Active Ratio Test (ART) as a Novel Diagnostic for Ovarian Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Clinical Samples
2.3. Generation of Monoclonal Antibodies against Human CXCL10
2.4. Surface Plasmon Resonance Imaging
2.5. Biotinylation of RA2 and RG2 Monoclonal Antibodies
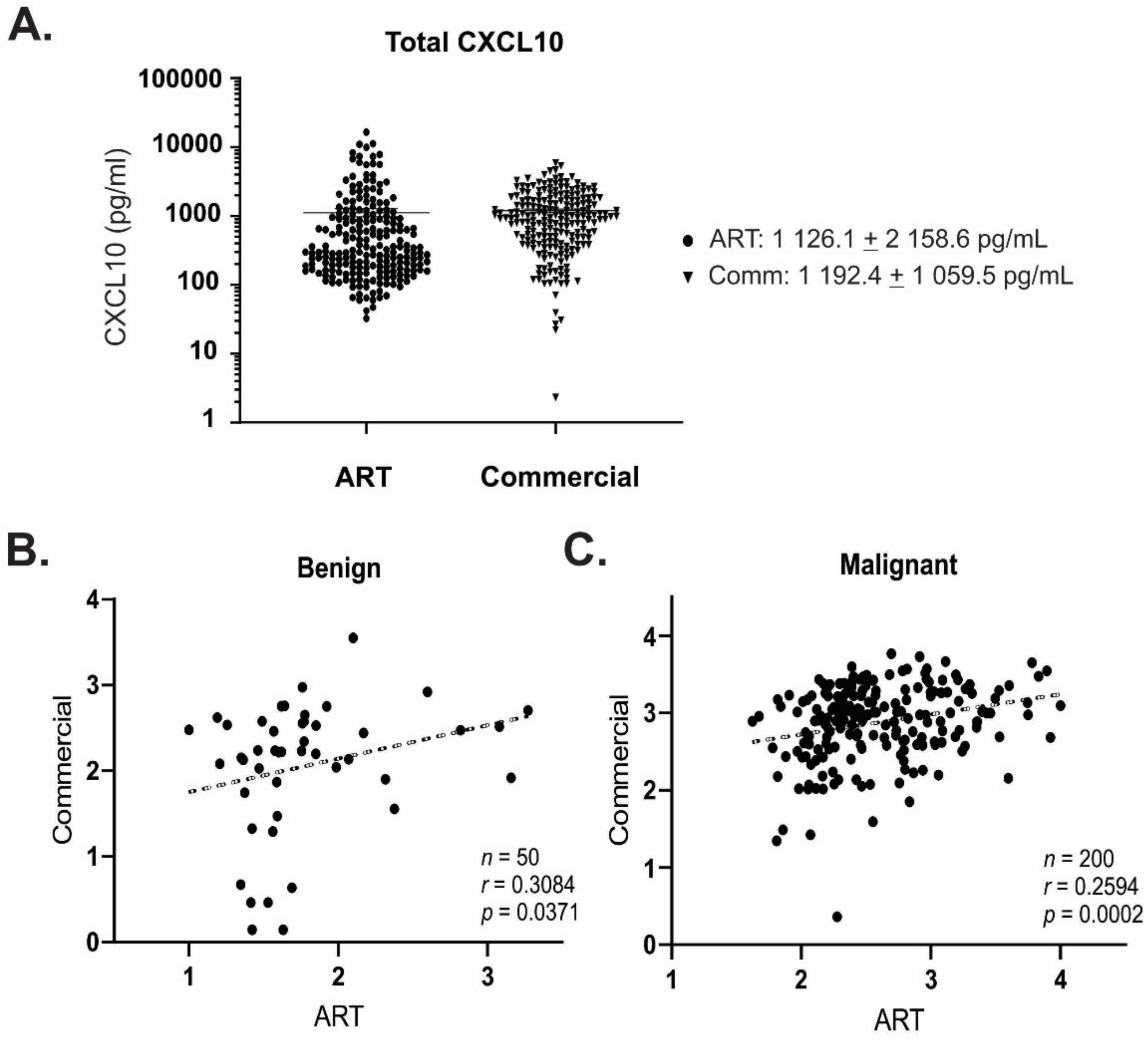
2.6. Quantitation of Active and Total CXCL10
2.7. Statistical Analyses
3. Results
3.1. Generation of a Functional Assay Differentiating Active from Total CXCL10
3.2. mAb-RA2 and mAb-RG2 Differentiate Functional from Non-Functional CXCL10
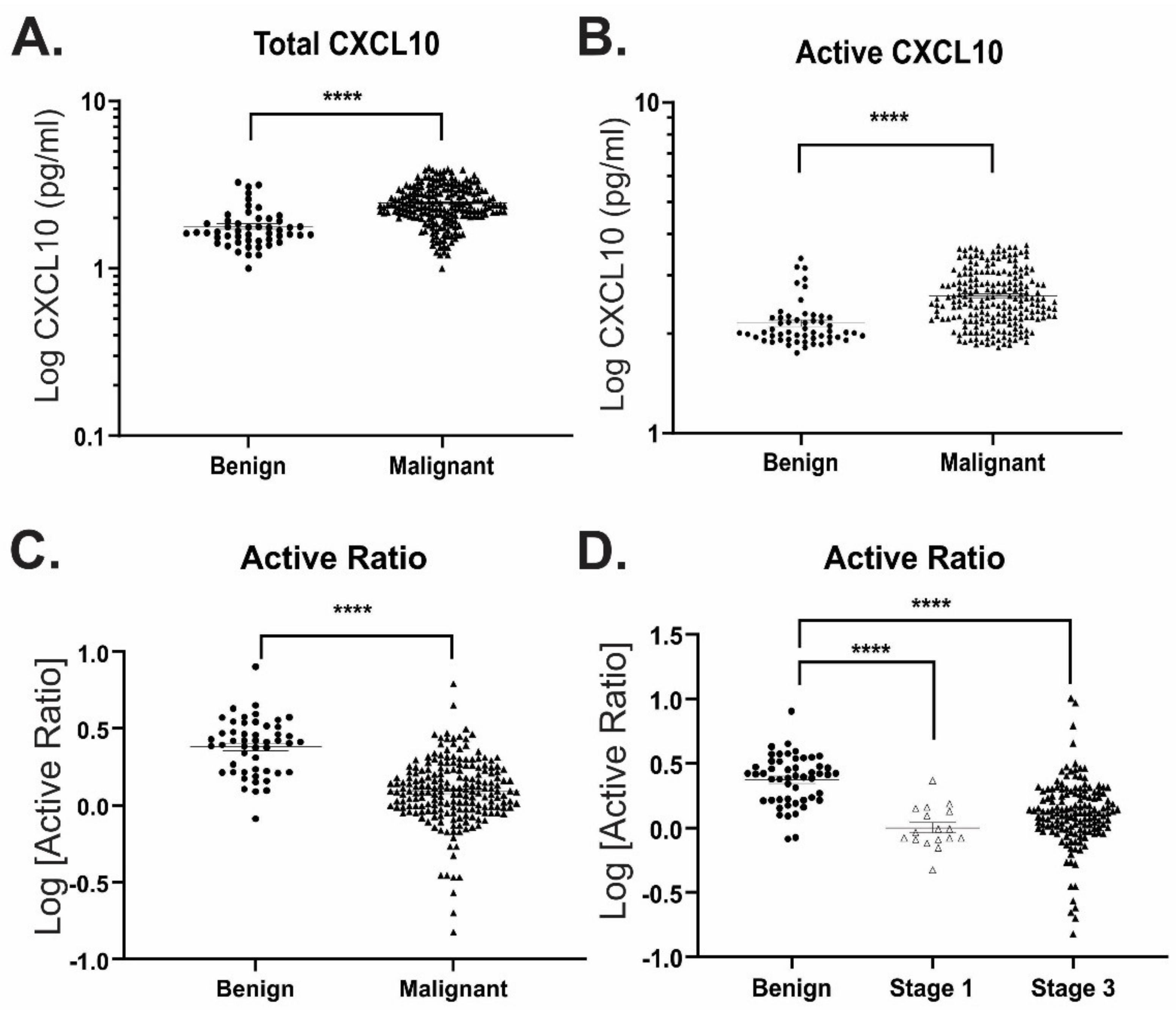
3.3. Active Ratio Test Differentiates Benign from Malignant Ovarian Cancer Samples
3.4. DPP4 Abundance and Activity Do Not Correlate with Functional CXCL10
3.5. Active Ratio Provides Prognostic Discrimination between Benign and Malignant Disease
3.6. Potential Application of ART as a Clinical Diagnostic
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bilandzic, M.; Rainczuk, A.; Green, E.; Fairweather, N.; Jobling, T.W.; Plebanski, M.; Stephens, A.N. Keratin-14 (KRT14) Positive Leader Cells Mediate Mesothelial Clearance and Invasion by Ovarian Cancer Cells. Cancers 2019, 11, 1228. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.; Ma, J.; Zou, Z.; Jemal, A. Cancer statistics, 2014. CA Cancer J. Clin. 2014, 64, 9–29. [Google Scholar] [CrossRef]
- Terry, K.L.; Schock, H.; Fortner, R.T.; Hüsing, A.; Fichorova, R.N.; Yamamoto, H.S.; Vitonis, A.F.; Johnson, T.; Overvad, K.; Tjonneland, A.; et al. A Prospective Evaluation of Early Detection Biomarkers for Ovarian Cancer in the European EPIC Cohort. Clin. Cancer Res. 2016, 22, 4664–4675. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, I.J.; Menon, U.; Ryan, A.; Gentry-Maharaj, A.; Burnell, M.; Kalsi, J.K.; Amso, N.N.; Apostolidou, S.; Benjamin, E.; Cruickshank, D.; et al. Ovarian cancer screening and mortality in the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS): A randomised controlled trial. Lancet 2016, 387, 945–956. [Google Scholar] [CrossRef]
- Rainczuk, A.; Rao, J.; Gathercole, J.; Stephens, A.N. The emerging role of CXC chemokines in epithelial ovarian cancer. Reproduction 2012, 144, 303–317. [Google Scholar] [CrossRef]
