uPAR PET/CT for Prognostication and Response Assessment in Patients with Metastatic Castration-Resistant Prostate Cancer Undergoing Radium-223 Therapy: A Prospective Phase II Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. PET/CT Acquisition
2.3. Image Analysis
2.4. Clinical Outcomes
2.5. Statistical Analysis
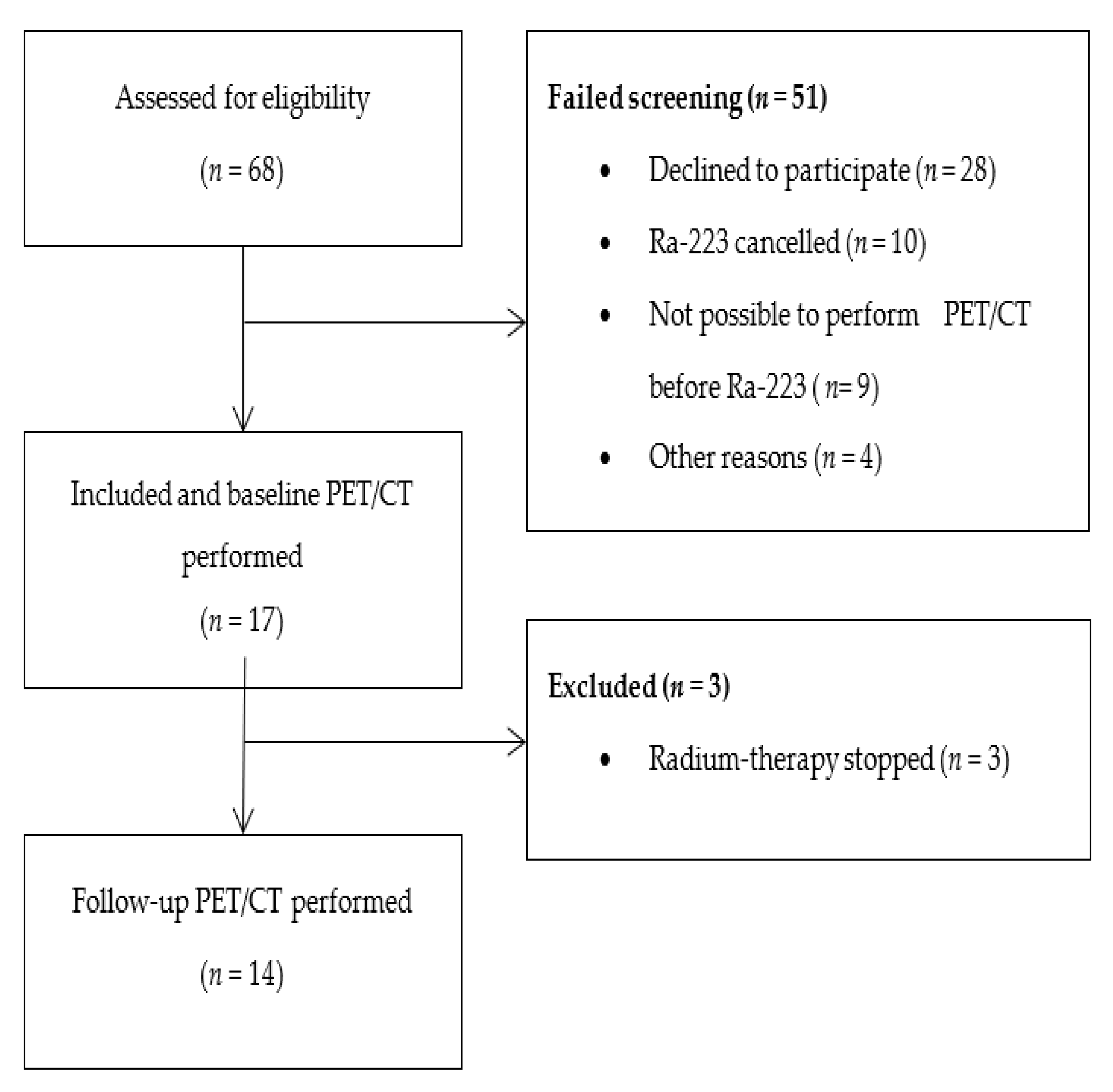
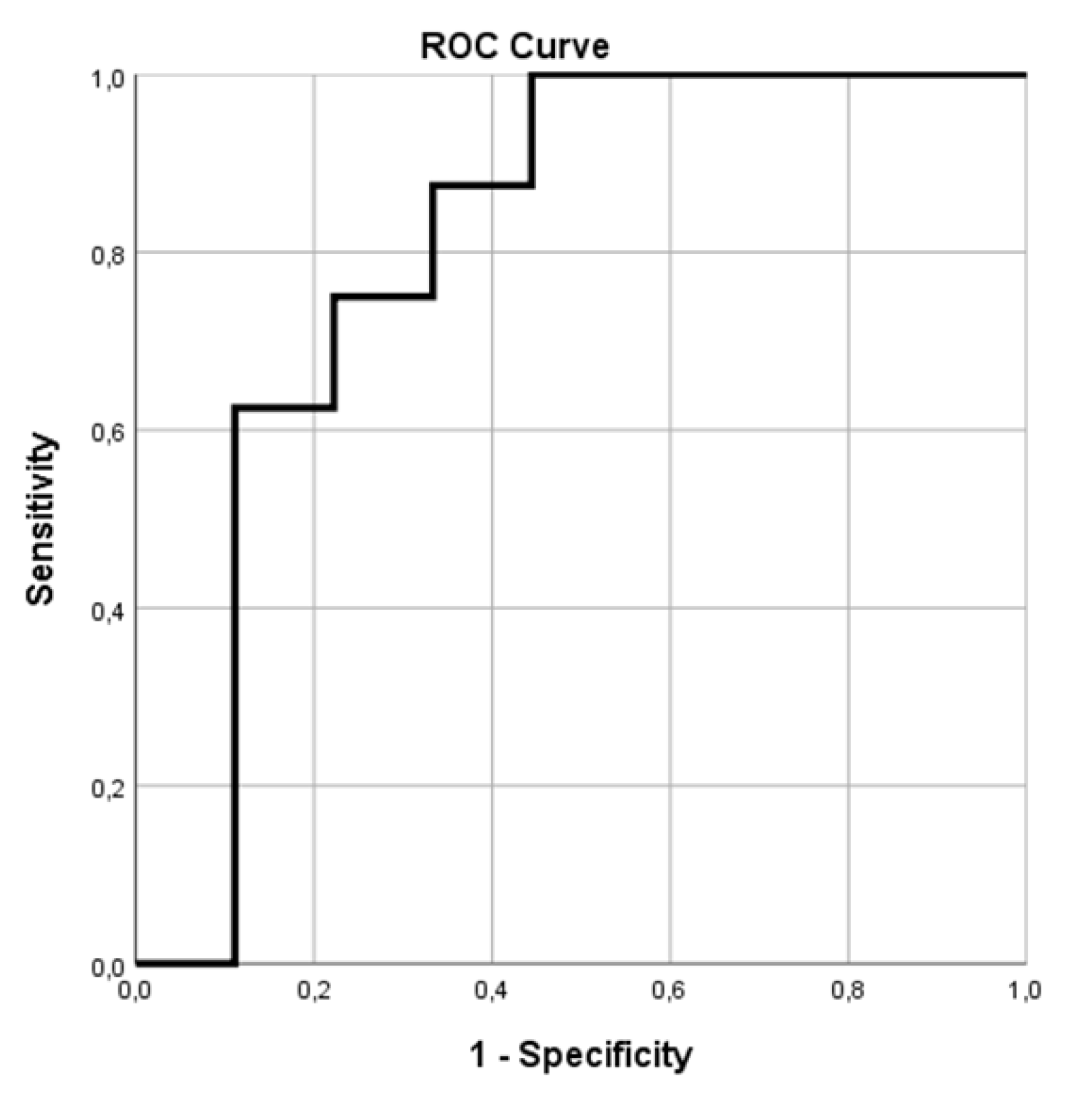
3. Results
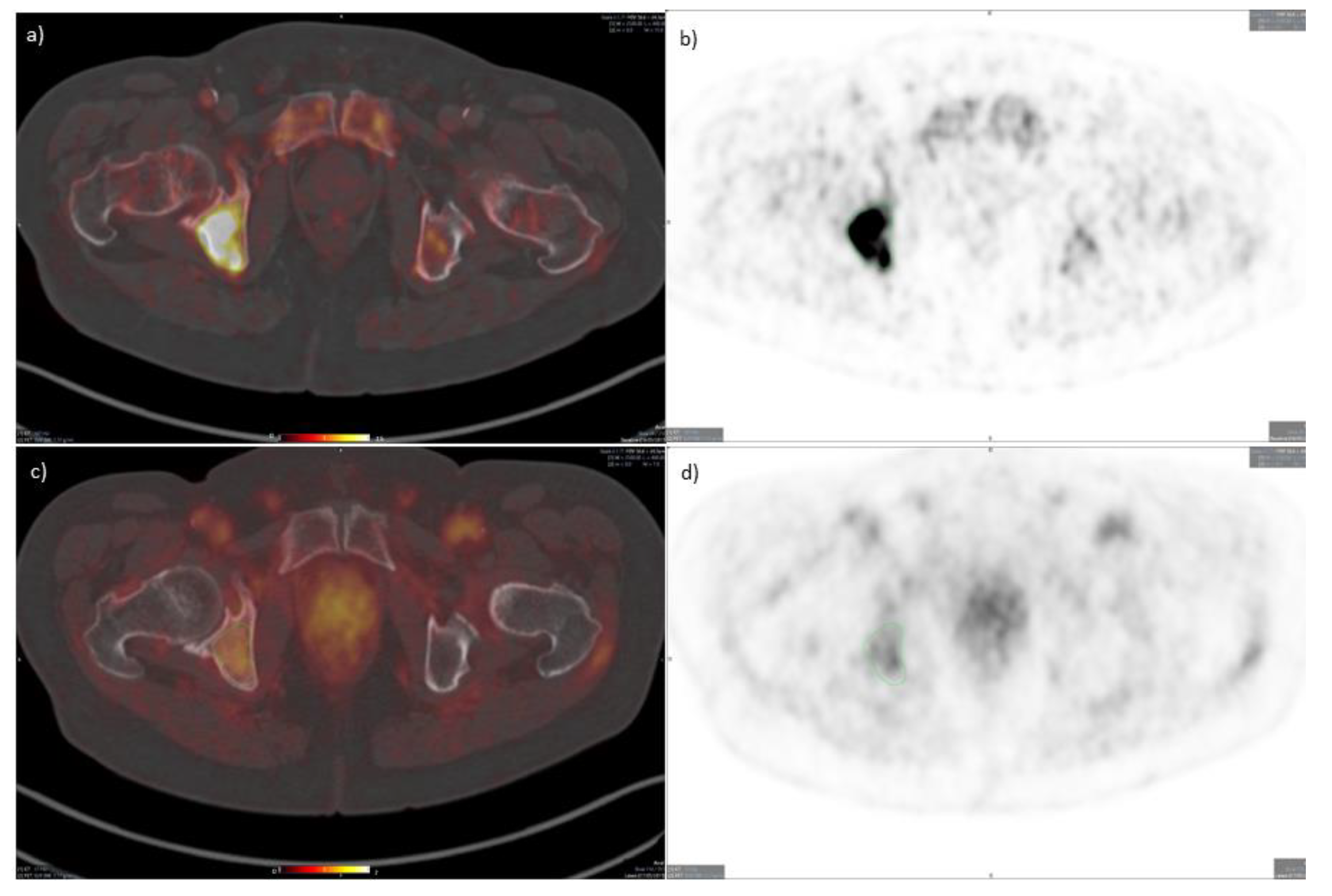
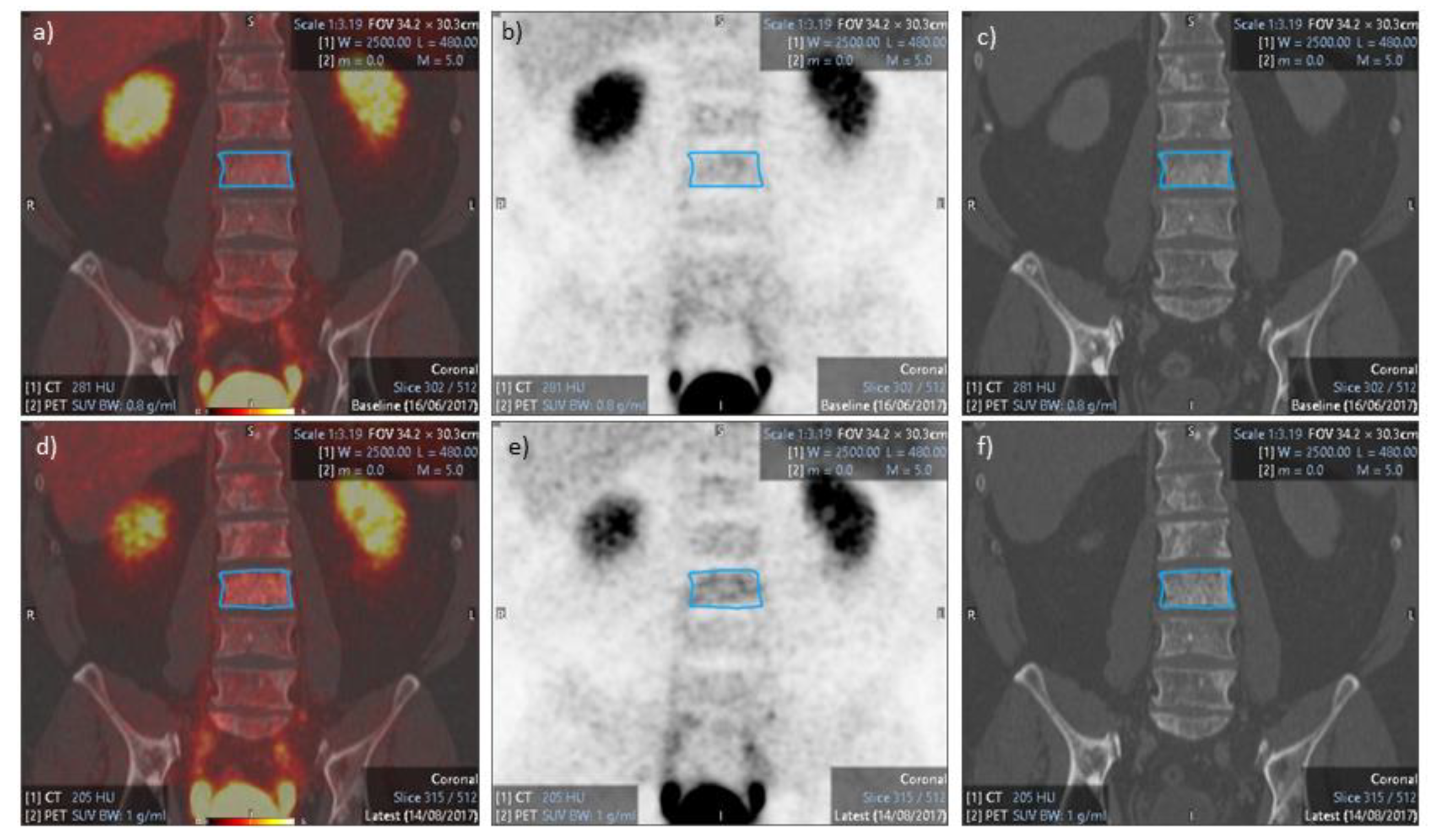
3.1. PET/CT Results
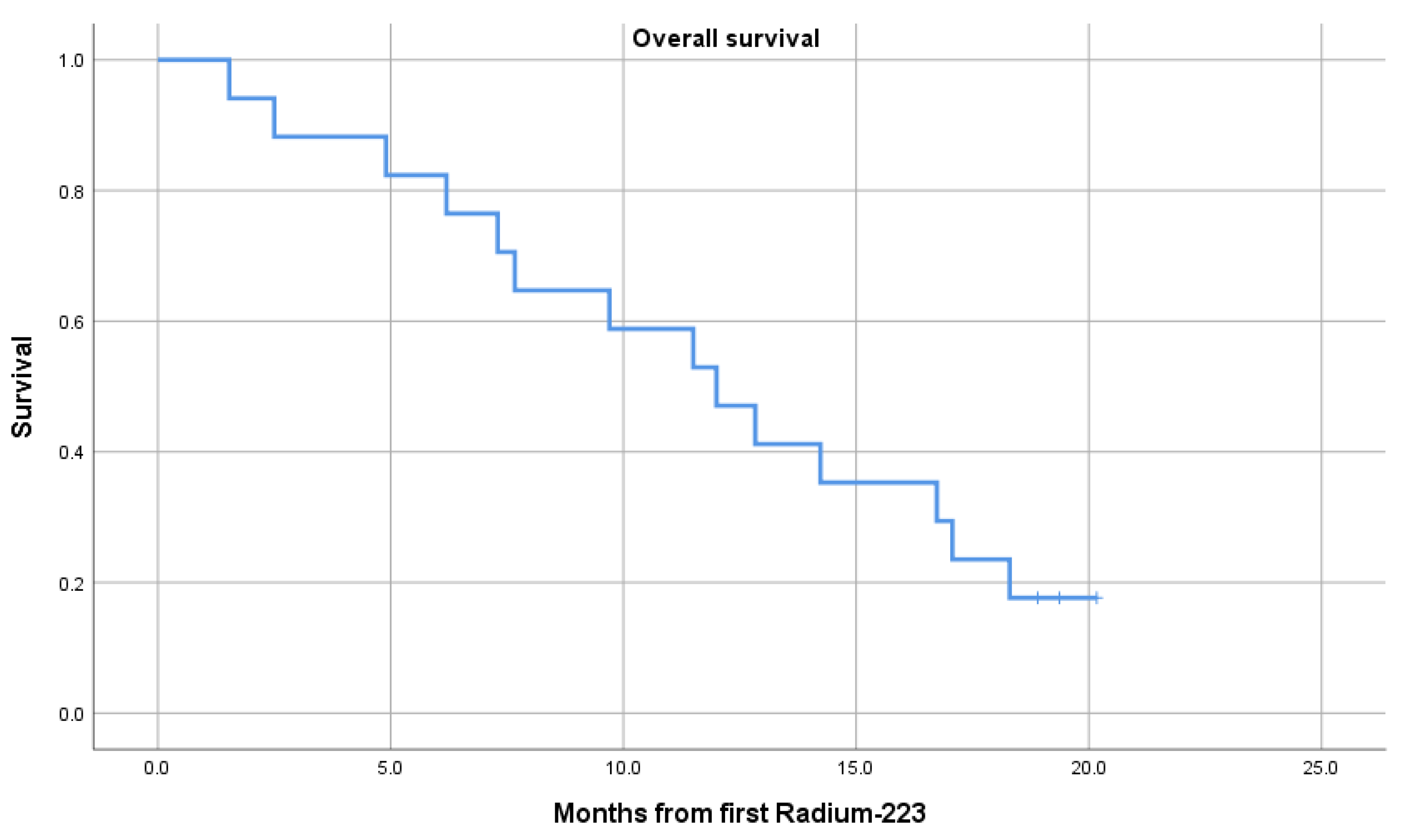
3.2. Overall Survival
3.3. Response
3.4. Symptomatic Skeletal Events
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Parker, C.; Nilsson, D.S.; Heinrich, S.D.; Helle, S.I.; O’Sullivan, J.M.; Fosså, S.D.; Chodacki, A.; Wiechno, P.; Logue, J.; Seke, M.; et al. Alpha Emitter Radium-223 and Survival in Metastatic Prostate Cancer. N. Engl. J. Med. 2013, 369, 213–223. [Google Scholar] [CrossRef]
