Abstract
Ultrasound-based assessment of the fetal nervous system is routinely recommended at the time of the mid-trimester anatomy scan or at different gestations based on clinical indications. This review evaluates the methodological quality of studies aimed at creating charts for fetal brain structures obtained by ultrasound, as poor methodology could explain substantial variability in percentiles reported. Electronic databases (MEDLINE, EMBASE, Cochrane Library, and Web of Science) were searched from January 1970 to January 2021 to select studies on singleton fetuses, where the main aim was to construct charts on one or more clinically relevant structures obtained in the axial plane: parieto-occipital fissure, Sylvian fissure, anterior ventricle, posterior ventricle, transcerebellar diameter, and cisterna magna. Studies were scored against 29 predefined methodological quality criteria to identify the risk of bias. In total, 42 studies met the inclusion criteria, providing data for 45,626 fetuses. Substantial heterogeneity was identified in the methodological quality of included studies, and this may explain the high variability in centiles reported. In 80% of the studies, a high risk of bias was found in more than 50% of the domains scored. In conclusion, charts to be used in clinical practice and research should have an optimal study design in order to minimise the risk of bias and to allow comparison between different studies. We propose to use charts from studies with the highest methodological quality.
1. Introduction
Ultrasound-based assessment of the fetal nervous system is routinely recommended in most settings at the time of the mid-trimester fetal anatomy scan or at different gestations based on clinical indications [1,2]. This usually includes routine measurement of the lateral ventricle anteriorly (AV) and posteriorly (PV), the transcerebellar diameter (TCD), and the cisterna magna (CM). Additional measurements as part of an extended neurosonography examination have been proposed in order to assess gyration and sulcation disorders, such as the parieto-occipital fissure (POF) and the Sylvian fissure (SF).
In previous systematic reviews of studies aimed at creating fetal and neonatal biometry charts, many studies were found to have high risks of bias. Such shortcomings of methodological design can become a source of substantial variability in percentiles reported, with differences in interpretation of the same measurement; ultimately, this can adversely influence clinical decision making [3,4]. Over the last five years, international prescriptive standards have been published in order to overcome the limitations inherent in such descriptive reference charts [5].
The objective of this systematic review was to evaluate the methodological quality of studies aimed to develop charts of fetal brain structures measured by ultrasound.
2. Materials and Methods
We conducted a systematic review of observational studies following the Preferred Reporting Item for Systematic Reviews and Meta-Analyses (PRISMA) IPD statement [6]. We searched the major electronic databases (MEDLINE, EMBASE, Cochrane Library, and Web of Science) and secondary reference sources from January 1970 to January 2021 to select studies on singleton fetuses aimed at creating charts on fetal brain structures growth.
Inclusion criteria for each study were (1) having as the main scope to construct charts on POF, SF, AV, PV, TCD, and CM; (2) published in English; (3) selection of normal singleton pregnancies; (4) acquisition of the image on routinely acquired transverse axial planes (transthalamic, transventricular, transcerebellar) [1]; and (5) growth charts developed beyond 14 weeks of gestation. No restriction for the ultrasound acquisition technique was applied (either from 2D pictures or images derived from 3D volumes and with transvaginal or transabdominal probe). Studies aiming at comparing different population groups or methods of imaging were excluded from the review.
The keyword search strategy was formulated in collaboration with a professional information specialist (NWR) and is presented in Table S1. Two reviewers (VD and RN) independently undertook a two-stage process to select the studies. In the first stage, they assessed abstracts and titles of all identified citations and selected potentially eligible studies. In the second stage, they obtained and assessed the full texts of the studies that fulfilled the inclusion criteria for evaluation. Disagreements regarding inclusion were resolved by consensus or by consultation with a third author (ATP). Reference lists of retrieved full-text articles were examined for additional, relevant citations.
Methodological quality criteria were defined a priori, using modified versions previously used to evaluate studies aimed at creating fetal growth charts and crown–rump length dating charts [3,4]. Table S2 reports the set of 29 quality criteria. Those criteria refer to three domains, namely, (1) study design (2) statistical methods, and (3) reporting methods.
All studies included were then scored against each criterion. The level of bias was defined as a dichotomous variable: 0 referred to a ‘high risk’, and 1 referred to a ‘low risk’. The overall risk score was defined by adding all scores across the whole set of criteria. Thus, the quality score for each item of the review could range from 0 (highest risk of bias) to 29 (lowest risk of bias). The assessment of the methodological quality was performed by two reviewers (RN and AC) for each study. Where disagreements arose, those were solved through consultation with a third reviewer (ATP).
