Sex-Related Differences in Cerebrospinal Fluid Plasma-Derived Proteins of Neurological Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Cohorts
2.3. Pre-Analytical Procedures
2.4. Samples Analysis
2.4.1. Cohort 1
- (1) linear IgG Index = QIgG/QAlb
- where QIgG = [IgG]CSF/[IgG]serum × 1000
- (2) the hyperbolic Reiber’s formula, IgGLoc = (QIgG − QLim) × [IgG]serum
- where QLim
2.4.2. Cohort 2
2.5. Statistical Analysis
3. Results
3.1. Patients’ Characteristics
3.2. Agreement Between Quantitative Method and the “Gold Standard” of Oligoclonal Bands for the Determination of an Intrathecal IgG Synthesis
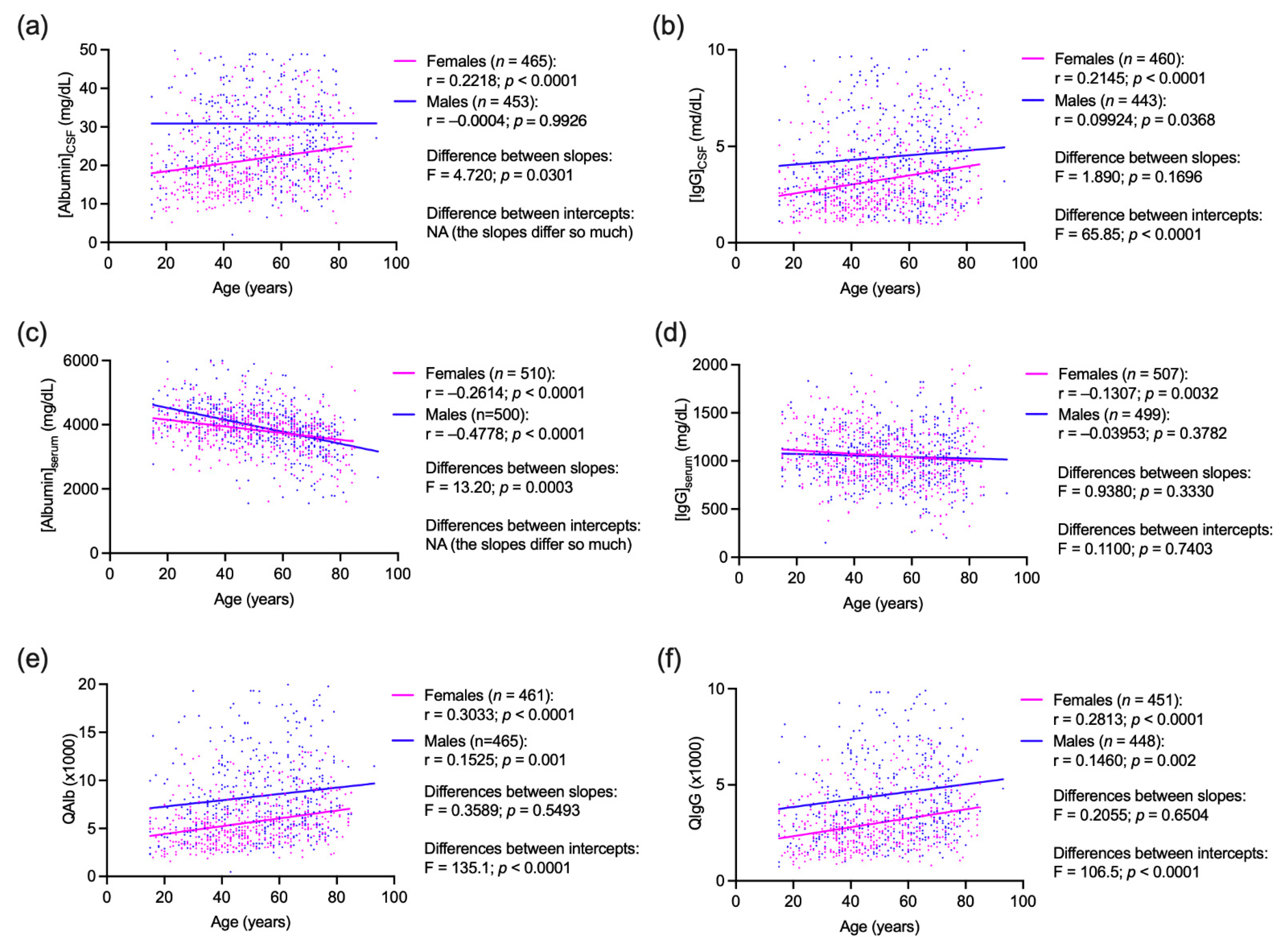
3.3. Correlations between Age, Sex and CSF and Serum Concentrations of Albumin and IgG
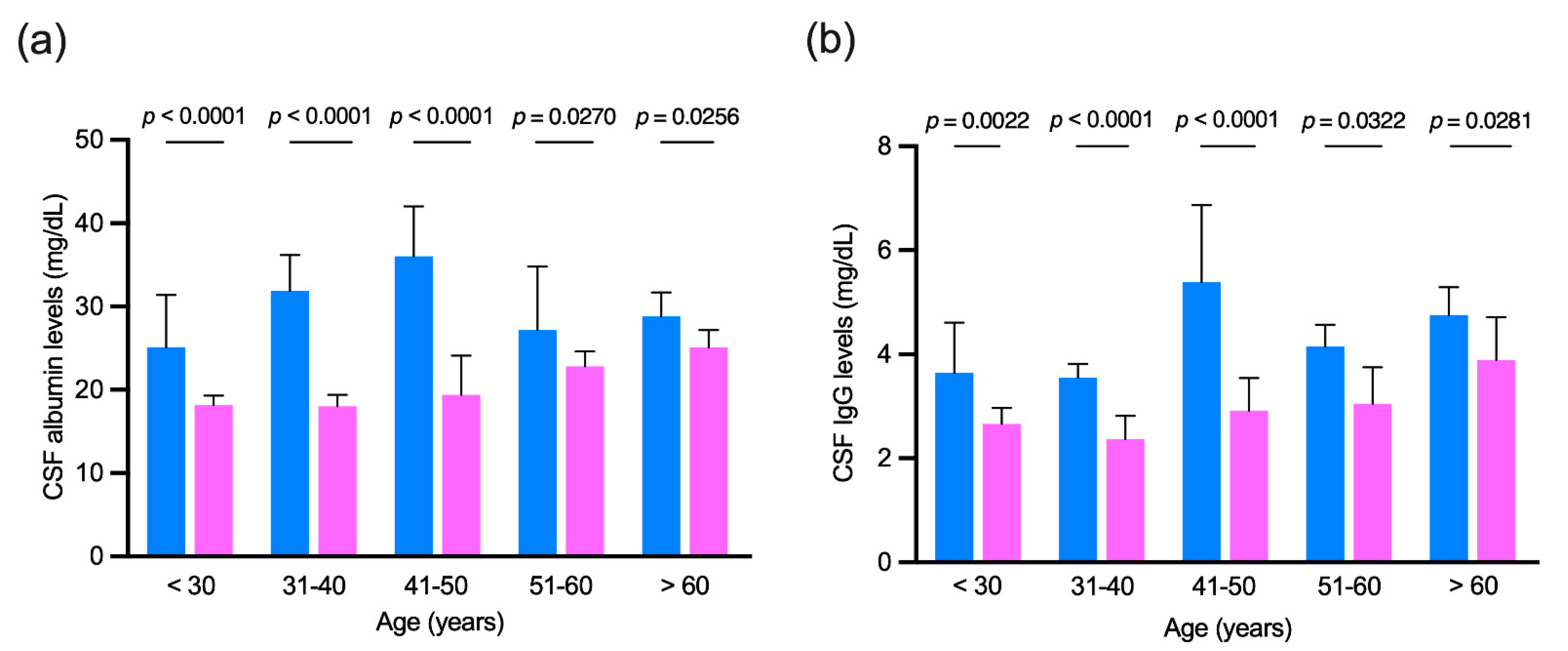
3.4. Age-Matched Comparison of Cerebrospinal Fluid Albumin and IgG Levels in Patients with no Evidence of Intrathecal IgG Synthesis
3.5. Multivariate Analysis
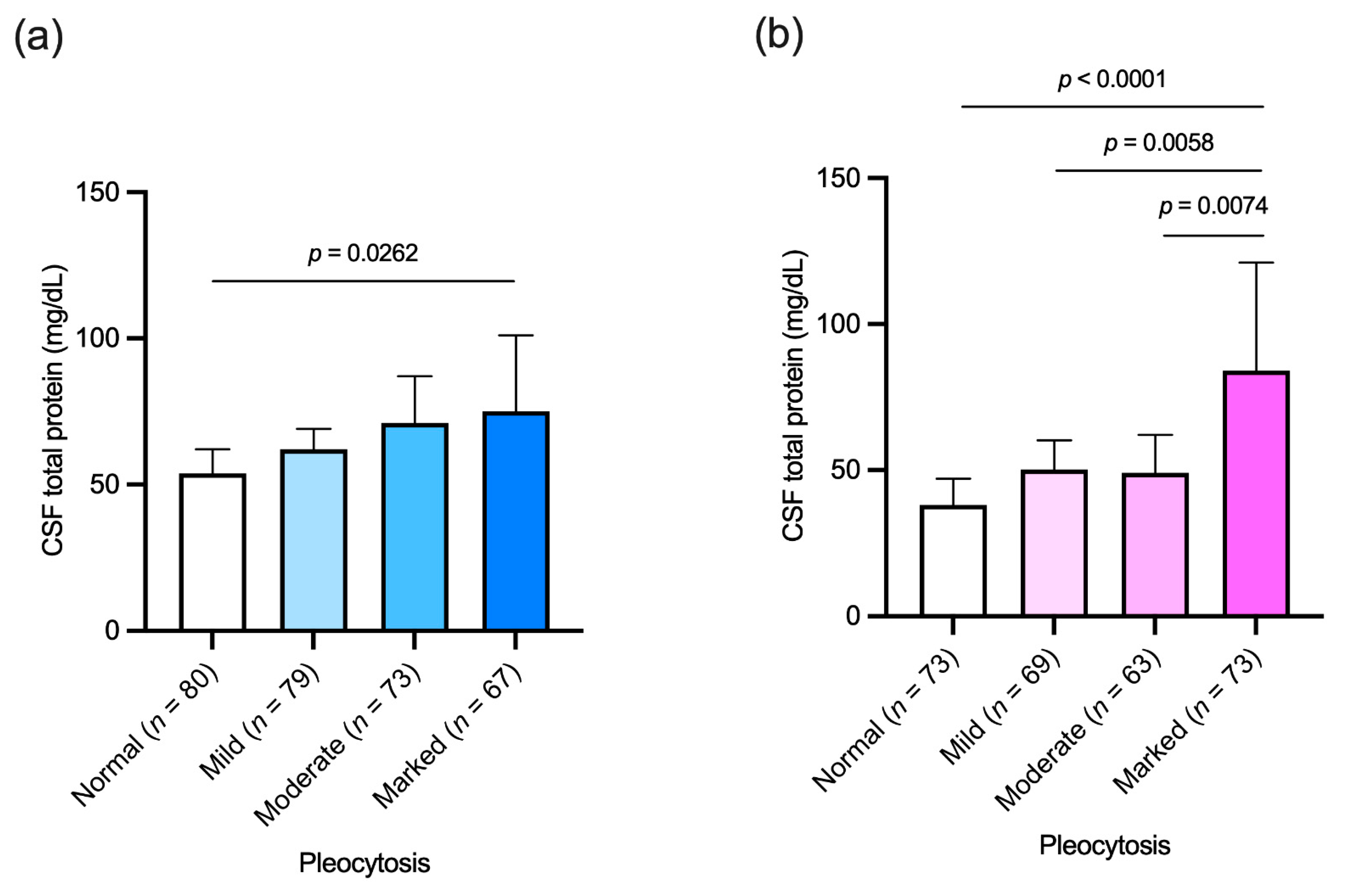
3.6. Association between Cerebrospinal Fluid Cellular and Protein Content
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sakka, L.; Coll, G.; Chazal, J. Anatomy and physiology of cerebrospinal fluid. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2011, 128, 309–316. [Google Scholar] [CrossRef]
- Hühmer, A.F.; Biringer, R.G.; Amato, H.; Fonteh, A.N.; Harrington, M.G. Protein analysis in human cerebrospinal fluid: Physiological aspects, current progress and future challenges. Dis. Markers 2006, 22, 3–26. [Google Scholar] [CrossRef] [PubMed]
- Obermeier, B.; Mentele, R.; Malotka, J.; Kellermann, J.; Kümpfel, T.; Wekerle, H.; Lottspeich, F.; Hohlfeld, R.; Dornmair, K. Matching of oligoclonal immunoglobulin transcriptomes and proteomes of cerebrospinal fluid in multiple sclerosis. Nat. Med. 2008, 14, 688–693. [Google Scholar] [CrossRef]