- Sartor, O.; Coleman, R.; Nilsson, S.; Heinrich, D.; I Helle, S.; O’Sullivan, J.M.; Fosså, S.D.; Chodacki, A.; Wiechno, P.; Logue, J.; et al. Effect of radium-223 dichloride on symptomatic skeletal events in patients with castration-resistant prostate cancer and bone metastases: Results from a phase 3, double-blind, randomised trial. Lancet Oncol. 2014, 15, 738–746. [Google Scholar] [CrossRef]
- Sartor, O.; Coleman, R.E.; Nilsson, S.; Heinrich, D.; Helle, S.I.; O’Sullivan, J.M.; Vogelzang, N.J.; Bruland, Ø.; Kobina, S.; Wilhelm, S.; et al. An exploratory analysis of alkaline phosphatase, lactate dehydrogenase, and prostate-specific antigen dynamics in the phase 3 ALSYMPCA trial with radium-223. Ann. Oncol. 2017, 28, 1090–1097. [Google Scholar] [CrossRef] [PubMed]
- Isensee, G.; Péporté, A.; Müller, J.; Schmid, S.; Gillessen, S.; Omlin, A. Is There a Flare Phenomenon on Bone Scintigraphy in Men With Advanced Prostate Cancer Treated With Radium-223? Clin. Genitourin. Cancer 2018, 16, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Wang, L.; Wang, Y.; Zhuo, Y.; Li, H.; Chen, J.; Chen, W. Overexpression of CD147 contributes to the chemoresistance of head and neck squamous cell carcinoma cells. J. Oral Pathol. Med. 2013, 42, 541–546. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Xing, Z.