Statistical Analysis
Data from the review were coded and transferred to an Excel spreadsheet (Microsoft Corporation 2007, Redmond, WA, USA). The quality score (0–29) was reported in percentage dividing the actual score by 29 and multiplying per 100. The distribution of the 5th and the 95th centile in studies with a low and high risk of bias was evaluated.
We also evaluated the impact on centiles’ heterogeneity associated with poor study methodology in the most commonly measured brain structure—TCD.
3. Results
From a total of 1005 records identified after database search, 73 were considered for potential inclusion (Figure 1). Excluded studies and reasons for exclusion are reported in Table S3. Finally, 42 studies, reported between January 1970 and 2021, met the inclusion criteria, and these provided data for 45,626 fetuses, included in the final analysis (Table 1) [7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48]. The median sample size of participating fetuses was 372.5 (range: 50 to 8313; 25th percentile: 175.8; 75th percentile: 709.3). Most studies created charts that covered a range of gestation; for example, if a study reported a chart from 20 to 40 weeks, this covered 21 weeks. The median of this coverage was 24 weeks (range: 7 to 30 weeks; 25th percentile: 18; 75th percentile: 27).
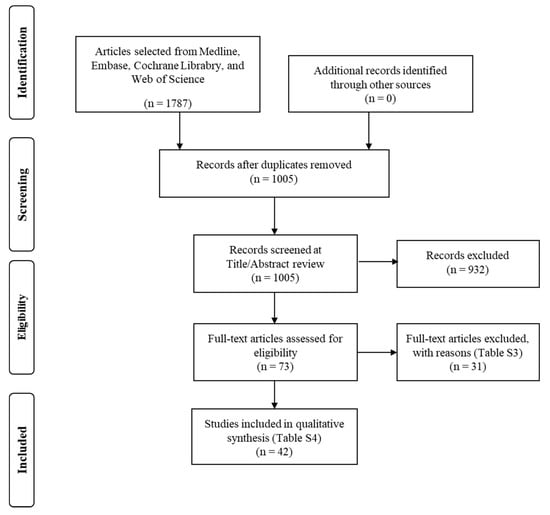
Figure 1.
Flow diagram of the studies’ selection process.

Table 1.
Main characteristics of studies included.
Nine studies reported more than one fetal brain structure [9,10,17,32,34,36,41,42,43]. In addition, 4 studies reported charts for POF [9,10,17,36], 6 for SF [9,10,17,35,36,44], 3 for AV [22,36,43], 14 for PV [7,8,14,19,23,26,27,30,32,34,36,38,40,43], 20 for TCD [12,15,16,18,20,21,24,25,28,31,32,34,39,41,42,43,45,46,47,48], and 10 studies reported the CM [11,13,29,33,34,36,37,41,42,43]. There was substantial heterogeneity in the methodology used in studies aimed at creating charts for fetal brain structures. In 34 studies (80%), high risk of bias was found in >50% of the domains scored (Figure 2, Table S4).
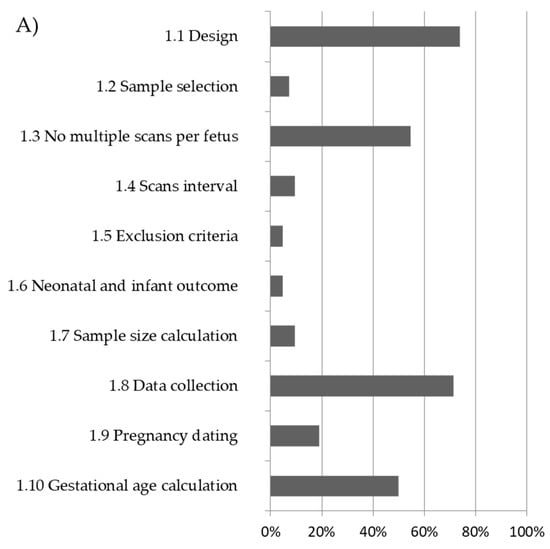
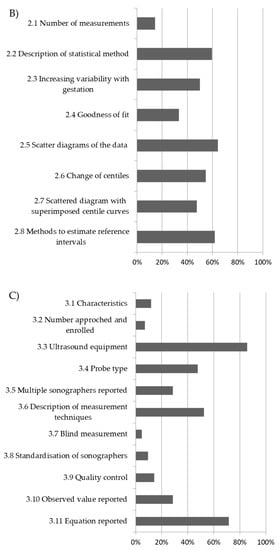
Figure 2.
