Development of a Prediction Model for the Occurrence of Stenosis or Occlusion after Percutaneous Deep Venous Arterialization
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. pDVA Procedure
2.3. Follow-Up
2.4. DUS Measurements
2.5. Data Collection and Study End Points
2.6. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. PSV and VF Measurements
3.3. Association between Patients’ Characteristics and Duplex Values
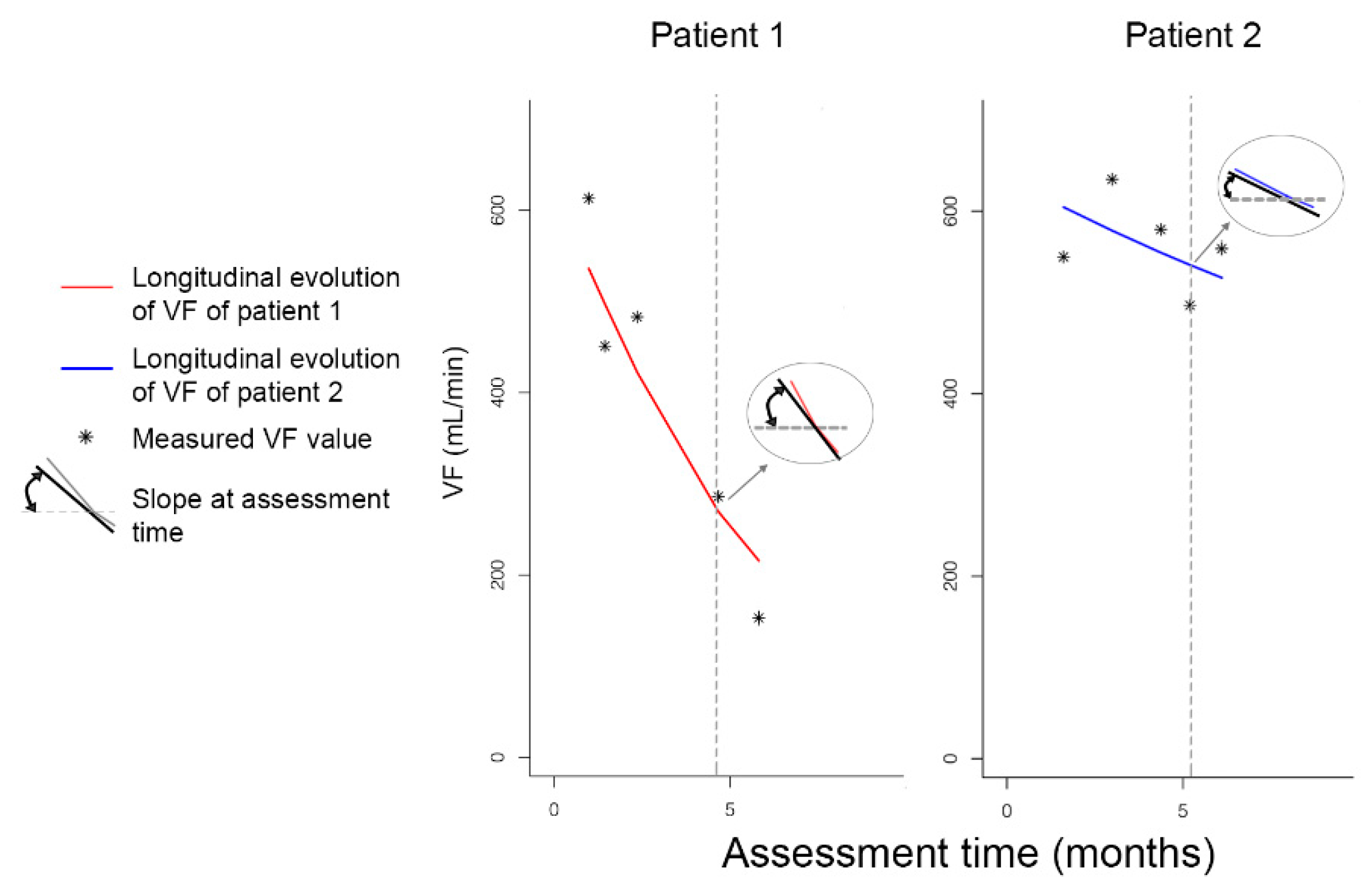
3.4. Pattern of Repeatedly Measured PSV and VF Values over Time
3.5. Association between Stenosis or Occlusion and Duplex Values
3.5.1. PSV Values
3.5.2. VF Values
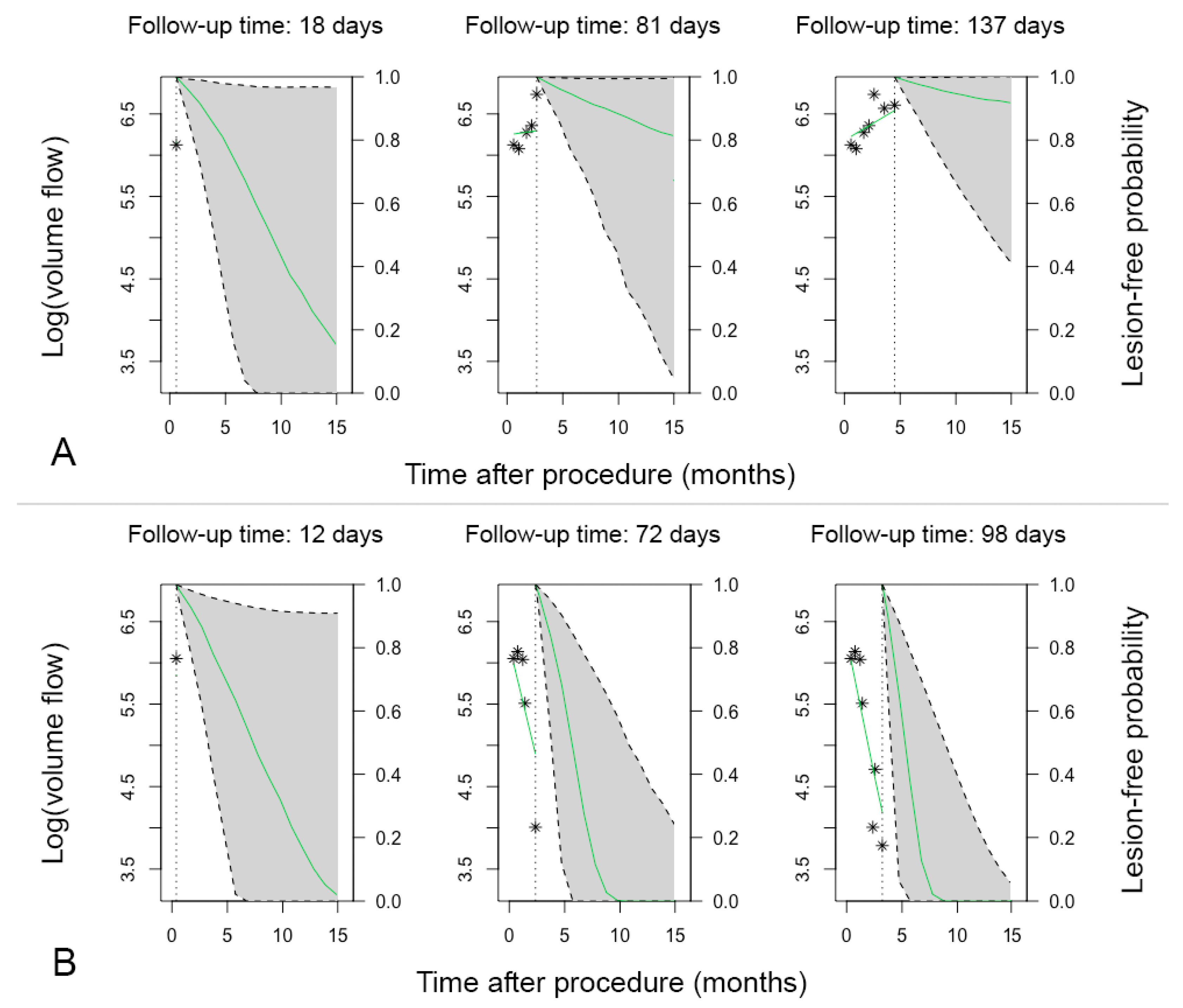
3.6. Patient-Specific Dynamic Prediction
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Appendix A
| Model | PSV Mid | PSV Distal | VF Mid | VF Distal | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Basic | Est. (95% CI) | p Value | Est. (95% CI) | p Value | Est. (95% CI) | p Value | Est. (95% CI) | p Value | ||
| Value | Time 1 | 0.96 (0.91–1.02) | 0.214 | 0.13 (0.09–0.19) | <0.0001 | 0.85 (0.80–0.91) | <0.0001 | 0.08 (0.02–0.30) | <0.001 | |
| Time 2 | 0.32 (0.25–0.42) | <0.0001 | 0.93 (0.07–11.94) | 0.953 | ||||||
| Age | 0.98 (0.97–0.99) | 0.005 | 0.98 (0.98–0.99) | <0.0001 | 0.99 (0.98–1.00) | 0.1007 | 0.98 (0.96–0.99) | 0.008 | ||
| Female sex | 0.71 (0.53–0.96) | 0.028 | 0.66 (0.57–0.78) | <0.0001 | 0.71 (0.51–0.99) | 0.0437 | 0.53 (0.16–1.74) | 0.295 | ||
| Reintervention | 0.98 (0.58–1.64) | 0.926 | 1.09 (0.92–1.31) | 0.314 | 0.84 (0.57–1.23) | 0.3724 | 1.12 (0.38–3.25) | 0.837 | ||
| Slope | Time 1 | 0.96 (0.92–1.01) | 0.103 | 0.20 (0.13–0.31) | <0.0001 | 0.84 (0.81–0.87) | <0.0001 | 0.05 (0.03–0.09) | <0.0001 | |
| Time 2 | 0.48 (0.33–0.69) | <0.001 | 0.69 (0.53–0.90) | 0.007 | ||||||
| Age | 0.98 (0.97–0.99) | 0.003 | 0.98 (0.97–0.99) | <0.0001 | 0.99 (0.98–1.01) | 0.2517 | 0.98 (0.97–0.98) | <0.0001 | ||