-H.; Jiang, Q.-W.; Yang, Y.; Huang, J.-R.; Yuan, M.-L.; Wei, M.-N.; Li, Y.; Wang, S.-T.; Liu, K.; et al. Targeting uPAR by CRISPR/Cas9 System Attenuates Cancer Malignancy and Multidrug Resistance. Front. Oncol. 2019, 9, 80. [Google Scholar] [CrossRef] [PubMed]
- Eastman, B.M.; Jo, M.; Webb, D.L.; Takimoto, S.; Gonias, S.L. A transformation in the mechanism by which the urokinase receptor signals provides a selection advantage for estrogen receptor-expressing breast cancer cells in the absence of estrogen. Cell. Signal. 2012, 24, 1847–1855. [Google Scholar] [CrossRef] [PubMed]
- Rabbani, S.A.; Ateeq, B.; Arakelian, A.; Valentino, M.L.; Shaw, D.E.; Dauffenbach, L.M.; Kerfoot, C.A.; Mazar, A.P. An Anti-Urokinase Plasminogen Activator Receptor Antibody (ATN-658) Blocks Prostate Cancer Invasion, Migration, Growth, and Experimental Skeletal Metastasis In Vitro and In Vivo. Neoplasia 2010, 12, 778–788. [Google Scholar] [CrossRef]
- Mahmood, N.; Mihalcioiu, C.; Rabbani, S.A. Multifaceted Role of the Urokinase-Type Plasminogen Activator (uPA) and Its Receptor (uPAR): Diagnostic, Prognostic, and Therapeutic Applications. Front. Oncol. 2018, 8, 24. [Google Scholar] [CrossRef] [PubMed]