- Deisenhammer, F.; Bartos, A.; Egg, R.; Gilhus, N.E.; Giovannoni, G.; Rauer, S.; Sellebjerg, F. Guidelines on routine cerebrospinal fluid analysis. Report from an EFNS task force. Eur. J. Neurol. 2006, 13, 913–922. [Google Scholar] [CrossRef] [PubMed]
- Leeman, M.; Choi, J.; Hansson, S.; Storm, M.U.; Nilsson, L. Proteins and antibodies in serum, plasma, and whole blood-size characterization using asymmetrical flow field-flow fractionation (AF4). Anal. Bioanal. Chem. 2018, 410, 4867–4873. [Google Scholar] [CrossRef] [PubMed]
- Freedman, M.S.; Thompson, E.J.; Deisenhammer, F.; Giovannoni, G.; Grimsley, G.; Keir, G.; Ohman, S.; Racke, M.K.; Sharief, M.; Sindic, C.J.; et al. Recommended standard of cerebrospinal fluid analysis in the diagnosis of multiple sclerosis: A consensus statement. Arch. Neurol. 2005, 62, 865–870. [Google Scholar] [CrossRef]
- Gastaldi, M.; Zardini, E.; Leante, R.; Ruggieri, M.; Costa, G.; Cocco, E.; De Luca, G.; Cataldo, I.; Biagioli, T.; Ballerini, C.; et al. Cerebrospinal fluid analysis and the determination of oligoclonal bands. Neurol. Sci. Off. J. Ital. Neurol. Soc. Ital. Soc. Clin. Neurophysiol. 2017, 38, 217–224. [Google Scholar] [CrossRef]
- Tumani, H.; Petereit, H.F.; Gerritzen, A.; Gross, C.C.; Huss, A.; Isenmann, S.; Jesse, S.; Khalil, M.; Lewczuk, P.; Lewerenz, J.; et al. S1 guidelines “lumbar puncture and cerebrospinal fluid analysis” (abridged and translated version). Neurol. Res. Pract. 2020, 2, 8. [Google Scholar] [CrossRef]
- Andersson, M.; Alvarez-Cermeno, J.; Bernardi, G.; Cogato, I.; Fredman, P.; Frederiksen, J.; Fredrikson, S.; Gallo, P.; Grimaldi, L.M.; Gronning, M.; et al. Cerebrospinal fluid in the diagnosis of multiple sclerosis: A consensus report. J. Neurol. Neurosurg. Psychiatry 1994, 57, 897–902. [Google Scholar] [CrossRef]