| Female sex | 0.71 (0.53–0.95) | 0.023 | 0.67 (0.53–0.85) | 0.001 | 0.73 (0.53–1.01) | 0.0553 | 0.47 (0.37–0.60) | <0.0001 | ||
| Reintervention | 0.94 (0.63–1.40) | 0.772 | 1.15 (0.90–1.47) | 0.274 | 0.76 (0.51–1.13) | 0.1751 | 1.34 (0.97–1.84) | 0.077 | ||
| Reintervention | Value | Time 1 | 0.10 (0.04–2.53) | <0.0001 | 0.14 (0.05–0.38) | <0.001 | 0.80 (0.76–0.85) | <0.0001 | 0.78 (0.72–0.84) | <0.0001 |
| Time 2 | 0.54 (0.29–1.01) | 0.053 | 1.71 (0.85–3.41) | 0.130 | ||||||
| Time relative | 1.01 (0.97–1.06) | 0.645 | 1.26 (0.92–1.71) | 0.147 | 1.05 (0.96–1.14) | 0.3294 | 1.09 (0.99–1.20) | 0.089 | ||
| Age | 0.99 (0.98–1.00) | 0.011 | 0.98 (0.97–0.99) | 0.001 | 0.99 (0.97–1.00) | 0.0411 | 0.98 (0.97–1.00) | 0.010 | ||
| Time × time relative | 0.73 (0.44–1.20) | 0.211 | ||||||||
| Time × time relative | 0.82 (0.66–1.03) | 0.089 | ||||||||
| Slope | Time 1 | 0.11 (0.05–0.25) | <0.0001 | 0.14 (0.05–0.41) | <0.001 | 0.79 (0.76–0.82) | <0.0001 | 0.76 (0.73–0.80) | <0.0001 | |
| Time 2 | 0.47 (0.29–0.77) | 0.003 | 1.69 (0.84–3.39) | 0.141 | ||||||
| Time relative | 1.02 (0.98–1.07) | 0.315 | 1.30 (0.94–1.79) | 0.114 | 1.09 (1.00–1.18) | 0.0591 | 1.13 (1.04–1.24) | 0.007 | ||
| Age | 0.99 (0.98–1.00) | 0.002 | 0.98 (0.97–0.99) | 0.001 | 0.99 (0.98–1.00) | 0.0383 | 0.98 (0.97–1.00) | 0.009 | ||
| Time × time relative | 0.71 (0.42–1.20) | 0.201 | ||||||||
| Time × time relative | 0.80 (0.64–1.01) | 0.057 | ||||||||
| Series | Value | Time 1 | 0.74 (0.73–0.76) | <0.0001 | 0.07 (0.04–0.14) | <0.0001 | 0.10 (0.04–0.28) | <0.0001 | 0.05 (0.02–0.09) | <0.0001 |
| Time 2 | 0.59 (0.43–0.81) | 0.001 | 0.23 (0.07–0.75) | 0.0148 | 0.26 (0.21–0.33) | <0.0001 | ||||
| Slope | Time 1 | 0.74 (0.73–0.76) | <0.0001 | 0.09 (0.06–0.13) | <0.0001 | 0.14 (0.09–0.22) | <0.0001 | 0.16 (0.10–0.25) | <0.0001 | |
| Time 2 | 0.57 (0.48–0.68) | <0.0001 | 0.31 (0.24–0.41) | <0.0001 | 0.39 (0.30–0.51) | <0.0001 | ||||
References
