Focal Pancreatic Lesions: Role of Contrast-Enhanced Ultrasonography
Abstract
1. Introduction
2. CEUS Technique
3. Pancreatic Solid Lesions
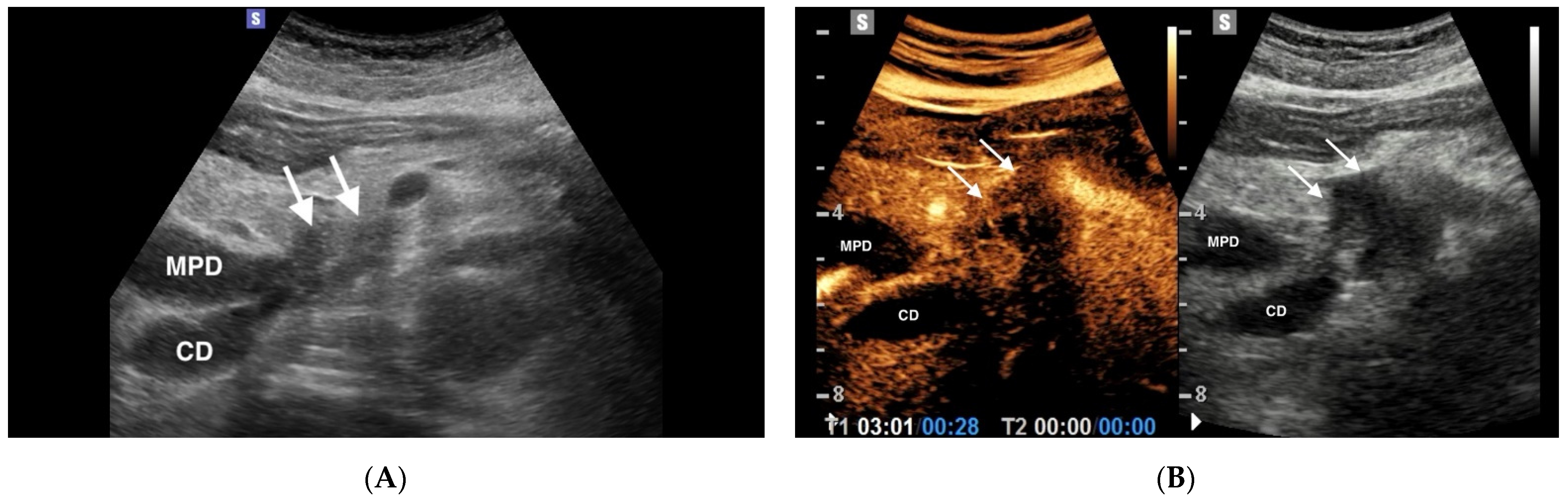
3.1. Pancreatic Ductal Adenocarcinoma
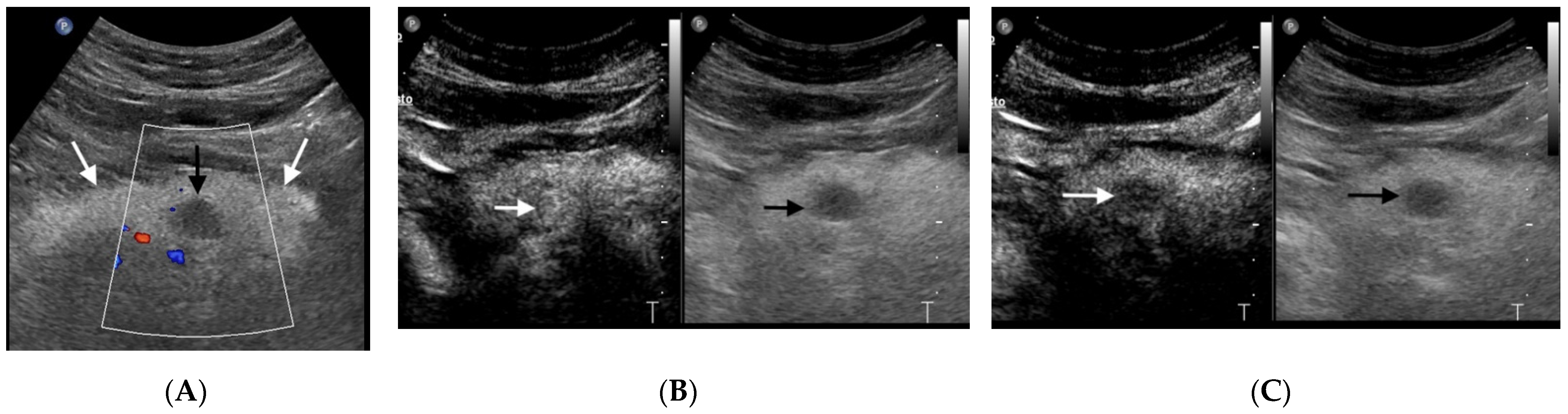
3.2. Pancreatic Neuroendocrine Tumors
4. Neoplastic Cystic Lesions
4.1. Mucinous Cystic Neoplasms
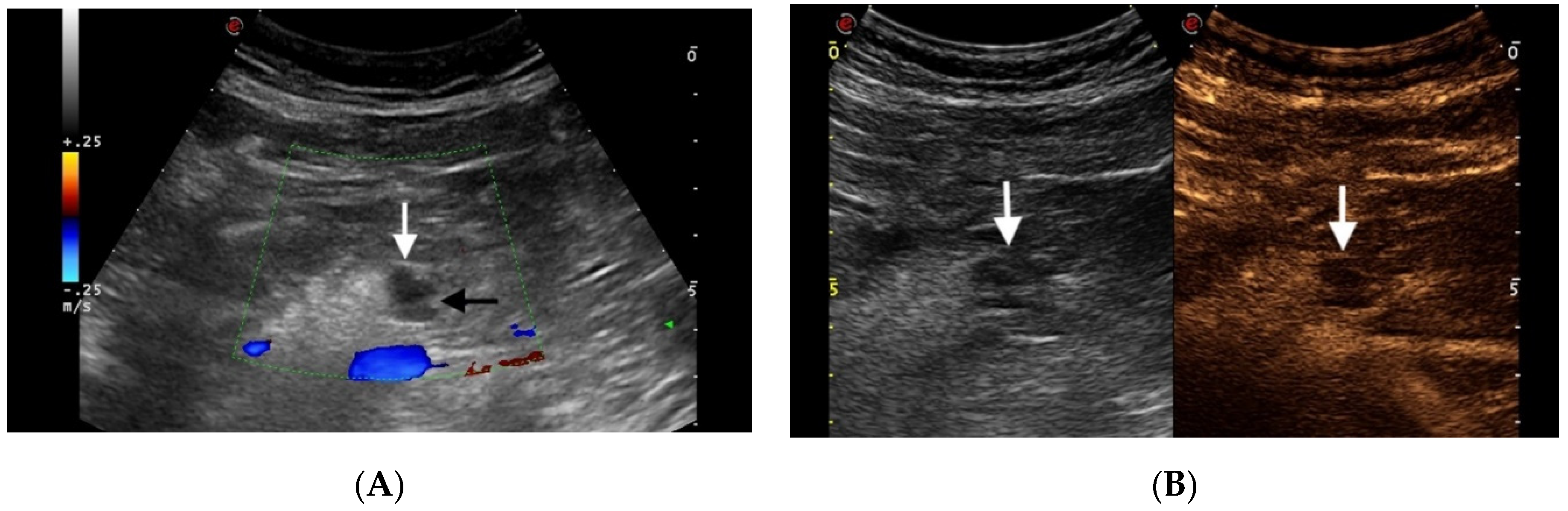
4.1.1. IPMN
4.1.2. MCNs
4.2. Serous Cystic Neoplasms
5. Non Neoplastic Cystic Lesions
Pseudocyst
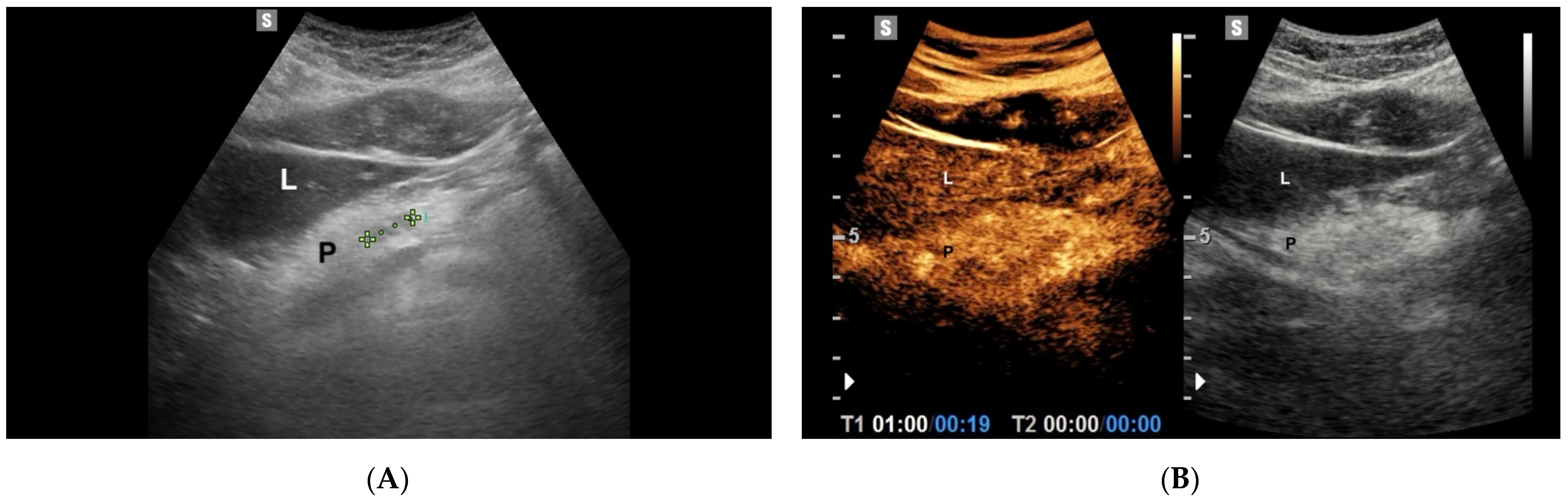
6. Chronic Pancreatitis
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Tedesco, G.; Sarno, A.; Rizzo, G.; Grecchi, A.; Testa, I.; Giannotti, G.; D’Onofrio, M. Clinical use of contrast-enhanced ultrasound beyond the liver: A focus on renal, splenic, and pancreatic applications. Ultrasonography 2019, 38, 278–288. [Google Scholar] [CrossRef] [PubMed]