- Yang, X.; Chu, Y.; Wang, Y.; Zhang, R.; Xiong, S. Targeted in vivo expression of IFN-gamma-inducible protein 10 induces specific antitumor activity. J. Leukoc. Biol. 2006, 80, 1434–1444. [Google Scholar] [CrossRef]
- Hu, Z.; Chen, J.; Zhou, S.; Yang, N.; Duan, S.; Zhang, Z.; Su, J.; He, J.; Zhang, Z.; Lu, X.; et al. Mouse IP-10 Gene Delivered by Folate-modified Chitosan Nanoparticles and Dendritic/tumor Cells Fusion Vaccine Effectively Inhibit the Growth of Hepatocellular Carcinoma in Mice. Theranostics 2017, 7, 1942–1952. [Google Scholar] [CrossRef]
- Chow, M.T.; Ozga, A.J.; Servis, R.L.; Frederick, D.T.; Lo, J.A.; Fisher, D.E.; Freeman, G.J.; Boland, G.M.; Luster, A.D. Intratumoral Activity of the CXCR3 Chemokine System Is Required for the Efficacy of Anti-PD-1 Therapy. Immunity 2019, 50, 1498–1512.e5. [Google Scholar] [CrossRef]
- Cancer Genome Atlas Research Network. Integrated genomic analyses of ovarian carcinoma. Nature 2011, 474, 609–615. [Google Scholar] [CrossRef]
- Kawada, K.; Sonoshita, M.; Sakashita, H.; Takabayashi, A.; Yamaoka, Y.; Manabe, T.; Inaba, K.; Minato, N.; Oshima, M.; Taketo, M.M. Pivotal Role of CXCR3 in Melanoma Cell Metastasis to Lymph Nodes. Cancer Res. 2004, 64, 4010–4017. [Google Scholar] [CrossRef] [PubMed]
- Lambeck, A.J.; Crijns, A.P.G.; Leffers, N.; Sluiter, W.J.; ten Hoor, K.A.; Braid, M.; van der Zee, A.G.J.; Daemen, T.; Nijman, H.W.; Kast, W.M. Serum cytokine profiling as a diagnostic and prognostic tool in ovarian cancer: A potential role for inter-leukin 7. Clin. Cancer Res. 2007, 13, 2385–2391. [Google Scholar] [CrossRef] [PubMed]
- Sato, E.; Olson, S.H.; Ahn, J.; Bundy, B.; Nishikawa, H.; Qian, F.; Jungbluth, A.A.; Frosina, D.; Gnjatic, S.; Ambrosone, C.; et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc. Natl. Acad. Sci. USA 2005, 102, 18538–18543. [Google Scholar] [CrossRef]
- Emerson, R.O.; Sherwood, A.M.; Rieder, M.J.; Guenthoer, J.; Williamson, D.W.; Carlson, C.S.; Drescher, C.W.; Tewari, M.; Bielas, J.H.; Robins, H.S. High-throughput sequencing of T-cell receptors reveals a homogeneous repertoire of tumour-infiltrating lymphocytes in ovarian cancer. J. Pathol. 2013, 231, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Rainczuk, A.; Rao, J.R.; Gathercole, J.; Fairweather, N.J.; Chu, S.; Masadah, R.; Jobling, T.W.; Deb-Choudhury, S.; Dyer, J.; Stephens, A.N. Evidence for the antagonistic form of CXC-motif chemokine CXCL10 in serous epithelial ovarian tumours. Int. J. Cancer 2013, 134, 530–541. [Google Scholar] [CrossRef]