Overall methodological quality of included studies and percentage of low risk of bias: (A) study design; (B) statistical methods; (C) reporting methods.
The overall quality score in terms of low risk of bias ranged between 5 and 74%, 15 and 65%, and 5 and 86% for criteria analysed in the study design, statistical methods, and reporting methods domain, respectively (Figure 2). Overall, 30 studies (72%) undertook prospective data collection. Only 5 studies (12%) had a longitudinal design, and 26 studies (62%) had a cross-sectional design, whereas in all the remaining 11 studies (26%) the design was not reported. Only two studies (5%) had inclusion and exclusion criteria that were clearly stated and applied, and also reported detailed neonatal or infant outcomes (Table S4). Considering the domain of ‘statistical methods’, there was a low risk of bias in only 25 studies (60%); a regression equation was reported in order to calculate expected centiles in 30 studies (72%), and only in 14 studies (33%) was performed goodness of fit of the proposed model (Table S4, Figure 2). Most of the studies scored low on criteria concerning the reporting methods such as the description of the measurement technique (50%). Only six studies (14%) adopted a comprehensive strategy for ultrasound quality control—in four cases (9%) sonographers were standardised to the measuring technique, and in two (5%), cases measurements were taken in a blinded fashion.
In Table 2, we present the centiles of each structure at three relevant gestational ages for those studies where this was possible—either reported by the authors or calculated when a relevant equation was reported. Figure 3 shows the distribution of the 5th and the 95th centile for the TCD in studies with a high risk of bias in more or less than 50% of the quality criteria. Studies with a lower risk of bias had a smaller distribution of centiles, compared with studies with a higher risk of bias at any of the three gestational ages analysed. The same analysis could not be reported for other structures in view of a low number of data points.

Table 2.
Comparison of centiles value.
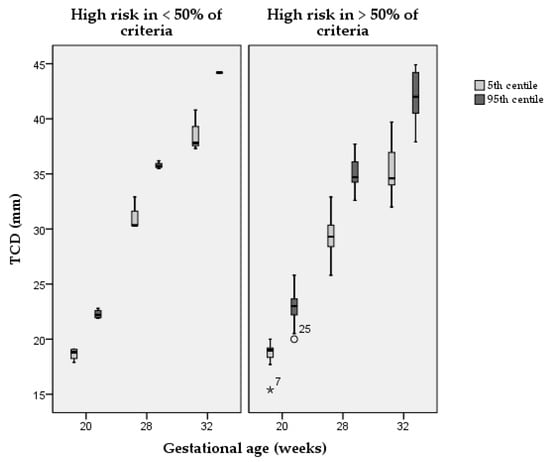
Figure 3.
Distribution of the 5th and the 95th centile in studies with a high risk of bias in more or less than 50% of the quality criteria. TCD = transcerebellar diameter; ° = outlier; * = extreme outlier.
4. Discussion
The aim of this systematic review was to evaluate the methodology used in studies aimed at creating charts on specific fetal brain structures measured by ultrasound. Using a set of 29 predefined quality criteria on study design, statistical methods, and reporting methods, studies were scored as having a low or high risk of bias. This approach has been previously proposed in order to evaluate the quality of existing charts on fetal biometry and first-trimester dating [3,4].
In 34 out of 42 studies (80%), a high risk of bias was found in >50% of the domains scored (Figure 2). Only the studies by Napolitano et al. and Rodriguez-Sibaja et al. were at low risk of bias in a significantly high number of quality criteria, respectively, in 93% and 86%; all other studies were below 60% [36,39].
The highest potential for bias was noted in most of the criteria regarding the ‘study design’. Specifically, only two studies reported a low risk of bias in the definition of inclusion/exclusion criteria. In addition, only two studies described detailed neonatal and development outcomes [36,39]. In two other studies, there was neurological follow-up described, but this was never assessed with a standardised approach in all fetuses included. Thus, in the study by Hilpert et al. telephone follow-up was obtained at a minimum of 2 years of age in fetuses with PV measurement of 10 mm or more [26], and in the study by Farrell et al., most fetuses with PV measurements more than 8 mm had an unspecified follow-up that varied from 2 days to 12 months [19]. We believe that infant follow-up is essential if the aim is to create charts of brain structures. This is because pathological conditions may be prevalent, possibly affecting the resulting charts, and because many cases of developmental delay cannot currently be predicted by antenatal ultrasound. The proportion of infants with abnormal development in a study should confirm that they are representative of a healthy population.