- Bartolotta, T.V.; Pinto, A.S.; Cannella, R.; Porrello, G.; Taravella, R.; Randazzo, A.; Taibbi, A. Focal liver lesions: Inter- and intraobserver agreement evaluation of 3D CEUS assisted volume measurement. Ultrasonography 2020. [CrossRef]
- Bartolotta, T.V.; Terranova, M.C.; Gagliardo, C.; Taibbi, A. CEUS LI-RADS: A pictorial review. Insights Imaging 2020, 11, 9. [Google Scholar] [CrossRef] [PubMed]
- Quaia, E. Microbubble ultrasound contrast agents: An update. Eur. Radiol. 2007, 17, 1995–2008. [Google Scholar] [CrossRef]
- Piscaglia, F.; Bolondi, L.; Italian Society for Ultrasound in Medicine and Biology (SIUMB) Study Group on Ultrasound Contrast Agents. The safety of Sonovue® in abdominal applications: Retrospective analysis of 23188 investigations. Ultrasound Med. Biol. 2006, 32, 1369–1375. [Google Scholar] [CrossRef] [PubMed]
- D’Onofrio, M.; Canestrini, S.; De Robertis, R.; Crosara, S.; Demozzi, E.; Ciaravino, V.; Mucelli, R.P.; Mirko, D.; Stefano, C.; Riccardoa, D.R.; et al. CEUS of the pancreas: Still research or the standard of care. Eur. J. Radiol. 2015, 84, 1644–1649. [Google Scholar] [CrossRef]
- D’Onofrio, M.; Megibow, A.J.; Faccioli, N.; Malagò, R.; Capelli, P.; Falconi, M.; Mucelli, R.P. Comparison of Contrast-Enhanced Sonography and MRI in Displaying Anatomic Features of Cystic Pancreatic Masses. Am. J. Roentgenol. 2007, 189, 1435–1442. [Google Scholar] [CrossRef] [PubMed]
- Sidhu, P.S.; Cantisani, V.; Dietrich, C.F.; Gilja, O.H.; Saftoiu, A.; Bartels, E.; Bertolotto, M.; Calliada, F.; Clevert, D.-A.; Cosgrove, D.; et al. The EFSUMB Guidelines and Recommendations for the Clinical Practice of Contrast-Enhanced Ultrasound (CEUS) in Non-Hepatic Applications: Update 2017 (Long Version). Ultraschall Med. Eur. J. Ultrasound 2018, 39, e2–e44. (In English) [Google Scholar] [CrossRef]
- Globocan. Available online: https://gco.iarc.fr/today/data/factsheets/cancers/13-Pancreas-fact-sheet.pdf (accessed on 3 April 2021).