- Barreira da Silva, R.; Laird, M.E.; Yatim, N.; Fiette, L.; Ingersoll, M.A.; Albert, M.L. Dipeptidylpeptidase 4 inhibition enhances lymphocyte trafficking, improving both naturally oc-curring tumor immunity and immunotherapy. Nat. Immunol. 2015, 16, 850–858. [Google Scholar] [CrossRef] [PubMed]
- Ragab, D.; Laird, M.; Duffy, D.; Casrouge, A.; Mamdouh, R.; Abass, A.; Shenawy, D.E.; Shebl, A.; Elkashef, W.F.; Zalata, K.R.; et al. CXCL10 antagonism and plasma sDPPIV correlate with increasing liver disease in chronic HCV genotype 4 infected patients. Cytokine 2013, 63, 105–112. [Google Scholar] [CrossRef]
- Riva, A.; Laird, M.; Casrouge, A.; Ambrozaitis, A.; Williams, R.; Naoumov, N.V.; Albert, M.L.; Chokshi, S. Truncated CXCL10 is associated with failure to achieve spontaneous clearance of acute hepatitis C infection. Hepatology 2014, 60, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Casrouge, A.; Bisiaux, A.; Stephen, L.; Schmolz, M.; Mapes, J.; Pfister, C.; Pol, S.; Mallet, V.; Albert, M.L. Discrimination of agonist and antagonist forms of CXCL10 in biological samples. Clin. Exp. Immunol. 2011, 167, 137–148. [Google Scholar] [CrossRef]
- Windmüller, C.; Zech, D.; Avril, S.; Boxberg, M.; Dawidek, T.; Schmalfeldt, B.; Schmitt, M.; Kiechle, M.; Bronger, H. CXCR3 mediates ascites-directed tumor cell migration and predicts poor outcome in ovarian cancer patients. Oncogenesis 2017, 6, e331. [Google Scholar] [CrossRef]
- Mortier, A.; Gouwy, M.; Van Damme, J.; Proost, P. Effect of posttranslational processing on the in vitro and in vivo activity of chemokines. Exp. Cell Res. 2011, 317, 642–654. [Google Scholar] [CrossRef]
- Loos, T.; Mortier, A.; Gouwy, M.; Ronsse, I.; Put, W.; Lenaerts, J.-P.; Van Damme, J.; Proost, P. Citrullination of CXCL10 and CXCL11 by peptidylarginine deiminase: A naturally occurring posttranslational modification of chemokines and new dimension of immunoregulation. Blood 2008, 112, 2648–2656. [Google Scholar] [CrossRef] [PubMed]