- Mustapha, J.A.; Saab, F.A.; Clair, D.; Schneider, P. Interim Results of the PROMISE I Trial to Investigate the LimFlow System of Percutaneous Deep Vein Arterialization for the Treatment of Critical Limb Ischemia. J. Invasive Cardiol. 2019, 31, 57–63. [Google Scholar] [PubMed]
- Kum, S.; Huizing, E.; Schreve, M.A.; Unlu, C.; Ferraresi, R.; Samarakoon, L.B.; van den Heuvel, D.A. Percutaneous deep venous arterialization in patients with critical limb ischemia. J. Cardiovasc. Surg. 2018, 59, 665–669. [Google Scholar] [CrossRef] [PubMed]
- Schreve, M.A.; Huizing, E.; Kum, S.; de Vries, J.-P.P.M.; de Borst, G.J.; Ünlü, Ç. Volume Flow and Peak Systolic Velocity of the Arteriovenous Circuit in Patients after Percutaneous Deep Venous Arterialization. Diagnostics 2020, 10, 760. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.; Schreve, M.A.; Huizing, E.; Del Giudice, C.; Branzan, D.; Ünlü, Ç.; Varcoe, R.L.; Ferraresi, R.; Kum, S. Midterm Outcomes of Percutaneous Deep Venous Arterialization With a Dedicated System for Patients With No-Option Chronic Limb-Threatening Ischemia: The ALPS Multicenter Study. J. Endovasc. Ther. 2020, 27, 658–665. [Google Scholar] [CrossRef] [PubMed]
- Conte, M.S.; Bradbury, A.W.; Kolh, P.; White, J.V.; Dick, F.; Fitridge, R.; Mills, J.L.; Ricco, J.-B.; Suresh, K.R.; Murad, M.H.; et al. Global vascular guidelines on the management of chronic limb-threatening ischemia. J. Vasc. Surg. 2019, 69, 3S–125S.e40. [Google Scholar] [CrossRef] [PubMed]
- Papageorgiou, G.; Mokhles, M.M.; Takkenberg, J.J.M.; Rizopoulos, D. Individualized dynamic prediction of survival with the presence of intermediate events. Stat Med. 2019, 38, 5623–5640. [Google Scholar] [CrossRef] [PubMed]
- Rizopoulos, D. Joint Models for Longitudinal and Time-to-Event Data: With Applications in R; Chapman & Hall/CRC Biostatistics Series; Chapman & Hall/CRC: Boca Raton, FL, USA, 2012. [Google Scholar]
- Rizopoulos, D. JM: An R Package for the Joint Modelling of Longitudinal and Time-to-Event Data. J. Stat. Softw. 2010, 35, 1–33. [Google Scholar] [CrossRef]