The reason for this high source for bias identified in these fields is also probably related to the retrospective design (around 30% of the studies). Furthermore, in only four studies sample size estimation was performed [18,36,39,44], and only three studies had population-based sample selection [36,39,47], with all other studies reporting either convenience sampling or arbitrary recruitment or sampling methods that were not reported (Figure 2).
One area of significant bias in fetal biometry is associated with calliper placement not done in a blinded fashion [49] (considered in only two studies [36,39]). It is difficult to quantify the magnitude of this factor, but one might assume that for values close to cut-offs for referral or investigation (e.g., 10 mm for the PV); such lack of blinding may have a relevant impact on the resulting charts. The sonographer tends to over- or underestimate to generate referrals or avoid abnormality diagnosis, respectively. In addition, there was a lack of ultrasound quality control in 80% of the studies, which has been previously demonstrated to be useful in reducing measurement variability [50].
The high risk of bias in most of the criteria assessed may explain the substantial heterogeneity in the resulting centiles. For example, in the case of TCD at 32 weeks of gestation, the 50th centile according to Vinkesteijn et al. was equivalent to the 95th centile according to Hayata et al. and Hata et al. [24,25,48]. Likewise, the 95th centile according to Smith et al. was smaller than the 5th centile according to Serhatlioglu et al. (Table 2) [41,42]. In Figure 3, we show that the higher is the risk of bias score the higher is the variability in centiles reported of TCD for the three gestational age ranges considered. It is clear that such differences are not desirable since they can lead to false-positive or false-negative results. This also makes different studies difficult to compare in research.
Only nine studies reported the regression equation of the standard deviation—instead, they either did not report the equation or they just reported the mean standard deviation throughout gestation. This is an important limitation since a clear increase in variability with advancing gestation from visual assessment of scatter diagrams in those studies was observed. Hence, the changing standard deviation with advancing gestation should be taken into account and is needed for the accurate calculation of centiles.
Strengths and Limitations of the Review
This is the first systematic review on the topic and it includes all currently published charts on POF, SF, AV, PV, TCD, and CM. A rigorous methodology based on predetermined quality criteria was applied and was based on previously published quality checklists used to evaluate studies on other aspects of fetal size [3,4]. We had no limitations on the year of inclusion of studies; it could be argued that, in a rapidly emerging field such as prenatal imaging, older studies should not be subjected to the same rigorous quality assessment as more recent ones. It is also fair to assume a gradual improvement in both the ultrasound technology and the statistical methods of data analysis over the decades. In fact, there was some evidence of improving study quality over time; the median quality score for the first half of the studies (between 1984 and 2008) was 9, while the median score for the latter half of the studies was 13. Nevertheless, the high risk of bias was time independent for many criteria such as inclusion/exclusion criteria, neonatal and infant outcome, sample selection, characteristics of the study population, measurement acquired blindly, and standardisation of the sonographers.
5. Conclusions
The use of a wide range of reference charts can affect both clinical assessment and research on the development of new technologies associated with antenatal ultrasound [51,52]. We have shown the lack of methodological quality in most existing studies aiming at creating fetal brain charts. Most studies have significant risks of bias, leading to large differences in size charts of normal brain structures. This provides the controversial background context to studies that suggest that differences in fetal brain measurements exist due to, for example, maternal ethnicity, country of origin, or fetal sex: it is not possible to ascribe such differences to biological causes when basic methods of study design, statistical analyses, and reporting are not optimised and when different studies utilise widely different methods.
This review of the literature has shown that 40 out of 42 studies had a low risk of bias in no more than 60% of the quality criteria. In order to allow a unified approach to clinical practice and research, we suggest using charts that have an optimal study design, use a prescriptive approach, and a methodology that is at low risk of bias, including fetuses from low-risk populations worldwide, and that follow infants up for developmental assessment to confirm that the population was appropriate for the construction of these charts.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/diagnostics11060916/s1, Table S1: Search strategy, Table S2: List of methodological quality criteria, Table S3: Excluded studies after full paper review, Table S4: Risk of bias score.