- Mlynska, A.; Salciuniene, G.; Zilionyte, K.; Garberyte, S.; Strioga, M.; Intaite, B.; Barakauskiene, A.; Lazzari, G.; Dobrovolskiene, N.; Krasko, J.A.; et al. Chemokine profiling in serum from patients with ovarian cancer reveals candidate biomarkers for recurrence and immune infiltration. Oncol. Rep. 2018, 41, 1238–1252. [Google Scholar] [CrossRef] [PubMed]
- Ulusoy, H.; Kamanli, A.; Ilhan, N.; Kuru, O.; Arslan, S.; Alkan, G.; Ozgocmen, S.; Kamanlı, A. Serum levels of soluble CD26 and CD30 and their clinical significance in patients with rheumatoid arthritis. Rheumatol. Int. 2012, 32, 3857–3862. [Google Scholar] [CrossRef]
- Zhang, L.; Conejo-Garcia, J.R.; Katsaros, D.; Gimotty, P.A.; Massobrio, M.; Regnani, G.; Makrigiannakis, A.; Gray, H.; Schlienger, K.; Liebman, M.N.; et al. Intratumoral T Cells, Recurrence, and Survival in Epithelial Ovarian Cancer. N. Engl. J. Med. 2003, 348, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Hu, R.; Zeng, Y.; Zhang, W.; Zhou, H.-H. Tumour immune cell infiltration and survival after platinum-based chemotherapy in high-grade serous ovarian cancer subtypes: A gene expression-based computational study. EBioMedicine 2020, 51, 102602. [Google Scholar] [CrossRef]
- McCool, K.W.; Freeman, Z.T.; Zhai, Y.; Wu, R.; Hu, K.; Liu, C.-J.; Tomlins, S.A.; Fearon, E.R.; Magnuson, B.; Kuick, R.; et al. Murine Oviductal High-Grade Serous Carcinomas Mirror the Genomic Alterations, Gene Expression Profiles, and Immune Microenvironment of Their Human Counterparts. Cancer Res. 2020, 80, 877–889. [Google Scholar] [CrossRef]
- Furuya, M.; Suyama, T.; Usui, H.; Kasuya, Y.; Nishiyama, M.; Tanaka, N.; Ishiwata, I.; Nagai, Y.; Shozu, M.; Kimura, S. Up-regulation of CXC chemokines and their receptors: Implications for proinflammatory microenvironments of ovarian carcinomas and endometriosis. Hum. Pathol. 2007, 38, 1676–1687. [Google Scholar] [CrossRef]
- Walser, T.C.; Rifat, S.; Ma, X.; Kundu, N.; Ward, C.; Goloubeva, O.; Johnson, M.G.; Medina, J.C.; Collins, T.L.; Fulton, A.M. Antagonism of CXCR3 Inhibits Lung Metastasis in a Murine Model of Metastatic Breast Cancer. Cancer Res. 2006, 66, 7701–7707. [Google Scholar] [CrossRef]
- Vanheule, V.; Metzemaekers, M.; Janssens, R.; Struyf, S.; Proost, P. How post-translational modifications influence the biological activity of chemokines. Cytokine 2018, 109, 29–51. [Google Scholar] [CrossRef]
