intern. Med. 2017, 37, 83–89. [Google Scholar] [CrossRef]
- Gomollón, F.; Dignass, A.; Annese, V.; Tilg, H.; Van Assche, G.; Lindsay, J.O.; Peyrin-Biroulet, L.; Cullen, G.J.; Daperno, M.; Kucharzik, T.; et al. 3rd European Evidence-based Consensus on the Diagnosis and Management of Crohn’s Disease 2016: Part 1: Diagnosis and Medical Management. J. Crohn’s Colitis 2017, 11, 3–25. [Google Scholar] [CrossRef] [PubMed]
- Haapamäki, J.; Roine, R.P.; Turunen, U.; Färkkilä, M.A.; Arkkila, P.E. Increased risk for coronary heart disease, asthma, and connective tissue diseases in inflammatory bowel disease. J. Crohn’s Colitis 2011, 5, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, S.L.; Ahlehoff, O.; Lindhardsen, J.; Erichsen, R.; Jensen, G.V.; Torp-Pedersen, C.; Nielsen, O.H.; Gislason, G.H.; Hansen, P.R. Disease activity in inflammatory bowel disease is associated with increased risk of myocardial infarction, stroke and cardiovascular death—A Danish nationwide cohort study. PLoS ONE 2013, 8, e56944. [Google Scholar] [CrossRef]
- Kristensen, S.L.; Ahlehoff, O.; Lindhardsen, J.; Erichsen, R.; Lamberts, M.; Khalid, U.; Nielsen, O.H.; Torp-Pedersen, C.; Gislason, G.H.; Hansen, P.R.; et al. Inflammatory bowel disease is associated with an increased risk of hospitalization for heart failure: A Danish Nationwide Cohort study. Circ. Heart Fail. 2014, 7, 717–722. [Google Scholar] [CrossRef] [PubMed]
- Prijić, R.; Premužić, V.; Brinar, M.; Krznarić, Ž.; Jelaković, B.; Čuković-Čavka, S. Increased arterial stiffness-similar findings in patients with inflammatory bowel disease without prior hypertension or diabetes and in patients with well-controlled hypertension. Blood Press. 2018, 27, 240–246. [Google Scholar] [CrossRef]
- Roifman, I.; Sun, Y.C.; Fedwick, J.P.; Panaccione, R.; Buret, A.G.; Liu, H.; Rostom, A.; Anderson, T.J.; Beck, P.L. Evidence of endothelial dysfunction in patients with inflammatory bowel disease. Clin. Gastroenterol. Hepatol. 2009, 7, 175–182. [Google Scholar] [CrossRef]
- Yarur, A.J.; Deshpande, A.R.; Pechman, D.M.; Tamariz, L.; Abreu, M.T.; Sussman, D.A. Inflammatory bowel disease is associated with an increased incidence of cardiovascular events. Am. J. Gastroenterol. 2011, 106, 741–747. [Google Scholar] [CrossRef]
- Harbord, M.; Eliakim, R.; Bettenworth, D.; Karmiris, K.; Katsanos, K.; Kopylov, U. Third European Evidence-based Consensus on Diagnosis and Management of Ulcerative Colitis. Part 2: Current Management. J. Crohn’s Colitis 2017, 11, 769–784. [Google Scholar] [CrossRef]