Author Contributions
R.N., V.D. and A.T.P. designed the study and defined the quality criteria a priori. N.W.R. made the literature search. R.N., V.D. and A.C. extracted the data and scored the studies. R.N., V.D., A.C., C.I. and A.T.P. analysed the data, interpreted the results, drafted the manuscript, and made the decision to submit. All authors assisted in drafting the article submitted and revising it for important intellectual content, and all edited and approved the final, submitted version. All authors have read and agreed to the published version of the manuscript.
Funding
This project was supported by funding for the INTERGROWTH-21st Project (Grant ID# 49038) from the Bill and Melinda Gates Foundation to the University of Oxford, for which we are very grateful. Aris T. Papageorghiou is supported by the National Institute for Health Research (NIHR) Oxford Biomedical Research Centre (BRC). Aris T. Papageorghiou is the Senior Scientific Advisor of Intelligent Ultrasound.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- ISUOG Committee. ISUOG Practice Guidelines (Updated): Sonographic examination of the fetal central nervous system. Part 1: Performance of screening examination and indications for targeted neurosonography. Ultrasound Obs. Gynecol. 2020, 56, 476–484. [Google Scholar] [CrossRef]
- Namburete, A.I.; Stebbing, R.V.; Kemp, B.; Yaqub, M.; Papageorghiou, A.T.; Noble, A.J. Learning-based prediction of gestational age from ultrasound images of the fetal brain. Med. Image Anal. 2015, 21, 72–86. [Google Scholar] [CrossRef]
- Napolitano, R.; Dhami, J.; Ohuma, E.O.; Ioannou, C.; Conde-Agudelo, A.; Kennedy, S.H.; Villar, J.; Papageorghiou, A.T. Pregnancy dating by fetal crown-rump length: A systematic review of charts. BJOG 2014, 121, 556–565. [Google Scholar] [CrossRef]
- Ioannou, C.; Talbot, K.; Ohuma, E.; Sarris, I.; Villar, J.; Conde-Agudelo, A.; Papageorghiou, A.T. Systematic review of methodology used in ultrasound studies aimed at creating charts of fetal size. BJOG 2012, 119, 1425–1439. [Google Scholar] [CrossRef]
- Papageorghiou, A.T.; Ohuma, E.O.; Altman, D.G.; Todros, T.; Cheikh Ismail, L.; Lambert, A.; Jaffer, Y.A.; Bertino, E.; Gravett, M.G.; Purwar, M.; et al. International Fetal and Newborn Growth Consortium for the 21st Century (INTERGROWTH-21st). International standards for fetal growth based on serial ultrasound measurements: The Fetal Growth Longitudinal Study of the INTERGROWTH-21st Project. Lancet 2014, 384, 869–879. [Google Scholar] [CrossRef]
- Stewart, L.A.; Clarke, M.; Rovers, M.; Riley, R.D.; Simmonds, M.; Stewart, G.; Tierney, J.F. PRISMA-IPD Development Group. Preferred Reporting Items for Systematic Review and Meta-Analyses of individual participant data: The PRISMA-IPD Statement. JAMA 2015, 313, 1657–1665. [Google Scholar] [CrossRef] [PubMed]
- Alagappan, R.; Browning, P.D.; Laorr, A.; McGahan, J.P. Distal lateral ventricular atrium: Reevaluation of normal range. Radiology 1994, 193, 405–408. [Google Scholar] [CrossRef] [PubMed]
- Almog, B.; Gamzu, R.; Achiron, R.; Fainaru, O.; Zalel, Y. Fetal lateral ventricular width: What should be its upper limit? A prospective cohort study and reanalysis of the current and previous data. J. Ultrasound Med. 2003, 22, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Alonso, I.; Borenstein, M.; Grant, G.; Narbona, I.; Azumendi, G. Depth of brain fissures in normal fetuses by prenatal ultrasound between 19 and 30 weeks of gestation. Ultrasound Obstet. Gynecol. 2010, 36, 693–699. [Google Scholar] [CrossRef] [PubMed]