- Link, H. The value of cerebrospinal fluid immunoglobulin analysis in clincial neurology. Riv. Patol. Nerv. Ment. 1976, 97, 323–340. [Google Scholar]
- Reiber, H.; Felgenhauer, K. Protein transfer at the blood cerebrospinal fluid barrier and the quantitation of the humoral immune response within the central nervous system. Clin. Chim. Acta; Int. J. Clin. Chem. 1987, 163, 319–328. [Google Scholar] [CrossRef]
- Cosgrove, K.P.; Mazure, C.M.; Staley, J.K. Evolving knowledge of sex differences in brain structure, function, and chemistry. Biol. Psychiatry 2007, 62, 847–855. [Google Scholar] [CrossRef]
- McCarthy, M.M.; Arnold, A.P.; Ball, G.F.; Blaustein, J.D.; De Vries, G.J. Sex differences in the brain: The not so inconvenient truth. J. Neurosci. Off. J. Soc. Neurosci. 2012, 32, 2241–2247. [Google Scholar] [CrossRef]
- Parrado-Fernandez, C.; Blennow, K.; Hansson, M.; Leoni, V.; Cedazo-Minguez, A.; Bjorkhem, I. Evidence for sex difference in the CSF/plasma albumin ratio in ~20,000 patients and 335 healthy volunteers. J. Cell Mol. Med. 2018, 22, 5151–5154. [Google Scholar] [CrossRef] [PubMed]
- Castellazzi, M.; Morotti, A.; Tamborino, C.; Alessi, F.; Pilotto, S.; Baldi, E.; Caniatti, L.M.; Trentini, A.; Casetta, I.; Granieri, E.; et al. Increased age and male sex are independently associated with higher frequency of blood-cerebrospinal fluid barrier dysfunction using the albumin quotient. Fluids Barriers CNS 2020, 17, 14. [Google Scholar] [CrossRef]
- Meixensberger, S.; Bechter, K.; Dersch, R.; Feige, B.; Maier, S.; Schiele, M.A.; Runge, K.; Denzel, D.; Nickel, K.; Spieler, D.; et al. Sex difference in cerebrospinal fluid/blood albumin quotients in patients with schizophreniform and affective psychosis. Fluids Barriers CNS 2020, 17, 67. [Google Scholar] [CrossRef] [PubMed]