- Proost, P.; Struyf, S.; Van Damme, J.; Fiten, P.; Ugarte-Berzal, E.; Opdenakker, G. Chemokine isoforms and processing in inflammation and immunity. J. Autoimmun. 2017, 85, 45–57. [Google Scholar] [CrossRef]
- Sullivan, G.M.; Feinn, R. Using Effect Size—Or Why the P Value Is Not Enough. J. Grad. Med Educ. 2012, 4, 279–282. [Google Scholar] [CrossRef]
- Scholler, N.; Crawford, M.; Sato, A.; Drescher, C.W.; O’Briant, K.C.; Kiviat, N.; Anderson, G.L.; Urban, N. Bead-Based ELISA for Validation of Ovarian Cancer Early Detection Markers. Clin. Cancer Res. 2006, 12, 2117–2124. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Bellone, S.; Siegel, E.R.; Altwerger, G.; Menderes, G.; Bonazzoli, E.; Egawa-Takata, T.; Pettinella, F.; Bianchi, A.; Riccio, F.; et al. A novel multiple biomarker panel for the early detection of high-grade serous ovarian carcinoma. Gynecol. Oncol. 2018, 149, 585–591. [Google Scholar] [CrossRef]
- Boccardi, V.; Marano, L.; Rossetti, R.R.A.; Rizzo, M.R.; Di Martino, N.; Paolisso, G. Serum CD26 levels in patients with gastric cancer: A novel potential diagnostic marker. BMC Cancer 2015, 15, 703. [Google Scholar] [CrossRef] [PubMed]
- Varin, E.M.; Mulvihill, E.E.; Beaudry, J.L.; Pujadas, G.; Fuchs, S.; Tanti, J.-F.; Fazio, S.; Kaur, K.; Cao, X.; Baggio, L.L.; et al. Circulating Levels of Soluble Dipeptidyl Peptidase-4 Are Dissociated from Inflammation and Induced by Enzymatic DPP4 Inhibition. Cell Metab. 2019, 29, 320–334.e5. [Google Scholar] [CrossRef] [PubMed]
- Carl-McGrath, S.; Lendeckel, U.; Ebert, M.; Röcken, C. Ectopeptidases in tumour biology: A review. Histol. Histopathol. 2006, 21, 1339–1353. [Google Scholar] [PubMed]
- Lettau, M.; Dietz, M.; Vollmers, S.; Armbrust, F.; Peters, C.; Dang, T.M.; Chitadze, G.; Kabelitz, D.; Janssen, O. Degranulation of human cytotoxic lymphocytes is a major source of proteolytically active soluble CD26/DPP4. Cell. Mol. Life Sci. 2019, 77, 751–764. [Google Scholar] [CrossRef] [PubMed]
- Nargis, T.; Kumar, K.; Ghosh, A.R.; Sharma, A.; Rudra, D.; Sen, D.; Chakrabarti, S.; Mukhopadhyay, S.; Ganguly, D.; Chakrabarti, P. KLK5 induces shedding of DPP4 from circulatory Th17 cells in type 2 diabetes. Mol. Metab. 2017, 6, 1529–1539. [Google Scholar] [CrossRef]