- Alves, C.M.; Araujo Junior, E.; Nardozza, L.M.; Goldman, S.M.; Martinez, L.H.; Martins, W.P.; Oliveira, P.S.; Moron, A.F. Reference ranges for fetal brain fissure development on 3-dimensional sonography in the multiplanar mode. J. Ultrasound Med. 2013, 32, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Araujo Junior, E.; Martins, W.P.; Rolo, L.C.; Pires, C.R.; Zanforlin Filho, S.M. Normative data for fetal cisterna magna length measurement between 18 and 24 weeks of pregnancy. Childs Nerv. Syst. 2014, 30, 9–12. [Google Scholar] [CrossRef] [PubMed]
- Araujo Junior, E.; Martins, W.P.; Nardozza, L.M.; Pires, C.R.; Filho, S.M. Reference range of fetal transverse cerebellar diameter between 18 and 24 weeks of pregnancy in a Brazilian population. J. Child Neurol. 2015, 30, 250–253. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.N. Reassessment of the normal fetal cisterna magna during gestation and an alternative approach to the definition of cisterna magna dilatation. Fetal Diagn. Ther. 2013, 34, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Cardoza, J.D.; Goldstein, R.B.; Filly, R.A. Exclusion of fetal ventriculomegaly with a single measurement: The width of the lateral ventricular atrium. Radiology 1988, 169, 711–714. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.H.; Chang, F.M.; Yu, C.H.; Ko, H.C.; Chen, H.Y. Three-dimensional ultrasound in the assessment of fetal cerebellar transverse and antero-posterior diameters. Ultrasound Med. Biol. 2000, 26, 175–182. [Google Scholar] [CrossRef]
- Chavez, M.R.; Ananth, C.V.; Smulian, J.C.; Lashley, S.; Kontopoulos, E.V.; Vintzileos, A.M. Fetal transcerebellar diameter nomogram in singleton gestations with special emphasis in the third trimester: A comparison with previously published nomograms. Am. J. Obs. Gynecol. 2003, 189, 1021–1025. [Google Scholar] [CrossRef]
- Chen, X.; Li, S.L.; Luo, G.Y.; Norwitz, E.R.; Ouyang, S.Y.; Wen, H.X.; Yuan, Y.; Tian, X.X.; He, J.M. Ultrasonographic Characteristics of Cortical Sulcus Development in the Human Fetus between 18 and 41 Weeks of Gestation. Chin. Med. J. 2017, 130, 920–928. [Google Scholar] [CrossRef] [PubMed]
- Eze, C.U.; Onwuzu, Q.E.; Uchechukwu, N.I. Sonographic Reference Values for Fetal Transverse Cerebellar Diameter in the Second and Third Trimesters in a Nigerian Population. J. Diagn. Med. Sonogr. 2017, 33, 174–181. [Google Scholar] [CrossRef]
- Farrell, T.A.; Hertzberg, B.S.; Kliewer, M.A.; Harris, L.; Paine, S.S. Fetal lateral ventricles: Reassessment of normal values for atrial diameter at US. Radiology 1994, 193, 409–411. [Google Scholar] [CrossRef]
- Goel, P.; Singla, M.; Ghai, R.; Jain, S.; Budhiraja, V.; Babu, C.S.R. Transverse Cerebellar Diameter—A Marker for Estimation of Gestational Age. J. Anat. Soc. India 2010, 59, 158–161. [Google Scholar] [CrossRef]
- Goldstein, I.; Reece, E.A.; Pilu, G.; Bovicelli, L.; Hobbins, J.C. Cerebellar measurements with ultrasonography in the evaluation of fetal growth and development. Am. J. Obstet. Gynecol. 1987, 156, 1065–1069. [Google Scholar] [CrossRef]
- Goldstein, I.; Reece, E.A.; Pilu, G.L.; Hobbins, J.C.; Bovicelli, L. Sonographic evaluation of the normal developmental anatomy of fetal cerebral ventricles: I. The frontal horn. Obstet. Gynecol. 1988, 72, 588–592. [Google Scholar] [PubMed]
- Goldstein, I.; Reece, E.A.; Pilu, G.L.; Hobbins, J.C. Sonographic evaluation of the normal developmental anatomy of fetal cerebral ventricles. IV.: The posterior horn. Am. J. Perinatol. 1990, 7, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Hata, K.; Hata, T.; Senoh, D.; Makihara, K.; Aoki, S.; Takamiya, O.; Kitao, M. Ultrasonographic measurement of the fetal transverse cerebellum in utero. Gynecol. Obstet. Invest. 1989, 28, 111–112. [Google Scholar] [CrossRef] [PubMed]