- Mattace-Raco, F.; Hofman, A.; Verwoert, G.C.; Wittemana, J.C.; Wilkinson, I.; Cockcroft, J.; McEniery, C.; Yasmin; Laurent, S.; Boutouyrie, P.; et al. Determinants of pulse wave velocity in healthy people and in the presence of cardiovascular risk factors: ‘establishing normal and reference values’. Eur. Heart J. 2010, 31, 2338–2350. [Google Scholar] [CrossRef]
- Ikonomidis, I.; Makavos, G.; Lekakis, J. Arterial stiffness and coronary artery disease. Curr. Opin. Cardiol. 2015, 30, 422–431. [Google Scholar] [CrossRef]
- Pereira, T.; Correia, C.; Cardoso, J. Novel Methods for Pulse Wave Velocity Measurement. J. Med. Biol. Eng. 2015, 35, 555–565. [Google Scholar] [CrossRef] [PubMed]
- Lambadiari, V.; Pavlidis, G.; Kousathana, F.; Maratou, E.; Georgiou, D.; Andreadou, I.; Kountouri, A.; Varoudi, M.; Balampanis, K.; Parissis, J.; et al. Effects of Different Antidiabetic Medications on Endothelial Glycocalyx, Myocardial Function, and Vascular Function in Type 2 Diabetic Patients: One Year Follow-Up Study. J. Clin. Med. 2019, 8, 983. [Google Scholar] [CrossRef] [PubMed]
- Reindl, M.; Tiller, C.; Holzknecht, M.; Lechner, I.; Beck, A.; Plappert, D.; Gorzala, M.; Pamminger, M.; Mayr, A.; Klug, G.; et al. Prognostic Implications of Global Longitudinal Strain by Feature-Tracking Cardiac Magnetic Resonance in ST-Elevation Myocardial Infarction. Circ. Cardiovasc. Imaging 2019, 12, e009404. [Google Scholar] [CrossRef] [PubMed]
- Smiseth, O.A.; Torp, H.; Opdahl, A.; Haugaa, K.H.; Urheim, S. Myocardial strain imaging: How useful is it in clinical decision making? Eur. Heart J. 2016, 37, 1196–1207. [Google Scholar] [CrossRef]
- Ikonomidis, I.; Makavos, G.; Papadavid, E.; Varoudi, M.; Andreadou, I.; Gravanis, K.; Theodoropoulos, K.; Pavlidis, G.; Triantafyllidi, H.; Parissis, J.; et al. Similarities in coronary function and myocardial deformation between psoriasis and coronary artery disease: The role of oxidative stress and inflammation. Can. J. Cardiol. 2015, 31, 287–295. [Google Scholar] [CrossRef]
- Ikonomidis, I.; Papadavid, E.; Makavos, G.; Andreadou, I.; Varoudi, M.; Gravanis, K.; Theodoropoulos, K.; Pavlidis, G.; Triantafyllidi, H.; Moutsatsou, P.; et al. Lowering Interleukin-12 Activity Improves Myocardial and Vascular Function Compared with Tumor Necrosis Factor—A Antagonism or Cyclosporine in Psoriasis. Circ. Cardiovasc. Imaging 2017, 10. [Google Scholar] [CrossRef]
- Makavos, G.; Ikonomidis, I.; Andreadou, I.; Varoudi, M.; Kapniari, I.; Loukeri, E.; Theodoropoulos, K.; Pavlidis, G.; Triantafyllidi, H.; Thymis, J.; et al. Effects of Interleukin 17A Inhibition on Myocardial Deformation and Vascular Function in Psoriasis. Can. J. Cardiol. 2020, 36, 100–111. [Google Scholar] [CrossRef]