- McCudden, C.R.; Brooks, J.; Figurado, P.; Bourque, P.R. Cerebrospinal Fluid Total Protein Reference Intervals Derived from 20 Years of Patient Data. Clin. Chem. 2017, 63, 1856–1865. [Google Scholar] [CrossRef] [PubMed]
- Castellazzi, M.; Pizzicotti, S.; Lombardo, I.; Alfiero, S.; Morotti, A.; Pellegatti, P.; Negri, G.; Natali, L.; Ferri, C.; Fainardi, E.; et al. Sexual dimorphism in the cerebrospinal fluid total protein content. Clin. Chem. Lab. Med. 2020. [Google Scholar] [CrossRef]
- Salden, H.J.; Bas, B.M.; Hermans, I.T.; Janson, P.C. Analytical performance of three commercially available nephelometers compared for quantifying proteins in serum and cerebrospinal fluid. Clin. Chem. 1988, 34, 1594–1596. [Google Scholar] [CrossRef] [PubMed]
- Tibbling, G.; Link, H.; Ohman, S. Principles of albumin and IgG analyses in neurological disorders. I. Establishment of reference values. Scand. J. Clin. Lab. Investig. 1977, 37, 385–390. [Google Scholar] [CrossRef]
- Reiber, H. External quality assessment in clinical neurochemistry: Survey of analysis for cerebrospinal fluid (CSF) proteins based on CSF/serum quotients. Clin. Chem. 1995, 41, 256–263. [Google Scholar] [CrossRef]
- Landis, J.R.; Koch, G.G. The measurement of observer agreement for categorical data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef] [PubMed]
- Thompson, E.J.; Keir, G. Laboratory investigation of cerebrospinal fluid proteins. Ann. Clin. Biochem. 1990, 27 Pt 5, 425–435. [Google Scholar] [CrossRef]