- Low, G.; Panu, A.; Millo, N.; Leen, E. Multimodality Imaging of Neoplastic and Nonneoplastic Solid Lesions of the Pancreas. Radiographics 2011, 31, 993–1015. [Google Scholar] [CrossRef]
- Bosman: WHO Classification of Tumours of the Digestive System, 4th ed.; World Health Organization: Geneva, Switzerland, 2010.
- Wang, Y.; Yan, K.; Fan, Z.; Sun, L.; Wu, W.; Yang, W. Contrast-Enhanced Ultrasonography of Pancreatic Carcinoma: Correlation with Pathologic Findings. Ultrasound Med. Biol. 2016, 42, 891–898. [Google Scholar] [CrossRef]
- D’Onofrio, M.; Barbi, E.; Dietrich, C.F.; Kitano, M.; Numata, K.; Sofuni, A.; Principe, F.; Gallotti, A.; Zamboni, G.A.; Mucelli, R.P. Pancreatic multicenter ultrasound study (PAMUS). Eur. J. Radiol. 2012, 81, 630–638. [Google Scholar] [CrossRef]
- D’Onofrio, M.; Biagioli, E.; Gerardi, C.; Canestrini, S.; Rulli, E.; Crosara, S.; De Robertis, R.; Floriani, I. Diagnostic Performance of Contrast-Enhanced Ultrasound (CEUS) and Contrast-Enhanced Endoscopic Ultrasound (ECEUS) for the Differentiation of Pancreatic Lesions: A Systematic Review and Meta-Analysis. Ultraschall Med. Eur. J. Ultrasound 2014, 35, 515–521. [Google Scholar] [CrossRef]
- D’Onofrio, M.; Crosara, S.; Signorini, M.; De Robertis, R.; Canestrini, S.; Principe, F.; Mucelli, R.P. Comparison between CT and CEUS in the Diagnosis of Pancreatic Adenocarcinoma. Ultraschall Med. Eur. J. Ultrasound 2013, 34, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Bartolotta, T.V.; Taibbi, A.; Picone, D.; Anastasi, A.; Midiri, M.; Lagalla, R. Detection of liver metastases in cancer patients with geographic fatty infiltration of the liver: The added value of contrast-enhanced sonography. Ultrasonography 2017, 36, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Jang, S.; Han, J.K.; Kim, H.; Kwon, W.; Jang, J.Y.; Lee, K.B.; Kim, H.; Lee, D.H. Preoperative assessment of the resectabil-ity of pancreatic ductal adenocarcinoma on CT according to the NCCN Guidelines focusing on SMA/SMV branch in-vasion. Eur. Radiol. 2021. [Google Scholar] [CrossRef]
- Wei, Y.; Yu, X.-L.; Liang, P.; Cheng, Z.-G.; Han, Z.-Y.; Liu, F.-Y.; Yu, J. Guiding and Controlling Percutaneous Pancreas Biopsies with Contrast-Enhanced Ultrasound: Target Lesions Are Not Localized on B-Mode Ultrasound. Ultrasound Med. Biol. 2015, 41, 1561–1569. [Google Scholar] [CrossRef]
- Tawada, K.; Yamaguchi, T.; Kobayashi, A.; Ishihara, T.; Sudo, K.; Nakamura, K.; Hara, T.; Denda, T.; Matsuyama, M.; Yokosuka, O. Changes in Tumor Vascularity Depicted by Contrast-Enhanced Ultraso-nography as a Predictor of Chemotherapeutic Effect in Patients With Unresectable Pancreatic Cancer. Pancreas 2009, 38, 30–35. [Google Scholar] [CrossRef]
- Fraenkel, M.; Kim, M.; Faggiano, A.; Valk, G. Epidemiology of gastroenteropancreatic neuroendocrine tumours. Best Pract. Res. Clin. Gastroenterol. 2012, 26, 691–703. [Google Scholar] [CrossRef] [PubMed]
- Öberg, K.; Eriksson, B. Endocrine tumours of the pancreas. Best Pract. Res. Clin. Gastroenterol. 2005, 19, 753–781. [Google Scholar] [CrossRef]