- Romacho, T.; Sell, H.; Indrakusuma, I.; Roehrborn, D.; Castañeda, T.R.; Jelenik, T.; Markgraf, D.; Hartwig, S.; Weiss, J.; Al-Hasani, H.; et al. DPP4 deletion in adipose tissue improves hepatic insulin sensitivity in diet-induced obesity. Am. J. Endocrinol. Physiol. Metab. 2020, 318, E590–E599. [Google Scholar] [CrossRef]
- Denney, H.; Clench, M.; Woodroofe, N. Cleavage of chemokines CCL2 and CXCL10 by matrix metalloproteinases-2 and -9: Implications for chemotaxis. Biochem. Biophys. Res. Commun. 2009, 382, 341–347. [Google Scholar] [CrossRef]






| Binding Affinity (KD) for CXCL10 Proteomics | Assay Parameters | ||||||
|---|---|---|---|---|---|---|---|
| Full Length | Intact N-term | VP-Truncated N-term | Limit of Detection (LOD) | Limit of Quantification (LOQ) | Intra-Assay Precision (%) | Inter-Assay Precision (%) | |
| mAb-RA2 | 4.90 × 10−10 | 7.36 × 10−10 | no binding | 95.9 pg mL−1 | 162.2 pg mL−1 | 2.8 | 9.9 |
| mAb-RG2 | 3.89 × 10−12 | 3.54 × 10−9 | 3.00 × 10−9 | 116.3 pg mL−1 | 272.7 pg mL−1 | 4.0 | 8.5 |
| Group | Pathology | Grade | Stage | Median CA125 (IQR) | Menopausal Status |
|---|---|---|---|---|---|
| Benign (n = 51) | Cystadenoma (n = 19) Fibroma (n = 10) Other (n = 22) | n/a | n/a | 52 (23–154) | Mixed |
| Malignant (n = 224) | Serous (n = 163) Mucinous (n = 10) Endometroid (n = 14) Clear cell (13) Mixed epithelial/poorly differentiated (n = 24) | 1–3 | I–IV | 641 (149–1591) | Mixed |
| Biomarkers (Cut-Off Points) | Specificity % (95% CI) | Sensitivity % (95% CI) | AUC | PPV | NPV |
|---|---|---|---|---|---|
| Active Ratio | |||||
| <1.43 | 90.0 (78.6–95.7) | 63.5 (56.6–69.9) | 0.8617 | 80.4 | 79.2 |
| <1.25 | 96.0 (86.5–99.3) | 52.0 (45.1–58.8) | 89.4 | 75.5 | |
| Total CXCL10 (pg mL−1) | |||||
| >241 | 90.2 (79.0–95.7) | 51.0 (44.2–57.6) | 0.8122 | 77.1 | 74.0 |
| >1204 | 94.1 (84.1–98.4) | 19.5 (14.7–25.4) | 68.2 | 64.4 | |
| Active CXCL10 (pg mL−1) | |||||
| >598 | 90.1 (80.4–96.1) | 35.6 (29.4–42.3) | 0.7872 | 70.0 | 68.4 |
| >1400 | 94.6 (85.2–98.5) | 17.3 (12.8–23.0) | 67.5 | 63.9 | |
| DPP4 (ng mL−1) | |||||
| >316 | 89.6 (77.8–95.5) | 12.2 (7.8–18.4) | 0.5598 | 43.2 | 61.2 |
| >389 | 95.8 (86.0–99.3) | 7.4 (4.2–12.8) | 53.3 | 61.5 | |
| Plasma CA125 (U mL−1) | |||||
| >548 | 90.0 (73.5–97.9) | 52.1 (44.7–59.5) | 0.8262 | 77.1 | 74.4 |
| >757 | 93.3 (77.9–99.2) | 44.7 (37.4–52.1) | 89.8 | 73.0 | |