- Smith, H.W. and Marshall, C.J. Regulation of cell signalling by uPAR. Nat. Rev. Mol. Cell Biol. 2010, 23–36. [Google Scholar] [CrossRef]
- Kumano, M.; Miyake, H.; Muramaki, M.; Furukawa, J.; Takenaka, A.; Fujisawa, M. Expression of urokinase-type plasminogen activator system in prostate cancer: Correlation with clinicopathological outcomes in patients undergoing radical prostatectomy. Urol. Oncol. Semin. Orig. Investig. 2009, 27, 180–186. [Google Scholar] [CrossRef]
- Cozzi, P.J.; Wang, J.; Delprado, W.; Madigan, M.C.; Fairy, S.; Russell, P.J.; Li, Y. Evaluation of urokinase plasminogen activator and its receptor in different grades of human prostate cancer☆. Hum. Pathol. 2006, 37, 1442–1451. [Google Scholar] [CrossRef] [PubMed]
- Skovgaard, D.; Persson, M.; Brandt-Larsen, M.; Christensen, C.; Madsen, J.; Klausen, T.L.; Holm, S.; Andersen, F.L.; Loft, A.; Berthelsen, A.K.; et al. Safety, Dosimetry, and Tumor Detection Ability of 68 Ga-NOTA-AE105: First-in-Human Study of a Novel Radioligand for uPAR PET Imaging. J. Nucl. Med. 2017, 58, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Fosbøl, M.