- Hayata, K.; Hiramatsu, Y.; Masuyama, H.; Etou, E.; Nobumoto, E.; Mitsui, T. Creation of a cerebellar diameter reference standard and its clinical application to the detection of cerebellar hypoplasia unique to trisomy 18. J. Obstet. Gynaecol. Res. 2015, 41, 1899–1904. [Google Scholar] [CrossRef]
- Hilpert, P.L.; Hall, B.E.; Kurtz, A.B. The atria of the fetal lateral ventricles: A sonographic study of normal atrial size and choroid plexus volume. Am. J. Roentgenol. 1995, 164, 731–734. [Google Scholar] [CrossRef][Green Version]
- Ishola, A.; Asaleye, C.M.; Ayoola, O.O.; Loto, O.M.; Idowu, B.M. Reference Ranges of Fetal Cerebral Lateral Ventricle Parameters by Ultrasonography. Rev. Bras. Ginecol. Obs. 2016, 38, 428–435. [Google Scholar] [CrossRef]
- Joshi, B.R. Fetal transcerebellar diameter nomogram in Nepalese population. J. Inst. Med. 2010, 32, 19–23. [Google Scholar] [CrossRef]
- Koktener, A.; Dilmen, G.; Kurt, A. The cisterna magna size in normal second-trimester fetuses. J. Perinat. Med. 2007, 35, 217–219. [Google Scholar] [CrossRef]
- Koktener, A.; Dilmen, G.; Yıldırım, M.; Kösehan, D.; Akın, K.; Çakır, B. Growth of the lateral ventricle in normal second-trimester fetuses: Is a nomogram practical? J. Pediatric Neuroradiol. 2012, 1, 37–41. [Google Scholar]
- Koning, I.V.; Dudink, J.; Groenenberg, I.A.L.; Willemsen, S.P.; Reiss, I.K.M.; Steegers-Theunissen, R.P.M. Prenatal cerebellar growth trajectories and the impact of periconceptional maternal and fetal factors. Hum. Reprod. 2017, 32, 1230–1237. [Google Scholar] [CrossRef] [PubMed]
- Lei, H.; Wen, S.W. Ultrasonographic examination of intrauterine growth for multiple fetal dimensions in a Chinese population. Central-South China Fetal Growth Study Group. Am. J. Obs. Gynecol. 1998, 178, 916–921. [Google Scholar] [CrossRef]
- Mahony, B.S.; Callen, P.W.; Filly, R.A.; Hoddick, W.K. The fetal cisterna magna. Radiology 1984, 153, 773–776. [Google Scholar] [CrossRef] [PubMed]
- Medvedev, M.V.; Kozlova, O.I.; Altynnik, N.A.; Dmitrashchenko, A.A.; Lubashev, A.Y. Normal range of fetal brain structures in the second trimester ultrasound screening. J. Pharm. Sci. Res. 2018, 9, 1156–1159. [Google Scholar]
- Mittal, P.; Goncalves, L.F.; Kusanovic, J.P.; Espinoza, J.; Lee, W.; Nien, J.K.; Soto, E.; Romero, R. Objective evaluation of sylvian fissure development by multiplanar 3-dimensional ultrasonography. J. Ultrasound Med. 2007, 26, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Napolitano, R.; Molloholli, M.; Donadono, V.; Ohuma, E.O.; Wanyonyi, S.Z.; Kemp, B.; Yaqub, M.K.; Ash, S.; Barros, F.C.; Carvalho, M.; et al. International Fetal and Newborn Growth Consortium for the 21st Century (INTERGROWTH-21st). International standards for fetal brain structures based on serial ultrasound measurements from Fetal Growth Longitudinal Study of INTERGROWTH-21. Ultrasound Obs. Gynecol. 2020, 56, 359–370. [Google Scholar] [CrossRef]
- Passos, A.P.; Araujo Júnior, E.; Bruns, R.F.; Nardozza, L.M.; Moron, A.F. Reference ranges of fetal cisterna magna length and area measurements by 3-dimensional ultrasonography using the multiplanar mode. J. Child. Neurol. 2015, 30, 209–215. [Google Scholar] [CrossRef]
- Peixoto, A.B.; Caldas, T.M.; Barbosa, M.F.; Romão, L.A.; Martins, W.P.; Araujo Júnior, E. Reference values for the fetal lateral ventricle atrium measurement in the second and third trimesters of pregnancy in a Brazilian population. J. Matern. Fetal Neonatal Med. 2016, 29, 2337–2340. [Google Scholar] [CrossRef]