| Combined Biomarkers | |||||
| Active ratio & DPP4 (<1.43 and > 316 ng mL−1) | 91.7 (73.0–99.0) | 77.3 (68.3–84.7) | 0.9114 | 85.8 | 86.2 |
| Active ratio & DPP4 (<1.25 and > 389 ng mL−1) | 95.8 (78.9–99.9) | 67.3 (57.7–75.9) | 91.2 | 81.9 | |
| Active ratio & plasma CA125 (<1.43 and > 548 U mL−1) | 91.7 (70.3–86.3) | 79.1 (70.3–86.3) | 0.9337 | 86.1 | 87.1 |
| Active ratio & plasma CA125 (<1.25 and > 757 U mL−1) | 95.8 (78.9–99.9) | 51.8 (42.1–61.5) | 88.9 | 75.4 | |
| Active ratio & DPP4 & plasma CA125 (<1.43 and > 316 ng mL−1 and > 548 U mL−1) | 91.7 (73.0–99.0) | 90.0 (82.8–94.9) | 0.9511 | 87.5 | 93.4 |
| Active ratio & DPP4 & plasma CA125 (<1.25 and > 389 ng mL−1 and > 757 U mL−1) | 95.8 (78.9–99.9) | 81.8 (73.3–88.5) | 93.4 | 94.7 |
| Biomarker | Sample Type | AUC | Cohen’s d | CV % (Malignant) | p-Value |
|---|---|---|---|---|---|
| Total CXCL10 (pg mL−1) | Ascites | 0.8122 | 0.6976 | 191.7 | <0.0001 |
| Active CXCL10 (pg mL−1) | Ascites | 0.7872 | 0.6062 | 134.1 | <0.0001 |
| Active Ratio | Ascites | 0.8617 | 0.8629 | 72.7 | <0.0001 |
| Active Ratio | CVS | 0.8036 | 1.0074 | 54.4 | <0.0001 |
| Active Ratio | Plasma | 0.7828 | 0.7905 | 52.0 | 0.0002 |
| DPP4 (ng mL−1) | Ascites | 0.5598 | 0.1124 | 75.8 | 0.2139 |
| CA125 | Plasma | 0.8262 | 0.6171 | 200.9 | <0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, S.-W.; Rainczuk, A.; Oehler, M.K.; Jobling, T.W.; Plebanski, M.; Stephens, A.N. Active Ratio Test (ART) as a Novel Diagnostic for Ovarian Cancer. Diagnostics 2021, 11, 1048. https://doi.org/10.3390/diagnostics11061048
Kang S-W, Rainczuk A, Oehler MK, Jobling TW, Plebanski M, Stephens AN. Active Ratio Test (ART) as a Novel Diagnostic for Ovarian Cancer. Diagnostics. 2021; 11(6):1048. https://doi.org/10.3390/diagnostics11061048
Chicago/Turabian StyleKang, Sung-Woog, Adam Rainczuk, Martin K. Oehler, Thomas W. Jobling, Magdalena Plebanski, and Andrew N. Stephens. 2021. "Active Ratio Test (ART) as a Novel Diagnostic for Ovarian Cancer" Diagnostics 11, no. 6: 1048. https://doi.org/10.3390/diagnostics11061048
APA StyleKang, S.-W., Rainczuk, A., Oehler, M. K., Jobling, T. W., Plebanski, M., & Stephens, A. N. (2021). Active Ratio Test (ART) as a Novel Diagnostic for Ovarian Cancer. Diagnostics, 11(6), 1048. https://doi.org/10.3390/diagnostics11061048