Ø.; Kurbegovic, S.; Johannesen, H.H.; Røder, M.A.; Hansen, A.E.; Mortensen, J.; Loft, A.; Petersen, P.M.; Madsen, J.; Brasso, K.; et al. Urokinase-Type Plasminogen Activator Receptor (uPAR) PET/MRI of Prostate Cancer for Noninvasive Evaluation of Aggressiveness: Comparison with Gleason Score in a Prospective Phase 2 Clinical Trial. J. Nucl. Med. 2021, 62, 354–359. [Google Scholar] [CrossRef] [PubMed]
- Soloway, M.S.; Hardeman, S.W.; Hickey, D.; Todd, B.; Soloway, S.; Raymond, J.; Moinuddin, M. Stratification of patients with metastatic prostate cancer based on extent of disease on initial bone scan. Cancer 1988, 61, 195–202. [Google Scholar] [CrossRef]
- Heinrich, D.; Bektic, J.; Bergman, A.M.; Caffo, O.; Cathomas, R.; Chi, K.N.; Daugaard, G.; Keizman, D.; Kindblom, J.; Kramer, G.; et al. The Contemporary Use of Radium-223 in Metastatic Castration-resistant Prostate Cancer. Clin. Genitourin. Cancer 2018, 16, e223–e231. [Google Scholar] [CrossRef]