- Dimitroulas, T.; Hodson, J.; Sandoo, A.; Smith, J.; Kitas, G.D. Endothelial injury in rheumatoid arthritis: A crosstalk between dimethylarginines and systemic inflammation. Arthritis Res. Ther. 2017, 19, 32. [Google Scholar] [CrossRef]
- Mak, A.; Kow, N.Y.; Schwarz, H.; Gong, L.; Tay, S.H.; Ling, L.H. Endothelial dysfunction in systemic lupus erythematosus—A case-control study and an updated meta-analysis and meta-regression. Sci. Rep. 2017, 7, 7320. [Google Scholar] [CrossRef]
- Wållberg-Jonsson, S.; Caidahl, K.; Klintland, N.; Nyberg, G.; Rantapää-Dahlqvist, S. Increased arterial stiffness and indication of endothelial dysfunction in long-standing rheumatoid arthritis. Scand. J. Rheumatol. 2008, 37, 1–5. [Google Scholar] [CrossRef]
- Yang, X.; Chang, Y.; Wei, W. Endothelial Dysfunction and Inflammation: Immunity in Rheumatoid Arthritis. Mediat. Inflamm. 2016, 2016, 6813016. [Google Scholar] [CrossRef]
- Ikonomidis, I.; Lekakis, J.; Revela, I.; Andreotti, F.; Nihoyannopoulos, P. Increased circulating C-reactive protein and macrophage-colony stimulating factor are complementary predictors of long-term outcome in patients with chronic coronary artery disease. Eur. Heart J. 2005, 26, 1618–1624. [Google Scholar] [CrossRef]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2018, 9, 7204–7218. [Google Scholar] [CrossRef]
- Hanada, T.; Yoshimura, A. Regulation of cytokine signaling and inflammation. Cytokine Growth Factor Rev. 2002, 13, 413–421. [Google Scholar] [CrossRef]
- Kelley, N.; Jeltema, D.; Duan, Y.; He, Y. The NLRP3 Inflammasome: An Overview of Mechanisms of Activation and Regulation. Int. J. Mol. Sci. 2019, 20, 3328. [Google Scholar] [CrossRef]
- Zheng, D.; Liwinski, T.; Elinav, E. Inflammasome activation and regulation: Toward a better understanding of complex mechanisms. Cell Discov. 2020, 6, 36. [Google Scholar] [CrossRef] [PubMed]
- Satoh, M.; Tabuchi, T.; Itoh, T.; Nakamura, M. NLRP3 inflammasome activation in coronary artery disease: Results from prospective and randomized study of treatment with atorvastatin or rosuvastatin. Clin. Sci. 2014, 126, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Bamias, G.; Arseneau, K.O.; Cominelli, F. Cytokines and mucosal immunity. Curr. Opin. Gastroenterol. 2014, 30, 547–552. [Google Scholar] [CrossRef] [PubMed]
- Bamias, G.; Pizarro, T.T.; Cominelli, F. Pathway-based approaches to the treatment of inflammatory bowel disease. Transl. Res. J. Lab. Clin. Med. 2016, 167, 104–115. [Google Scholar] [CrossRef]
- Pagnini, C.; Siakavellas, S.I.; Bamias, G. Systematic Review with Network Meta-Analysis: Efficacy of Induction Therapy with a Second Biological Agent in Anti-TNF-Experienced Crohn’s Disease Patients. Gastroenterol. Res. Pract. 2018, 2018, 6317057. [Google Scholar] [CrossRef]