- Gastaldi, M.; Zardini, E.; Franciotta, D. An update on the use of cerebrospinal fluid analysis as a diagnostic tool in multiple sclerosis. Expert Rev. Mol. Diagn. 2017, 17, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Tourtellotte, W.W.; Potvin, A.R.; Fleming, J.O.; Murthy, K.N.; Levy, J.; Syndulko, K.; Potvin, J.H. Multiple sclerosis: Measurement and validation of central nervous system IgG synthesis rate. Neurology 1980, 30, 240–244. [Google Scholar] [CrossRef] [PubMed]
- Rath, J.; Zulehner, G.; Schober, B.; Grisold, A.; Krenn, M.; Cetin, H.; Zimprich, F. Cerebrospinal fluid analysis in Guillain-Barré syndrome: Value of albumin quotients. J. Neurol. 2021. [Google Scholar] [CrossRef]
- Uher, T.; Horakova, D.; Tyblova, M.; Zeman, D.; Krasulova, E.; Mrazova, K.; Seidl, Z.; Vaneckova, M.; Krasensky, J.; Weinstock-Guttman, B.; et al. Increased albumin quotient (QAlb) in patients after first clinical event suggestive of multiple sclerosis is associated with development of brain atrophy and greater disability 48 months later. Mult. Scler. 2016, 22, 770–781. [Google Scholar] [CrossRef]
- Liang, S.; Qin, Q.; Tang, Y.; Liao, W.; Yang, Y.; He, J.; Li, L. Impact of blood-brain barrier disruption on newly diagnosed neuromyelitis optica spectrum disorder symptoms and prognosis. Ann. Palliat. Med. 2020, 9, 324–330. [Google Scholar] [CrossRef]
- Breiner, A.; Bourque, P.R.; Allen, J.A. Updated cerebrospinal fluid total protein reference values improve chronic inflammatory demyelinating polyneuropathy diagnosis. Muscle Nerve 2019, 60, 180–183. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Wan, Q.; Tian, Y.; Zhao, B.; Deng, Y. Female Hormone 17beta-Estradiol Downregulated MMP-2 Expression and Upregulated A1PI Expression in Human Corneal Stromal Cells. Cell Biochem. Biophys. 2018, 76, 265–271. [Google Scholar] [CrossRef]
- Gu, C.; Wang, F.; Hou, Z.; Lv, B.; Wang, Y.; Cong, X.; Chen, X. Sex-related differences in serum matrix metalloproteinase-9 screening non-calcified and mixed coronary atherosclerotic plaques in outpatients with chest pain. Heart Vessel. 2017, 32, 1424–1431. [Google Scholar] [CrossRef] [PubMed]