- Parker, C.; Heidenreich, A.; Nilsson, S.; Shore, N. Current approaches to incorporation of radium-223 in clinical practice. Prostate Cancer Prostatic Dis. 2018, 21, 37–47. [Google Scholar] [CrossRef]
- Etchebehere, E.; Brito, A.E.; Rezaee, A.; Langsteger, W.; Beheshti, M. Therapy assessment of bone metastatic disease in the era of 223radium. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 84–96. [Google Scholar] [CrossRef]
- Smith, M.; Parker, C.; Saad, F.; Miller, K.; Tombal, B.; Ng, Q.S.; Boegemann, M.; Matveev, V.; Piulats, J.M.; Zucca, L.E.; et al. Addition of radium-223 to abiraterone acetate and prednisone or prednisolone in patients with castration-resistant prostate cancer and bone metastases (ERA 223): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019, 20, 408–419. [Google Scholar] [CrossRef]
- Mottet, N.; van den Bergh, R.C.N.; Briers, E.; van den Broeck, T.; Cumberbatch, M.G.; De Santis, M.; Fanti, S.; Fossati, N.; Gandaglia, G.; Gillesen, S.; et al. EAU Guidelines. Edn. presented at the EAU Annual Congress Barcelona 2019. Available online: http://uroweb.org/guidelines/compilations-of-all-guidelines/ (accessed on 26 April 2021).