- Mills, J.L.S.; Conte, M.S.; Armstrong, D.G.; Pomposelli, F.B.; Schanzer, A.; Sidawy, A.N.; Andros, G. The Society for Vascular Surgery Lower Extremity Threatened Limb Classification System: Risk stratification based on wound, ischemia, and foot infection (WIfI). J. Vasc. Surg. 2014, 59, 220–222. [Google Scholar] [CrossRef] [PubMed]
- Rizopoulos, D. The R Package JMbayes for Fitting Joint Models for Longitudinal and Time-to-Event Data Using MCMC. J. Stat. Softw. 2016, 72, 1–46. [Google Scholar] [CrossRef]

| Total | Stenosis/Occlusion | |||
|---|---|---|---|---|
| Variable | No | Yes | p Value | |
| Patients | 23 | 6 (26) | 17 (74) | |
| Men | 9 (40) | 4 (67) | 5 (29) | 0.162 |
| Age, years | 63 (56–82) | 55 (35–73) | 72 (58–85) | 0.107 |
| Comorbidities | ||||
| Hypertension | 18 (79) | 3 (50) | 15 (88) | 0.089 |
| Diabetes | 16 (70) | 3 (50) | 13 (77) | 0.318 |
| Hyperlipidemia | 17 (74) | 2 (33) | 15 (88) | 0.021 |
| Cerebrovascular accident | 4 (17) | 0 (0) | 4 (24) | 0.539 |
| Coronary artery disease | 7 (30) | 1 (17) | 6 (35) | 0.621 |
| Dialysis dependent | 2 (9) | 1 (27) | 1 (6) | 0.462 |
| Body mass index, kg/m2 | 23 (19–25) | 24 (22–26) | 21 (19–24) | 0.100 |
| Laboratory results | ||||
| Creatinine, mg/dL | 86 (66–145) | 108 (82–179) | 84 (65–152) | 0.302 |
| eGFR <30 mL/min/1.73 m2 | 5 (24) | 1 (20) | 4 (25) | 1.000 |
| Rutherford | ||||
| 4 | 1 (4) | 0 (0) | 1 (6) | 1.000 |
| 5 | 15 (65) | 5 (83) | 10 (59) | 0.369 |
| 6 | 7 (30) | 1 (17) | 6 (35) | 0.621 |
| SVS WIfI risk staging | ||||
| Low risk | 1 (4) | 0 (0) | 1 (6) | 1.000 |
| Moderate risk | 5 (22) | 1 (17) | 4 (24) | 1.000 |
| High risk | 17 (74) | 5 (83) | 12 (71) | 1.000 |
| PSV Mid | PSV Distal | VF Mid | VF Distal | |||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | p Value | HR (95% CI) | p Value | HR (95% CI) | p Value | HR (95% CI) | p Value | |
| Basic model | ||||||||
| Value | 1.34 (1.05–1.71) | 0.018 | 1.37 (1.14–1.65) | 0.001 | 1.15 (1.04–1.28) | 0.007 | 1.15 (1.01–1.31) | 0.032 |