- Orditura, M.; Petrillo, A.; Ventriglia, J.; Diana, A.; Laterza, M.M.; Fabozzi, A.; Savastano, B.; Franzese, E.; Conzo, G.; Santini, L.; et al. Pancreatic neuroendocrine tumors: Nosography, management and treatment. Int. J. Surg. 2016, 28 (Suppl. 1), S156–S162. [Google Scholar] [CrossRef]
- Falconi, M.; Bartsch, D.K.; Eriksson, B.; Klöppel, G.; Lopes, J.M.; O’Connor, J.M.; Salazar, R.; Taal, B.G.; Vullierme, M.P.; O’Toole, D.; et al. ENETS Consensus Guidelines for the Management of Patients with Digestive Neuroendocrine Neoplasms of the Digestive System: Well-Differentiated Pancreatic Non-Functioning Tumors. Neuroendocrinology 2012, 95, 120–134. [Google Scholar] [CrossRef]
- Abdelkader, A.; Hunt, B.; Hartley, C.P.; Panarelli, N.C.; Giorgadze, T. Cystic Lesions of the Pancreas: Differential Diagnosis and Cytologic-Histologic Correlation. Arch. Pathol. Lab. Med. 2020, 144, 47–61. [Google Scholar] [CrossRef]
- Kim, Y.H.; Saini, S.; Sahani, D.; Hahn, P.F.; Mueller, P.R.; Auh, Y.H. Imaging diagnosis of cystic pancreatic lesions: Pseudocyst versus non pseudocyst. Radiographics 2005, 25, 671–685. [Google Scholar] [CrossRef] [PubMed]
- Freeny, P.C.; Saunders, M.D. Moving beyond Morphology: New Insights into the Characterization and Management of Cystic Pancreatic Lesions. Radiology 2014, 272, 345–363. [Google Scholar] [CrossRef] [PubMed]
- Gaujoux, S.; Brennan, M.F.; Gonen, M.; D’Angelica, M.I.; De Matteo, R.; Fong, Y.; Schattner, M.; Di Maio, C.; Janakos, M.; Jarnagin, W.R.; et al. Cystic Lesions of the Pancreas: Changes in the Presentation and Management of 1,424 Patients at a Single Institution over a 15-Year Time Period. J. Am. Coll. Surg. 2011, 212, 590–600. [Google Scholar] [CrossRef]
- Megibow, A.J.; Baker, M.E.; Morgan, D.E.; Kamel, I.R.; Sahani, D.V.; Newman, E.; Brugge, W.R.; Berland, L.L.; Pandharipande, P.V. Management of Incidental Pancreatic Cysts: A White Paper of the ACR Incidental Findings Committee. J. Am. Coll. Radiol. 2017, 14, 911–923. [Google Scholar] [CrossRef]
- De Jong, K.; Nio, C.Y.; Hermans, J.J.; Dijkgraaf, M.G.; Gouma, D.J.; Van Eijck, C.H.; Van Heel, E.; Klass, G.; Fockens, P.; Bruno, M.J. High Prevalence of Pancreatic Cysts Detected by Screening Magnetic Resonance Imaging Examinations. Clin. Gastroenterol. Hepatol. 2010, 8, 806–811. [Google Scholar] [CrossRef]
- Ip, I.K.; Mortele, K.J.; Prevedello, L.M.; Khorasani, R. Focal Cystic Pancreatic Lesions: Assessing Variation in Radiologists’ Management Recommendations. Radiology 2011, 259, 136–141. [Google Scholar] [CrossRef]
- Laffan, T.A.; Horton, K.M.; Klein, A.P.; Berlanstein, B.; Siegelman, S.S.; Kawamoto, S.; Johnson, P.T.; Fishman, E.K.; Hruban, R.H. Prevalence of Unsuspected Pancreatic Cysts on MDCT. AJR Am. J. Roentgenol. 2008, 191, 802–807. [Google Scholar] [CrossRef]
- Yoon, J.G.; Smith, D.; Ojili, V.; Paspulati, R.M.; Ramaiya, N.H.; Tirumani, S.H. Pancreatic cystic neoplasms: A review of current recommendations for surveillance and management. Abdom. Radiol. 2021, 1–17. [Google Scholar] [CrossRef]