- Persson, M.; Hosseini, M.; Madsen, J.; Jørgensen, T.J.D.; Jensen, K.J.; Kjaer, A.; Ploug, M. Improved PET Imaging of uPAR Expression Using new 64Cu-labeled Cross-Bridged Peptide Ligands: Comparative in vitro and in vivo Studies. Theranostics 2013, 3, 618–632. [Google Scholar] [CrossRef]
- Persson, M.; Madsen, J.; Østergaard, S.; Jensen, M.M.; Jørgensen, J.T.; Juhl, K.; Lehmann, C.; Ploug, M.; Kjaer, A. Quantitative PET of Human Urokinase-Type Plasminogen Activator Receptor with 64Cu-DOTA-AE105: Implications for Visualizing Cancer Invasion. J. Nucl. Med. 2012, 53, 138–145. [Google Scholar] [CrossRef]
- Persson, M.; Madsen, J.; Østergaard, S.; Ploug, M.; Kjaer, A. 68Ga-labeling and in vivo evaluation of a uPAR binding DOTA- and NODAGA-conjugated peptide for PET imaging of invasive cancers. Nucl. Med. Biol. 2012, 39, 560–569. [Google Scholar] [CrossRef] [PubMed]
- Margheri, F.; D’Alessio, S.; Serratì, S.; Pucci, M.; Annunziato, F.; Cosmi, L.; Liotta, F.; Angeli, R.; Angelucci, A.; Gravina, G.L.; et al. Effects of blocking urokinase receptor signaling by antisense oligonucleotides in a mouse model of experimental prostate cancer bone metastases. Gene Ther. 2005, 12, 702–714. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Saliganan, A.D.; Meng, H.; Nabha, S.M.; Sabbota, A.L.; Sheng, S.; Bonfil, R.D.; Cher, M.L. Prostate Cancer Cell-Derived Urokinase-Type Plasminogen Activator Contributes to Intraosseous Tumor Growth and Bone Turnover. Neoplasia 2008, 10, 439–449. [Google Scholar] [CrossRef]
- Uprimny, C.; Kroiss, A.; Nilica, B.; Buxbaum, S.; Decristoforo, C.; Horninger, W.; Virgolini, I.J. 68Ga-PSMA ligand PET versus 18F-NaF PET: Evaluation of response to 223Ra therapy in a prostate cancer patient. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 362–363. [Google Scholar] [CrossRef]
- Fanti, S.; Hadaschik, B.; Herrmann, K. Proposal for Systemic-Therapy Response-Assessment Criteria at the Time of PSMA PET/CT Imaging: The PSMA PET Progression Criteria. J. Nucl. Med. 2019, 61, 678–682. [Google Scholar] [CrossRef] [PubMed]
| Pt. No. | Age (Years) | ECOG Performance Status | Number of Bone Metastases † | s-PSA (µg/L) | s-ALP † (U/L) | Previous Systemic Therapies for mCRPC |
|---|---|---|---|---|---|---|
| 1 | 76 | 1 | >20 | 931 | NA | Doce, Caba, Enza, Abi |
| 2 | 73 | 0 | 6–20 | 26 | 66 | Doce |
| 3 | 76 | 1 | >20 | 7 | 82 | Doce, Enza |
| 4 | 78 | 1 | >20 | 494 | 231 | Doce, Caba, Enza, Abi |
| 5 | 69 | 1 | >20 | 318 | 99 | Doce, Enza |
| 6 | 81 | 1 | 6–20 | 109 | 73 | Doce, Caba, Abi |
| 7 | 63 | 0 | <6 | 5 | 69 | Doce |
| 8 | 69 | 1 | >20 | 235 | 271 | Doce, Caba |
| 9 | 76 | 1 | >20 | 68 | 507 | Doce, Caba |
| 10 | 71 | 1 | >20 | 52 | 350 | Doce |
| 11 | 82 | 0 | >20 | 87 | 75 | Enza, Abi |
| 12 | 76 | 0 | >20 | 119 | 85 | Doce, Abi |
| 13 | 78 | 1 | >20 | 74 | 265 | Doce, Enza |
| 14 | 76 | 1 | >20 | 942 | 108 | Doce, Caba, Enza, Abi |
| 15 | 79 | 1 | >20 | 99 | 77 | Enza, Abi |
| 16 | 83 | 1 | >20 | 77 | 98 | Doce, Enza |