- Ikonomidis, I.; Lekakis, J.P.; Nikolaou, M.; Paraskevaidis, I.; Andreadou, I.; Kaplanoglou, T.; Katsimbri, P.; Skarantavos, G.; Soucacos, P.N.; Kremastinos, D.T.; et al. Inhibition of interleukin-1 by anakinra improves vascular and left ventricular function in patients with rheumatoid arthritis. Circulation 2008, 117, 2662–2669. [Google Scholar] [CrossRef] [PubMed]
- Ikonomidis, I.; Tzortzis, S.; Lekakis, J.; Paraskevaidis, I.; Andreadou, I.; Nikolaou, M.; Kaplanoglou, T.; Katsimbri, P.; Skarantavos, G.; Soucacos, P.; et al. Lowering interleukin-1 activity with anakinra improves myocardial deformation in rheumatoid arthritis. Heart 2009, 95, 1502–1507. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Howard, C.P.; Walter, V.; Everett, B.; Libby, P.; Hensen, J.; Thuren, T.; CANTOS Pilot Investigative Group. Effects of interleukin-1β inhibition with canakinumab on hemoglobin A1c, lipids, C-reactive protein, interleukin-6, and fibrinogen: A phase IIb randomized, placebo-controlled trial. Circulation 2012, 126, 2739–2748. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M. From C-Reactive Protein to Interleukin-6 to Interleukin-1: Moving Upstream to Identify Novel Targets for Atheroprotection. Circ. Res. 2016, 118, 145–156. [Google Scholar] [CrossRef]
- Bu, J.; Wang, Z. Cross-Talk between Gut Microbiota and Heart via the Routes of Metabolite and Immunity. Gastroenterol. Res. Pract. 2018, 2018, 6458094. [Google Scholar] [CrossRef]
- DiRienzo, D.B. Effect of probiotics on biomarkers of cardiovascular disease: Implications for heart-healthy diets. Nutr. Rev. 2014, 72, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Ebel, B.; Lemetais, G.; Beney, L.; Cachon, R.; Sokol, H.; Langella, P.; Gervais, P. Impact of probiotics on risk factors for cardiovascular diseases. A review. Crit. Rev. Food Sci. Nutr. 2014, 54, 175–189. [Google Scholar] [CrossRef]
- Miglioranza Scavuzzi, B.; Miglioranza, L.H.; Henrique, F.C.; Pitelli Paroschi, T.; Lozovoy, M.A.; Simão, A.N.; Dichi, I. The role of probiotics on each component of the metabolic syndrome and other cardiovascular risks. Expert Opin. Ther. Targets 2015, 19, 1127–1138. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Buys, N. Effects of probiotics consumption on lowering lipids and CVD risk factors: A systematic review and meta-analysis of randomized controlled trials. Ann. Med. 2015, 47, 430–440. [Google Scholar] [CrossRef]
- Thushara, R.M.; Gangadaran, S.; Solati, Z.; Moghadasian, M.H. Cardiovascular benefits of probiotics: A review of experimental and clinical studies. Food Funct. 2016, 7, 632–642. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Guo, M.J.; Gao, Q.; Yang, J.F.; Yang, L.; Pang, X.L.; Jiang, X.J. The effects of probiotics on total cholesterol: A meta-analysis of randomized controlled trials. Medicine 2018, 97, e9679. [Google Scholar] [CrossRef] [PubMed]