- D’Onofrio, M.; Zamboni, G.; Faccioli, N.; Capelli, P.; Mucelli, R.P. Ultrasonography of the pancreas. 4. Contrast enhanced imaging. Abdom. Imaging 2007, 32, 171–181. [Google Scholar] [CrossRef]
- Zhang, X.-M.; Mitchell, D.G.; Dohke, M.; Holland, G.A.; Parker, L. Pancreatic Cysts: Depiction on Single-Shot Fast Spin-Echo MR Images. Radiology 2002, 223, 547–553. [Google Scholar] [CrossRef]
- Sahani, D.V.; Kadavigere, R.; Saokar, A.; Fernandez-del Castillo, C.; Brugge, W.R.; Hahn, P.F. Cystic pancreatic lesions: A sim-ple imaging-based classification system for guiding management. Radiographics 2005, 25, 1471–1484. [Google Scholar] [CrossRef]
- Lewin, M.; Hoeffel, C.; Azizi, L.; Lacombe, C.; Monnier-Cholley, L.; Raynal, M.; Arrivé, L.; Tubiana, J.M. Imaging of incidental cystic lesions of the pancreas. J. Radiol. 2008, 89, 197–207. [Google Scholar] [CrossRef]
- Zamboni, G.; Kloppel, G.; Hruban, R.H.; Longnecker, D.S.; Adler, G. Mucinous cystic neoplasms of the pancreas. World Health Organization classification of tumors. In Pathology and Genetics of Tumors of the Digestive System; Hamilton, S.R.A.L., Ed.; IARC Press: Lyon, Franch, 2000; pp. 237–240. [Google Scholar]
- Procacci, C.; Megibow, A.J.; Carbognin, G.; Guarise, A.; Spoto, E.; Biasiutti, C.; Pistolesi, G.F. Intraductal Papillary Mucinous Tumor of the Pancreas: A Pictorial Essay. Radiographics 1999, 19, 1447–1463. [Google Scholar] [CrossRef] [PubMed]
- Van Huijgevoort, N.C.M.; Del Chiaro, M.; Wolfgang, C.L.; Van Hooft, J.E.; Besselink, M.G. Diagnosis and management of pancreatic cystic neoplasms: Current evidence and guidelines. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 676–689. [Google Scholar] [CrossRef]
- Zamboni, G.A.; Ambrosetti, M.C.; D’Onofrio, M.; Mucelli, R.P. Ultrasonography of the Pancreas. Radiol. Clin. N. Am. 2012, 50, 395–406. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Chari, S.; Adsay, V.; Fernandez-del Castillo, C.; Falconi, M.; Shimizu, M.; Yamaguchi, K.; Yamao, K.; Matsuno, S.; International Association of Pancreatology. International Consensus Guidelines for Management of Intraductal Papillary Mucinous Neoplasms and Mucinous Cystic Neoplasms of the Pancreas. Pancreatology 2006, 6, 17–32. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Xie, X.-Y.; Liu, G.-J.; Xu, H.-X.; Xu, Z.-F.; Huang, G.-L.; Chen, P.-F.; Luo, J.; Lu, M.-D. The application value of contrast-enhanced ultrasound in the differential diagnosis of pancreatic solid-cystic lesions. Eur. J. Radiol. 2012, 81, 1432–1437. [Google Scholar] [CrossRef] [PubMed]
- Scheiman, J.M.; Hwang, J.H.; Moayyedi, P. American Gastroenterological Association Technical Review on the Diagnosis and Management of Asymptomatic Neoplastic Pancreatic Cysts. Gastroenterology 2015, 148, 824–848.e22. [Google Scholar] [CrossRef]
- Greer, J.B.; Ferrone, C.R. Spectrum and Classification of Cystic Neoplasms of the Pancreas. Surg. Oncol. Clin. N. Am. 2016, 25, 339–350. [Google Scholar] [CrossRef]