- Rodriguez-Sibaja, M.J.; Villar, J.; Ohuma, E.O.; Napolitano, R.; Heyl, S.; Carvalho, M.; Jaffer, Y.A.; Noble, J.A.; Oberto, M.; Purwar, M.; et al. Fetal cerebellar growth and Sylvian fissure maturation: International standards from the Fetal Growth Longitudinal Study of the INTERGROWTH-21. Ultrasound Obs. Gynecol. 2021, 57, 614–623. [Google Scholar] [CrossRef]
- Salomon, L.J.; Bernard, J.P.; Ville, Y. Reference ranges for fetal ventricular width: A non-normal approach. Ultrasound Obs. Gynecol. 2007, 30, 61–66. [Google Scholar] [CrossRef]
- Serhatlioglu, S.; Kocakoc, E.; Kiris, A.; Sapmaz, E.; Boztosun, Y.; Bozgeyik, Z. Sonographic measurement of the fetal cerebellum, cisterna magna, and cavum septum pellucidum in normal fetuses in the second and third trimesters of pregnancy. J. Clin. Ultrasound 2003, 31, 194–200. [Google Scholar] [CrossRef]
- Smith, P.A.; Johansson, D.; Tzannatos, C.; Campbell, S. Prenatal measurement of the fetal cerebellum and cisterna cerebellomedullaris by ultrasound. Prenat. Diagn. 1986, 6, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Snijders, R.J.; Nicolaides, K.H. Fetal biometry at 14–40 weeks’ gestation. Ultrasound Obs. Gynecol. 1994, 4, 34–48. [Google Scholar] [CrossRef]
- Spinelli, M.; Sica, C.; Ghezzi, F.; Cromi, A.; Surbek, D.; Raio, L. Nomograms of the Fetal Sylvian Fissure and Insular Lobe throughout Gestation: A Multicentric, Ultrasonographic Cross-Sectional Study. Fetal Diagn. 2019, 45, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Takano, M.; Nakata, M.; Oji, A.; Nagasaki, S.; Umemura, N.; Maemura, T.; Morita, M. Utility of fetal anteroposterior to transverse cerebellar diameter ratio to exclude cerebellar hypoplasia in trisomy 18. J. Obs. Gynaecol. Res. 2018, 44, 1031–1035. [Google Scholar] [CrossRef] [PubMed]
- Uerpairojkit, B.; Charoenvidhya, D.; Manotaya, S.; Tanawattanachareon, S.; Wacharaprechanont, T.; Tannirandorn, Y. Fetal transverse cerebellar diameter in Thai population. J. Med. Assoc. Thai. 2001, 84, S346–S351. [Google Scholar]
- Verburg, B.O.; Steegers, E.A.; De Ridder, M.; Snijders, R.J.; Smith, E.; Hofman, A.; Moll, H.A.; Jaddoe, V.W.; Witteman, J.C. New charts for ultrasound dating of pregnancy and assessment of fetal growth: Longitudinal data from a population-based cohort study. Ultrasound Obs. Gynecol. 2008, 31, 388–396. [Google Scholar] [CrossRef]
- Vinkesteijn, A.S.; Mulder, P.G.; Wladimiroff, J.W. Fetal transverse cerebellar diameter measurements in normal and reduced fetal growth. Ultrasound Obs. Gynecol. 2000, 15, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Napolitano, R.; Donadono, V.; Ohuma, E.O.; Knight, C.L.; Wanyonyi, S.Z.; Kemp, B.; Norris, T.; Papageorghiou, A.T. Scientific basis for standardization of fetal head measurements by ultrasound: A reproducibility study. Ultrasound Obs. Gynecol. 2016, 48, 80–85. [Google Scholar] [CrossRef]
- Cavallaro, A.; Ash, S.T.; Napolitano, R.; Wanyonyi, S.; Ohuma, E.O.; Molloholli, M.; Sande, J.; Sarris, I.; Ioannou, C.; Norris, T.; et al. Quality control of ultrasound for fetal biometry: Results from the INTERGROWTH-21st Project. Ultrasound Obs. Gynecol. 2018, 52, 332–339. [Google Scholar] [CrossRef]
- Kuklisova-Murgasova, M.; Cifor, A.; Napolitano, R.; Papageorghiou, A.; Quaghebeur, G.; Rutherford, M.A.; Hajnal, J.V.; Noble, J.A.; Schnabel, J.A. Registration of 3D fetal neurosonography and MRI. Med. Image Anal. 2013, 17, 1137–1150. [Google Scholar] [CrossRef] [PubMed]
- Maraci, M.A.; Bridge, C.P.; Napolitano, R.; Papageorghiou, A.; Noble, J.A. A framework for analysis of linear ultrasound videos to detect fetal presentation and heartbeat. Med. Image Anal. 2017, 37, 22–36. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).