- Celiberto, L.S.; Bedani, R.; Rossi, E.A.; Cavallini, D.C. Probiotics: The scientific evidence in the context of inflammatory bowel disease. Crit. Rev. Food Sci. Nutr. 2017, 57, 1759–1768. [Google Scholar] [CrossRef]
- Eom, T.; Kim, Y.S.; Choi, C.H.; Sadowsky, M.J.; Unno, T. Current understanding of microbiota- and dietary-therapies for treating inflammatory bowel disease. J. Microbiol. 2018, 56, 189–198. [Google Scholar] [CrossRef]
- Wasilewski, A.; Zielińska, M.; Storr, M.; Fichna, J. Beneficial Effects of Probiotics, Prebiotics, Synbiotics, and Psychobiotics in Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2015, 21, 1674–1682. [Google Scholar] [CrossRef]
- Derwa, Y.; Gracie, D.J.; Hamlin, P.J.; Ford, A.C. Systematic review with meta-analysis: The efficacy of probiotics in inflammatory bowel disease. Aliment. Pharmacol. Ther. 2017, 46, 389–400. [Google Scholar] [CrossRef]
- Parker, E.A.; Roy, T.; D’Adamo, C.R.; Wieland, L.S. Probiotics and gastrointestinal conditions: An overview of evidence from the Cochrane Collaboration. Nutrition 2018, 45, 125–134.e11. [Google Scholar] [CrossRef]
- Rondanelli, M.; Faliva, M.A.; Perna, S.; Giacosa, A.; Peroni, G.; Castellazzi, A.M. Using probiotics in clinical practice: Where are we now? A review of existing meta-analyses. Gut Microbes 2017, 8, 521–543. [Google Scholar] [CrossRef] [PubMed]
- Corb Aron, R.A.; Abid, A.; Vesa, C.M.; Nechifor, A.C.; Behl, T.; Ghitea, T.C.; Munteanu, M.A.; Fratila, O.; Andronie-Cioara, F.L.; Toma, M.M.; et al. Recognizing the Benefits of Pre-/Probiotics in Metabolic Syndrome and Type 2 Diabetes Mellitus Considering the Influence of Akkermansia muciniphila as a Key Gut Bacterium. Microorganisms 2021, 9, 618. [Google Scholar] [CrossRef] [PubMed]
- Dindelegan, C.M.; Faur, D.; Purza, L.; Bumbu, A.; Sabau, M. Distress in neurocognitive disorders due to Alzheimer’s disease and stroke. Exp. Ther. Med. 2020, 20, 2501–2509. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Cannon, C.P.; Morrow, D.; Rifai, N.; Rose, L.M.; McCabe, C.H.; Pfeffer, M.A.; Braunwald, E. C-reactive protein levels and outcomes after statin therapy. N. Engl. J. Med. 2005, 352, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N. Engl. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef] [PubMed]

| Characteristics/Markers | Total Population | Surgical Group (A) | Pharmaceutical Group (B) |
|---|---|---|---|
| Enrolled patients | 60 | 35 | 25 |
| Male | 32 (53%) | 19 (54%) | 13 (52%) |
| Female | 28 (47%) | 16 (46%) | 12 (48%) |