- Crippa, S.; Salvia, R.; Warshaw, A.L.; Domínguez, I.; Bassi, C.; Falconi, M.; Thayer, S.P.; Zamboni, G.; Lauwers, G.Y.; Mino-Kenudson, M.; et al. Mucinous Cystic Neoplasm of the Pancreas is Not an Aggressive Entity: Lessons from 163 resected patients. Ann. Surg. 2008, 247, 571–579. [Google Scholar] [CrossRef]
- Fan, Z.; Yan, K.; Wang, Y.; Qiu, J.; Wu, W.; Yang, L.; Chen, M. Application of Contrast-Enhanced Ultrasound in Cystic Pancreatic Lesions Using a Simplified Classification Diagnostic Criterion. BioMed Res. Int. 2015, 2015, 974621. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sun, Y.; Yang, S.; Qi, E.; Liu, F.; Zhou, F.; Lu, Y.; Liang, P.; Ye, H.; Yu, X. Comparative Diagnostic Evaluation with Contrast-Enhanced Ultrasound, Computed Tomography and Magnetic Resonance Imaging in Patients with Pancreatic Cystic Neoplasms. Cancer Manag. Res. 2020, 12, 2889–2898. [Google Scholar] [CrossRef] [PubMed]
- Sakorafas, G.H.; Smyrniotis, V.; Reid-Lombardo, K.M.; Sarr, M.G. Primary pancreatic cystic neoplasms revisited, part I: Se-rous cystic neoplasms. Surg. Oncol. 2011, 20, e84–e92. [Google Scholar] [CrossRef]
- Khurana, B.; Mortele, K.J.; Glickman, J.; Silverman, S.G.; Ros, P.R. Macrocystic Serous Adenoma of the Pancreas: Radiologic—Pathologic Correlation. AJR Am. J. Roentgenol. 2003, 181, 119–123. [Google Scholar] [CrossRef]
- Procacci, C.; Biasiutti, C.; Carbognin, G.; Accordini, S.; Bicego, E.; Guarise, A.; Spoto, E.; Andreis, I.A.; De Marco, R.; Megibow, A.J. Characterization of Cystic Tumors of the Pancreas: CT Accuracy. J. Comput. Assist. Tomogr. 1999, 23, 906–912. [Google Scholar] [CrossRef] [PubMed]
- Itoh, T.; Hirooka, Y.; Itoh, A.; Hashimoto, S.; Kawashima, H.; Hara, K.; Kanamori, A.; Ohmiya, N.; Niwa, Y.; Goto, H. Usefulness of contrast?enhanced trans-abdominal ultrasonography in the diagnosis of intraductal papillary mucinous tumors of the pancreas. Am. J. Gastroenterol. 2005, 100, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Procacci, C.; Biasiutti, C.; Carbognin, G.; Capelli, P.; El-Dalati, G.; Falconi, M.; Misiani, G.; Ghirardi, C.; Zamboni, G. Pancreatic neoplasms and tumor-like conditions. Eur. Radiol. 2001, 11 (Suppl. 2), S167–S192. [Google Scholar]
- Niederau, C.; Grendell, J.H. Diagnosis of chronic pancreatitis. Gastroenterology 1985, 88, 1973–1995. [Google Scholar] [CrossRef]
- Hessel, S.J.; Siegelman, S.S.; McNeil, B.J.; Sanders, R.; Adams, D.F.; Alderson, P.O.; Finberg, H.J.; Abrams, H.L. A prospective evaluation of computed tomography and ultrasound of the pancreas. Radiology 1982, 143, 129–133. [Google Scholar] [CrossRef] [PubMed]
- van Gulik, T.M.; Reeders, J.W.; Bosma, A.; Moojen, T.M.; Smits, N.J.; Allema, J.H.; Rauws, E.A.; Offerhaus, G.J.A.; Obertop, H.; Gouma, D.J. Incidence and clinical findings of benign, inflammatory disease in patients resected for presumed pancreatic head cancer. Gastrointest. Endosc. 1997, 46, 417–423. [Google Scholar] [CrossRef]
- D’Onofrio, M.; Zamboni, G.; Tognolini, A.; Malagò, R.; Faccioli, N.; Frulloni, L.; Mucelli, R.P. Mass-forming pancreatitis: Value of con-trast-enhanced ultrasonography. World J. Gastroenterol. 2006, 12, 4181–4284. [Google Scholar] [CrossRef] [PubMed]