- Castellazzi, M.; Ligi, D.; Contaldi, E.; Quartana, D.; Fonderico, M.; Borgatti, L.; Bellini, T.; Trentini, A.; Granieri, E.; Fainardi, E.; et al. Multiplex Matrix Metalloproteinases Analysis in the Cerebrospinal Fluid Reveals Potential Specific Patterns in Multiple Sclerosis Patients. Front. Neurol. 2018, 9, 1080. [Google Scholar] [CrossRef] [PubMed]
- Na, W.; Lee, J.Y.; Kim, W.S.; Yune, T.Y.; Ju, B.G. 17beta-Estradiol Ameliorates Tight Junction Disruption via Repression of MMP Transcription. Mol. Endocrinol. 2015, 29, 1347–1361. [Google Scholar] [CrossRef] [PubMed]
- Seyfert, S.; Kunzmann, V.; Schwertfeger, N.; Koch, H.C.; Faulstich, A. Determinants of lumbar CSF protein concentration. J. Neurol. 2002, 249, 1021–1026. [Google Scholar] [CrossRef] [PubMed]
| Women (n = 859) | Men (n = 660) | p | |
|---|---|---|---|
| Age, years: median (IQR) | 43 (33–57) | 50 (37–65) | <0.0001 |
| CSF IgG (mg/dL): median (IQR) | 3.59 (2.30–5.76) | 4.63 (2.91–7.57) | <0.0001 |
| CSF albumin (mg/dL): median (IQR) | 19.2 (14.6–27.4) | 29.7 (20.4–43.6) | <0.0001 |
| Serum IgG (mg/dL): median (IQR) | 1050 (876–1260) | 1030 (861–1220) | 0.1963 |
| Serum albumin (mg/dL): median (IQR) | 3940 (3630–4260) | 4000 (3583–4408) | 0.0866 |
| Albumin quotient (QAlb) value: median (IQR) | 4.93 (3.70–7.15) | 7.50 (5.12–11.48) | <0.0001 |
| IgG Index (positive): n (%) | 309 (36.0) | 160 (24.2) | <0.0001 |
| Reiber’s formula (positive): n (%) | 315 (36.7) | 127 (19.2) | <0.0001 |
| CSF IgG OCB patterns: n (%) | |||
| Pattern 1 (negative): | 445 (51.8) | 429 (65.0) | <0.0001 |
| Pattern 2 (local synthesis): | 323 (37.6) | 133 (20.2) | <0.0001 |
| Pattern 3 (mixed): | 24 (2.8) | 23 (3.5) | 0.4579 |