| Crohn Disease | 46 (77%) | 30 (86%) | 16 (64%) |
| Ulcerative Colitis | 14 (23%) | 5 (14%) | 9 (36%) |
| Age, y | 40 ± 13 | 40.5 ± 13.7 | 39.8 ± 12.5 |
| WBC, /mm3 | 8510 ± 578 | 8801 ± 640 | 7990 ± 1156 |
| CRP, mg/L | 11.4 ± 1.8 | 11.9 ± 2.5 | 10.6 ± 2.6 |
| MDA, nmol/mg | 4.17 ± 0.4 | 4.04 ± 0.53 | 4.36 ± 0.63 |
| TBARS, μmol/L | 4.13 ± 0.52 | 4.32 ± 0.92 | 3.93 ± 0.54 |
| ABTS, mmol/L | 24.9 ± 0.96 | 25 ± 1.42 | 24.8 ± 1.35 |
| RP, μmol/mL | 0.97 ± 0.17 | 0.96 ± 0.02 | 0.98 ± 0.03 |
| PWV peripheral, m/s | 7.5 ± 0.48 | 7.44 ± 0.75 | 7.58 ± 0.5 |
| PWV central, m/s | 9.1 ± 0.3 | 8.7 ± 0.28 | 9.86 ± 0.62 |
| PBR5-25, μm | 2.26 ± 0.46 | 2.23 ± 0.06 | 2.31 ± 0.07 |
| PBR5-9, μm | 1.22 ± 0.15 | 1.22 ± 0.02 | 1.21 ± 0.02 |
| PBR10-19, μm | 2.4 ± 0.05 | 2.38 ± 0.07 | 2.44 ± 0.08 |
| PBR20-25, μm | 2.87 ± 0.08 | 2.83 ± 0.1 | 2.94 ± 0.1 |
| CFRv | 2.49 ± 0.06 | 2.45 ± 0.07 | 2.55 ± 0.09 |
| CFRvti | 2.08 ± 0.06 | 1.97 ± 0.06 | 2.27 ± 0.1 |
| FMD, % | 6.73 ± 0.45 | 6.73 ± 0.55 | 6.74 ± 0.81 |
| GLS, % | −18.9 ± 0.3 | −19.5 ± 0.39 | −18 ± 0.44 |
| PWV/GLS, m/s% | −0.49 ± 0.02 | −0.45 ± 0.02 | −0.55 ± 0.03 |
| L4chS, % | −18.8 ± 0.35 | −19.1 ± 0.4 | −18.2 ± 0.64 |
| GcircS, % | −18.8 ± 0.56 | −18.7 ± 0.62 | −18.9 ± 1.1 |
| Markers | Total Population | Surgical Group (A) | Pharmaceutical Group (B) | |||
|---|---|---|---|---|---|---|
| Pre-Treatment | Post-Treatment | Pre-Treatment | Post-Treatment | Pre-Treatment | Post-Treatment | |
| WBC, /mm3 | 8510 ± 578 | 6547 ± 314 ‡ | 8801 ± 640 | 6792 ± 420 ‡ | 7990 ± 1156 | 6110 ± 445 * |
| CRP, mg/L | 11.4 ± 1.8 | 3.3 ± 0.8 ‡ | 11.9 ± 2.5 | 3.8 ± 1.2 † | 10.6 ± 2.6 | 2.3 ± 0.56 * |
| MDA, nmol/mg | 4.17 ± 0.4 | 3.35 ± 0.26 * | 4.04 ± 0.53 | 3.22 ± 0.3 | 4.36 ± 0.63 | 3.57 ± 0.47 * |
| TBARS, μmol/L | 4.13 ± 0.52 | 4.07 ± 0.48 | 4.32 ± 0.92 | 3.98 ± 0.82 | 3.93 ± 0.54 | 4.15 ± 0.54 |
| ABTS, mmol/L | 24.9 ± 0.96 | 25.9 ± 1.17 | 25 ± 1.42 | 25 ± 1.95 | 24.8 ± 1.35 | 26.8 ± 1.26 |
| RP, μmol/mL | 0.97 ± 0.17 | 0.96 ± 0.16 | 0.96 ± 0.02 | 0.94 ± 0.01 | 0.98 ± 0.03 | 0.99 ± 0.03 |
| PWV peripheral, m/s | 7.5 ± 0.48 | 7.16 ± 0.33 | 7.44 ± 0.75 | 7.14 ± 0.5 | 7.58 ± 0.5 | 7.17 ± 0.4 |
| PWV central, m/s | 9.1 ± 0.3 | 8.8 ± 0.3 | 8.7 ± 0.28 | 8.7 ± 0.37 | 9.86 ± 0.62 | 9.1 ± 0.55 * |
| PBR5-25, μm | 2.26 ± 0.46 | 2.02 ± 0.45 ‡ | 2.23 ± 0.06 | 2.02 ± 0.05 † | 2.31 ± 0.07 | 2.02 ± 0.08 † |
| PBR5-9, μm | 1.22 ± 0.15 | 1.15 ± 0.17 * | 1.22 ± 0.02 | 1.16 ± 0.02 * | 1.21 ± 0.02 | 1.14 ± 0.03 * |
| PBR10-19, μm | 2.4 ± 0.05 | 2.18 ± 0.06 † | 2.38 ± 0.07 | 2.18 ± 0.07 * | 2.44 ± 0.08 | 2.17 ± 0.1 * |
| PBR20-25, μm | 2.87 ± 0.08 | 2.55 ± 0.07 † | 2.83 ± 0.1 | 2.51 ± 0.07 * | 2.94 ± 0.1 | 2.6 ± 0.1 * |