| Slope | 1.57 (0.75–3.29) | 0.229 | 1.10 (0.93–1.31) | 0.276 | 5.02 (1.31–19.32) | 0.019 | 1.08 (0.62–1.88) | 0.790 |
| Reintervention model | ||||||||
| Value | 1.51 (1.12–2.05) | 0.008 | 1.34 (1.04–1.72) | 0.024 | 1.28 (1.07–1.54) | 0.008 | 1.23 (1.05–1.45) | 0.012 |
| Slope | 2.76 (1.09–7.02) | 0.033 | 1.66 (0.71–3.89) | 0.243 | 3.18 (0.97–10.38) | 0.056 | 3.06 (0.97–9.63) | 0.056 |
| Per series model | ||||||||
| Value | 1.32 (1.17–1.50) | <0.001 | 1.36 (1.19–1.56) | <0.001 | 1.11 (1.03–1.21) | 0.009 | 1.22 (1.06–1.14) | <0.001 |
| Slope | 3.49 (1.56–7.80) | 0.002 | 1.30 (1.06–1.58) | 0.010 | 0.95 (0.54–1.65) | 0.845 | 1.22 (0.52–2.86) | 0.642 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huizing, E.; Schreve, M.A.; Kum, S.; Papageorgiou, G.; de Vries, J.-P.P.M.; de Borst, G.J.; Ünlü, Ç. Development of a Prediction Model for the Occurrence of Stenosis or Occlusion after Percutaneous Deep Venous Arterialization. Diagnostics 2021, 11, 1008. https://doi.org/10.3390/diagnostics11061008
Huizing E, Schreve MA, Kum S, Papageorgiou G, de Vries J-PPM, de Borst GJ, Ünlü Ç. Development of a Prediction Model for the Occurrence of Stenosis or Occlusion after Percutaneous Deep Venous Arterialization. Diagnostics. 2021; 11(6):1008. https://doi.org/10.3390/diagnostics11061008
Chicago/Turabian StyleHuizing, Eline, Michiel A. Schreve, Steven Kum, Grigorios Papageorgiou, Jean-Paul P. M. de Vries, Gert J. de Borst, and Çağdaş Ünlü. 2021. "Development of a Prediction Model for the Occurrence of Stenosis or Occlusion after Percutaneous Deep Venous Arterialization" Diagnostics 11, no. 6: 1008. https://doi.org/10.3390/diagnostics11061008
APA StyleHuizing, E., Schreve, M. A., Kum, S., Papageorgiou, G., de Vries, J.-P. P. M., de Borst, G. J., & Ünlü, Ç. (2021). Development of a Prediction Model for the Occurrence of Stenosis or Occlusion after Percutaneous Deep Venous Arterialization. Diagnostics, 11(6), 1008. https://doi.org/10.3390/diagnostics11061008









