How Smart Manufacturing Can Help Combat the COVID-19 Pandemic
1. Smart Manufacturing and R2R Control for COVID-19 Vaccine Manufacturing
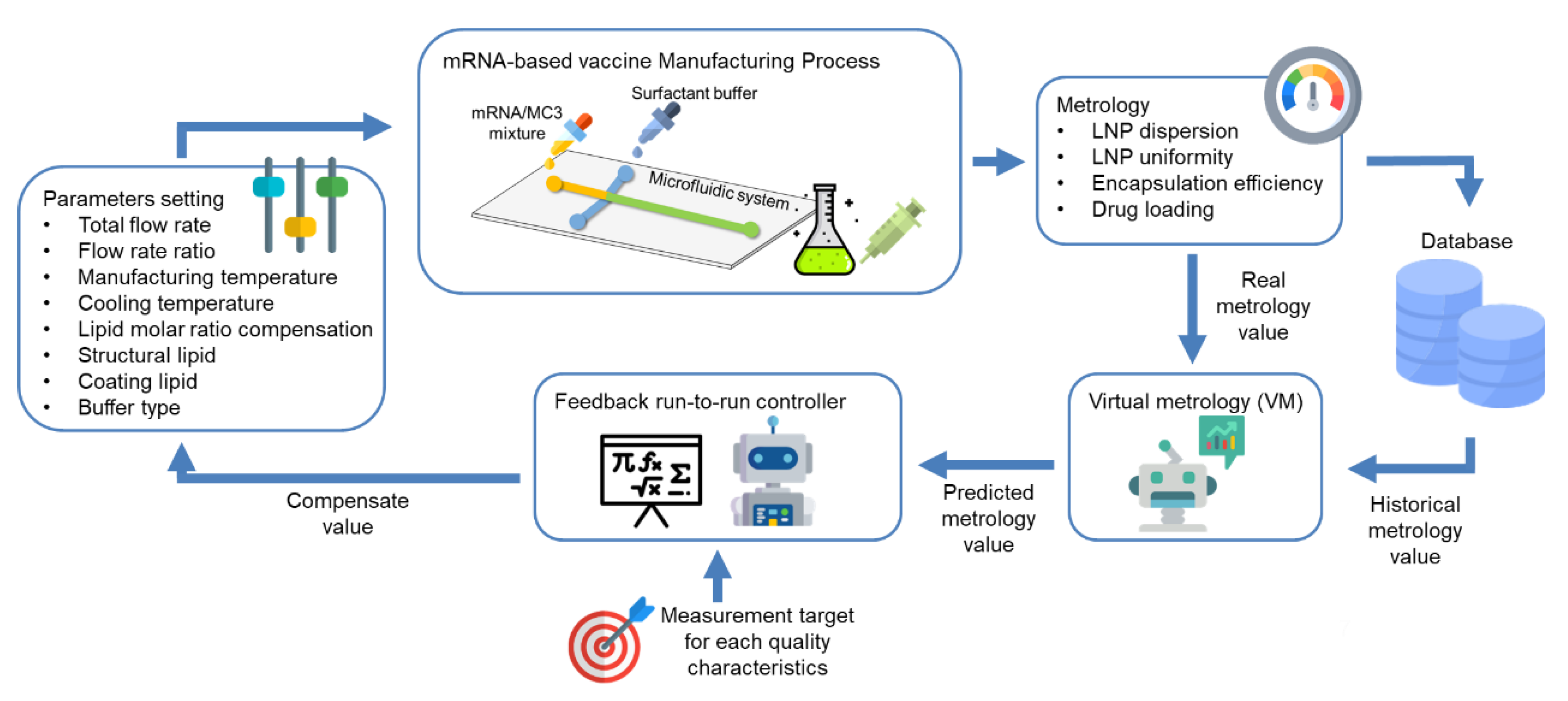
2. Concept of R2R Control for mRNA-LNP Vaccine Manufacturing
Funding
Conflicts of Interest
References
- Yang, T.; Wang, Y.-C.; Shen, C.-F.; Cheng, C.-M. Point-of-Care RNA-Based Diagnostic Device for COVID-19. Diagnostics 2020, 10, 165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, N.-N.; Li, X.-F.; Deng, Y.-Q.; Zhao, H.; Huang, Y.-J.; Yang, G.; Huang, W.-J.; Gao, P.; Zhou, C.; Zhang, R.-R.; et al. A Thermostable mRNA Vaccine against COVID-19. Cell 2020, 182, 1271–1283.e16. [Google Scholar] [CrossRef] [PubMed]
- Roces, C.B.; Lou, G.; Jain, N.; Abraham, S.; Thomas, A.; Halbert, G.W.; Perrie, Y. Manufacturing Considerations for the Development of Lipid Nanoparticles Using Microfluidics. Pharmaceutics 2020, 12, 1095. [Google Scholar] [CrossRef] [PubMed]
- Chien, C.F.; Shih, C.; Chang, J. The TSMC Way: Meeting Customer Needs at Taiwan Semiconductor Manufacturing Co. In Harvard Business School Technology & Operations Mgt. Unit Case 610-003; Harvard Business School Publishing: Boston, MA, USA, 2009. [Google Scholar]
- O’Meara, S. From plastic toys to Industry 4.0: How Taiwan is using science to upgrade its manufacturing. Nat. Cell Biol. 2020, 577, S1–S3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chien, C.-F.; Chen, Y.-J.; Hsu, C.-Y.; Wang, H.-K. Overlay Error Compensation Using Advanced Process Control with Dynamically Adjusted Proportional-Integral R2R Controller. IEEE Trans. Autom. Sci. Eng. 2014, 11, 473–484. [Google Scholar] [CrossRef]
- Khakifirooz, M.; Chien, C.-F.; Chen, Y.-J. Dynamic Support Vector Regression Control System for Overlay Error Compensation with Stochastic Metrology Delay. IEEE Trans. Autom. Sci. Eng. 2019, 17, 502–512. [Google Scholar] [CrossRef]
- Chien, C.-F.; Chen, Y.-J.; Hsu, C.-Y. A novel approach to hedge and compensate the critical dimension variation of the developed-and-etched circuit patterns for yield enhancement in semiconductor manufacturing. Comput. Oper. Res. 2015, 53, 309–318. [Google Scholar] [CrossRef]
- Moyne, J.; Del Castillo, E.; Hurwitz, A.M. Run-to-Run Control in Semiconductor Manufacturing; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Lee, C.-Y.; Chien, C.-F. Pitfalls and protocols of data science in manufacturing practice. J. Intell. Manuf. 2020, 2020, 1–19. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Y.-S.; Cheng, C.-M.; Chien, C.-F. How Smart Manufacturing Can Help Combat the COVID-19 Pandemic. Diagnostics 2021, 11, 885. https://doi.org/10.3390/diagnostics11050885
Lin Y-S, Cheng C-M, Chien C-F. How Smart Manufacturing Can Help Combat the COVID-19 Pandemic. Diagnostics. 2021; 11(5):885. https://doi.org/10.3390/diagnostics11050885
Chicago/Turabian StyleLin, Yun-Siang, Chao-Min Cheng, and Chen-Fu Chien. 2021. "How Smart Manufacturing Can Help Combat the COVID-19 Pandemic" Diagnostics 11, no. 5: 885. https://doi.org/10.3390/diagnostics11050885
APA StyleLin, Y.-S., Cheng, C.-M., & Chien, C.-F. (2021). How Smart Manufacturing Can Help Combat the COVID-19 Pandemic. Diagnostics, 11(5), 885. https://doi.org/10.3390/diagnostics11050885