| CFRv | 2.49 ± 0.06 | 3.05 ± 0.08 ‡ | 2.45 ± 0.07 | 3.1 ± 0.1 ‡ | 2.55 ± 0.09 | 2.96 ± 0.14 † |
| CFRvti | 2.08 ± 0.06 | 2.47 ± 0.0 6 ‡ | 1.97 ± 0.06 | 2.43 ± 0.63 ‡ | 2.27 ± 0.1 | 2.54 ± 0.1 * |
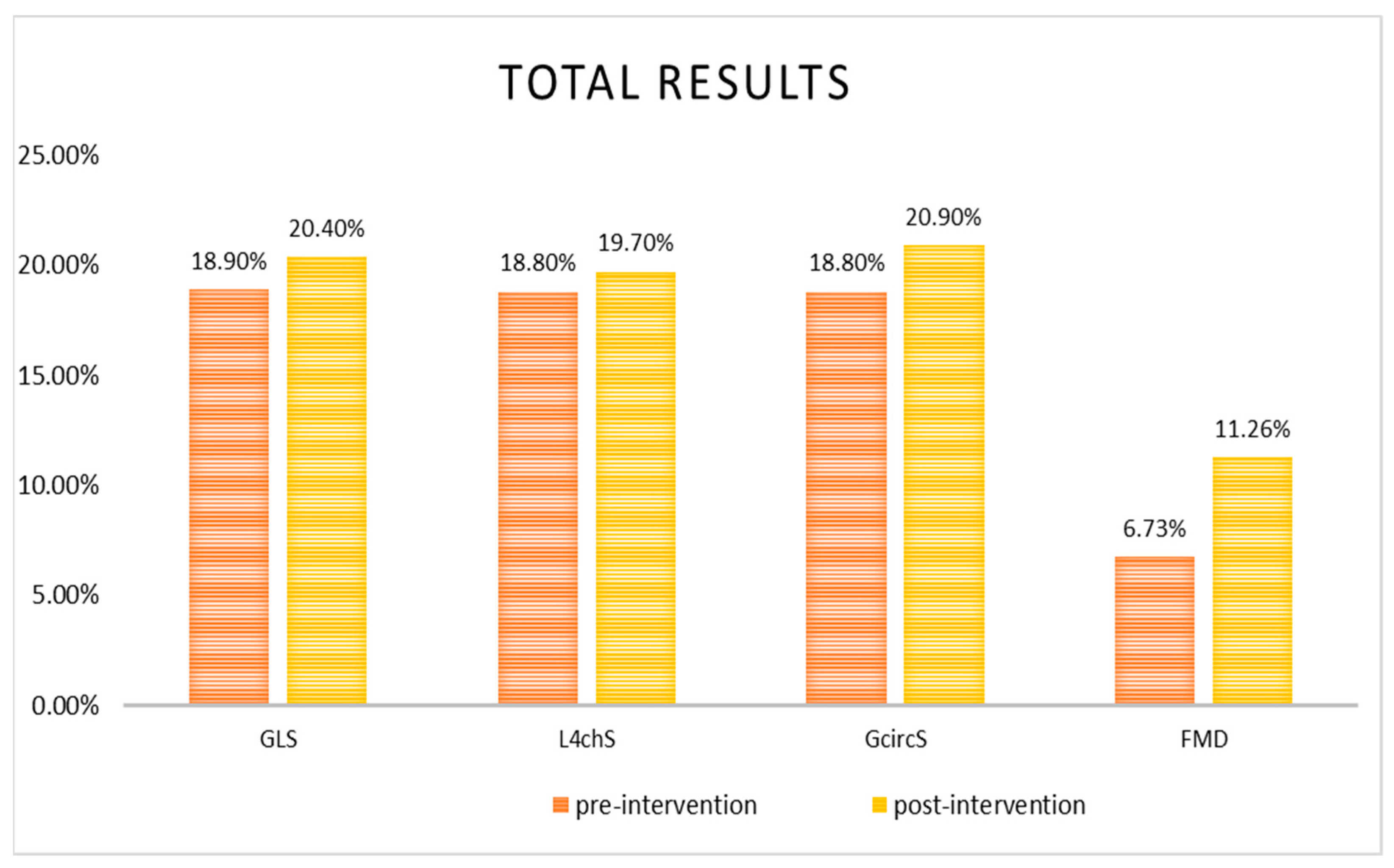
| FMD, % | 6.73 ± 0.45 | 11.26 ± 0.95 ‡ | 6.73 ± 0.55 | 12.75 ± 1.27 ‡ | 6.74 ± 0.81 | 8.8 ± 1.2 |
| GLS, % | −18.9 ± 0.3 | −20.4 ± 0.3 ‡ | −19.5 ± 0.39 | −20.7 ± 0.38 * | −18 ± 0.44 | −19.8 ± 0.43 † |
| PWV/GLS, m/s% | −0.49 ± 0.02 | −0.43 ± 0.02 † | −0.45 ± 0.02 | −0.42 ± 0.02 * | −0.55 ± 0.03 | −0.47 ± 0.03 * |
| L4chs, % | −18.8 ± 0.35 | −19.7 ± 0.32 † | −19.1 ± 0.4 | −19.8 ± 0.4 * | −18.2 ± 0.64 | −19.4 ± 0.55 * |
| GcircS, % | −18.8 ± 0.56 | −20.9 ± 0.68 † | −18.7 ± 0.62 | −20.6 ± 0.82 * | −18.9 ± 1.1 | −21.6 ± 1.2 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Triantafyllou, C.; Nikolaou, M.; Ikonomidis, I.; Bamias, G.; Kouretas, D.; Andreadou, I.; Tsoumani, M.; Thymis, J.; Papaconstantinou, I. Effects of Anti-Inflammatory Treatment and Surgical Intervention on Endothelial Glycocalyx, Peripheral and Coronary Microcirculatory Function and Myocardial Deformation in Inflammatory Bowel Disease Patients: A Two-Arms Two-Stage Clinical Trial. Diagnostics 2021, 11, 993. https://doi.org/10.3390/diagnostics11060993
Triantafyllou C, Nikolaou M, Ikonomidis I, Bamias G, Kouretas D, Andreadou I, Tsoumani M, Thymis J, Papaconstantinou I. Effects of Anti-Inflammatory Treatment and Surgical Intervention on Endothelial Glycocalyx, Peripheral and Coronary Microcirculatory Function and Myocardial Deformation in Inflammatory Bowel Disease Patients: A Two-Arms Two-Stage Clinical Trial. Diagnostics. 2021; 11(6):993. https://doi.org/10.3390/diagnostics11060993
Chicago/Turabian StyleTriantafyllou, Charilaos, Maria Nikolaou, Ignatios Ikonomidis, Giorgos Bamias, Dimitrios Kouretas, Ioanna Andreadou, Maria Tsoumani, John Thymis, and Ioannis Papaconstantinou. 2021. "Effects of Anti-Inflammatory Treatment and Surgical Intervention on Endothelial Glycocalyx, Peripheral and Coronary Microcirculatory Function and Myocardial Deformation in Inflammatory Bowel Disease Patients: A Two-Arms Two-Stage Clinical Trial" Diagnostics 11, no. 6: 993. https://doi.org/10.3390/diagnostics11060993
APA StyleTriantafyllou, C., Nikolaou, M., Ikonomidis, I., Bamias, G., Kouretas, D., Andreadou, I., Tsoumani, M., Thymis, J., & Papaconstantinou, I. (2021). Effects of Anti-Inflammatory Treatment and Surgical Intervention on Endothelial Glycocalyx, Peripheral and Coronary Microcirculatory Function and Myocardial Deformation in Inflammatory Bowel Disease Patients: A Two-Arms Two-Stage Clinical Trial. Diagnostics, 11(6), 993. https://doi.org/10.3390/diagnostics11060993