| 17 | 77 | 1 | 6–20 | 113 | 140 | Doce, Enza |
| Pt. No. | SUVmax ^ Baseline | SUVmax ^ 2 Cycles | ΔSUVmax | No. of 223Ra Cycles | ΔPSA from Baseline to EOT (%) | ΔALP from Baseline to EOT (%) | Radiographic Progression during Therapy | SSE (12 mo. from First 223Ra) | OS (mo. from First 223Ra) | Follow-Up (mo. from First 223Ra) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 3.92 | NA * | NA * | 1 | NA | NA | NA | - | 1.5 | |
| 2 | 2.09 | 1.65 | −0.44 | 6 | 246.2 | 18.2 | - | - | 19.4 | |
| 3 | 2.89 | 3.10 | 0.21 | 3 | 47.0 | -23.2 | Soft tissue | EBRT | 18.9 | |
| 4 | 1.90 | NA * | NA * | 1 | NA | NA | Soft tissue | - | 9.7 | |
| 5 | 2.53 | 2.45 | −0.08 | 3 | 73.0 | 55.6 | - | EBRT | 14.2 | |
| 6 | 2.79 | 2.86 | 0.07 | 3 | 166.1 | 46.6 | Bone | EBRT | 11.5 | |
| 7 | 1.22 | 1.39 | 0.17 | 6 | 269.6 | −13.0 | - | - | 20.2 | |
| 8 | 2.11 | 2.55 | 0.44 | 5 | 86.0 | 21.0 | Bone | - | 7.3 | |
| 9 | 2.39 | NA * | NA * | 1 | NA | NA | Soft tissue | Fracture | 4.9 | |
| 10 | 2.79 | 2.92 | 0.13 | 4 | 73.1 | −64.3 | - | EBRT | 16.7 | |
| 11 | 1.67 | 1.70 | 0.03 | 6 | 34.5 | −60.0 | - | - | 17.1 | |
| 12 | 3.82 | 2.75 | −1.07 | 3 | 106.7 | −16.5 | - | EBRT | 7.7 | |
| 13 | 2.29 | 3.23 | 0.95 | 4 | 604.1 | −30.9 | - | - | 12.0 | |
| 14 | 2.43 | 2.77 | 0.34 | 2 | 30.5 | 25.9 | Soft tissue | - | 2.5 | |
| 15 | 2.22 | 2.35 | 0.13 | 5 | 796.0 | 94.8 | Bone + Soft tissue | Spinal cord compression | 12.8 | |
| 16 | 3.52 | 3.11 | −0.41 | 3 | 10.8 | 28.6 | Bone + Soft tissue | EBRT | 6.2 | |
| 17 | 2.59 | 2.93 | 0.35 | 6 | 252.2 | −40.7 | - | - | 18.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fosbøl, M.Ø.; Mortensen, J.; Petersen, P.M.; Loft, A.; Madsen, J.; Kjaer, A. uPAR PET/CT for Prognostication and Response Assessment in Patients with Metastatic Castration-Resistant Prostate Cancer Undergoing Radium-223 Therapy: A Prospective Phase II Study. Diagnostics 2021, 11, 1087. https://doi.org/10.3390/diagnostics11061087
Fosbøl MØ, Mortensen J, Petersen PM, Loft A, Madsen J, Kjaer A. uPAR PET/CT for Prognostication and Response Assessment in Patients with Metastatic Castration-Resistant Prostate Cancer Undergoing Radium-223 Therapy: A Prospective Phase II Study. Diagnostics. 2021; 11(6):1087. https://doi.org/10.3390/diagnostics11061087
Chicago/Turabian StyleFosbøl, Marie Øbro, Jann Mortensen, Peter Meidahl Petersen, Annika Loft, Jacob Madsen, and Andreas Kjaer. 2021. "uPAR PET/CT for Prognostication and Response Assessment in Patients with Metastatic Castration-Resistant Prostate Cancer Undergoing Radium-223 Therapy: A Prospective Phase II Study" Diagnostics 11, no. 6: 1087. https://doi.org/10.3390/diagnostics11061087
APA StyleFosbøl, M. Ø., Mortensen, J., Petersen, P. M., Loft, A., Madsen, J., & Kjaer, A. (2021). uPAR PET/CT for Prognostication and Response Assessment in Patients with Metastatic Castration-Resistant Prostate Cancer Undergoing Radium-223 Therapy: A Prospective Phase II Study. Diagnostics, 11(6), 1087. https://doi.org/10.3390/diagnostics11061087