- Takeda, K.; Goto, H.; Hirooka, Y.; Itoh, A.; Hashimoto, S.; Niwa, K.; Hayakawa, T. Contrast-enhanced transabdominal ultra-sonography in the diagnosis of pancreatic mass lesions. Acta Radiol. 2003, 44, 103–106. [Google Scholar] [CrossRef] [PubMed]
| EFSUMB Recommendation for CEUS |
|---|
|
| Hypo-Vascular Heterogeneous | Hyper-Vascular Heterogeneous | Iso-Vascular Heterogeneous | Hyper-Vascular Homogeneous | Hypo-Vascular Homogeneous | Iso-Vascular Homogeneous | |
|---|---|---|---|---|---|---|
| Solid | Adenocarcinoma | Neuroendocrine tumors | Adenocarcinoma | Neuroendocrine tumors | Adenocarcinoma | Adenocarcinoma |
| Adenocarcinoma | Adenocarcinoma | |||||
| Cystic simple | - | Neuroendocrine tumors | Mucinous cystadenoma | Neuroendocrine tumors | Pseudocyst | Pseudocyst |
| IPMN 1 | IPMN | |||||
| Serous cystadenoma | Serous cystadenoma | |||||
| Cystic with septa | Adenocarcinoma | - | Adenocarcinoma | - | Adenocarcinoma | Adenocarcinoma |
| Mucinous cystadenoma | IPMN | IPMN | ||||
| Serous cystadenoma | Serous cystadenoma | |||||
| Cystic with nodules | Cystadenocarcinoma | Cystadenocarcinoma | Cystadenocarcinoma | Neuroendocrine tumors | Cystadenocarcinoma | Mucinous cystadenoma |
| Mucinous cystadenoma | Neuroendocrine tumors | Mucinous cystadenoma | Cystadenocarcinoma | Mucinous cystadenoma | ||
| Cystic with thick wall | IPMN | Neuroendocrine tumors | IPMN | Neuroendocrine tumors | IPMN | IPMN |
| Pseudocyst | Pseudocyst | Pseudocyst | ||||
| Mucinous cystadenoma | Serous cystadenoma | Adenocarcinoma | Serous cystadenoma | Serous cystadenoma | ||
| Adenocarcinoma | Mucinous cystadenoma | Mucinous cystadenoma | Mucinous cystadenoma | |||
| Cystic with septa, nodules and thick wall | IPMN | Neuroendocrine tumors | IPMN | Neuroendocrine tumors | IPMN | IPMN |
| Adenocarcinoma | Adenocarcinoma | Adenocarcinoma | Adenocarcinoma | |||
| Cystadenocarcinoma | Cystadenocarcinoma | Cystadenocarcinoma |
| Pancreatic Cystic Lesions: Imaging Features in Favor of Malignancy |
|---|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bartolotta, T.V.; Randazzo, A.; Bruno, E.; Alongi, P.; Taibbi, A. Focal Pancreatic Lesions: Role of Contrast-Enhanced Ultrasonography. Diagnostics 2021, 11, 957. https://doi.org/10.3390/diagnostics11060957
Bartolotta TV, Randazzo A, Bruno E, Alongi P, Taibbi A. Focal Pancreatic Lesions: Role of Contrast-Enhanced Ultrasonography. Diagnostics. 2021; 11(6):957. https://doi.org/10.3390/diagnostics11060957
Chicago/Turabian StyleBartolotta, Tommaso Vincenzo, Angelo Randazzo, Eleonora Bruno, Pierpaolo Alongi, and Adele Taibbi. 2021. "Focal Pancreatic Lesions: Role of Contrast-Enhanced Ultrasonography" Diagnostics 11, no. 6: 957. https://doi.org/10.3390/diagnostics11060957
APA StyleBartolotta, T. V., Randazzo, A., Bruno, E., Alongi, P., & Taibbi, A. (2021). Focal Pancreatic Lesions: Role of Contrast-Enhanced Ultrasonography. Diagnostics, 11(6), 957. https://doi.org/10.3390/diagnostics11060957