| Pattern 4 (mirror): | 57 (6.6) | 61 (9.2) | 0.0320 |
| Pattern 5 (paraproteinemic): | 10 (1.2) | 14 (2.1) | 0.1505 |
| Women (n = 859) | Men (n = 660) | ||||
|---|---|---|---|---|---|
| Values | 95% CI | Values | 95% CI | p | |
| IgG Index | |||||
| Kappa (SE) | 0.587 (0.028) | 0.531–0.642 | 0.559 (0.038) | 0.485–0.633 | |
| Sensitivity | 0.7032 | 0.6531–0.7488 | 0.6731 | 0.5961–0.7418 | 0.5308 |
| Specificity | 0.8730 | 0.8414–0.8991 | 0.8909 | 0.8606–0.9152 | 0.3837 |
| Reiber’s Formula | |||||
| Kappa (SE) | 0.635 (0.027) | 0.582–0.688 | 0.565 (0.0399) | 0.488–0.642 | |
| Sensitivity | 0.7262 | 0.6770–0.7705 | 0.5962 | 0.5177–0.6699 | 0.0049 |
| Specificity | 0.8965 | 0.8671–0.9200 | 0.9325 | 0.9072–0.9513 | 0.0437 |
| Women (n = 512) | Men (n = 504) | Mann–Whitney Statistics p | Quade Statistics p | |
|---|---|---|---|---|
| CSFalbumin (mg/dL): median (IQR) | 20.35 (14.90–30.08) | 30.50 (20.13–45.05) | <0.0001 | F = 83.329 <0.000001 |
| CSF IgG (mg/dL): median (IQR) | 2.93 (2.02–4.99) | 4.21 (2.73–7.14) | <0.0001 | F = 52.827 <0.000001 |
| Women | p | Men | p | |
|---|---|---|---|---|
| Pleocytosis: | ||||
| Mild: 6–25 cells/μL (n = 148) | r = 0.1722 (n = 69) | 0.1571 | r = 0.1351 (n = 79) | 0.2353 |
| Moderate: 26–100 cells/μL (n = 136) | r = 0.02903 (n = 63) | 0.8213 | r = 0.1028 (n = 73) | 0.3868 |
| Marked: >100 cells/μL (n = 140) | r = 0.2013 (n = 73) | 0.0876 | r = 0.1172 (n = 67) | 0.3448 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castellazzi, M.; Ferri, C.; Alfiero, S.; Lombardo, I.; Laudisi, M.; Tecilla, G.; Boni, M.; Pizzicotti, S.; Fainardi, E.; Bellini, T.; et al. Sex-Related Differences in Cerebrospinal Fluid Plasma-Derived Proteins of Neurological Patients. Diagnostics 2021, 11, 884. https://doi.org/10.3390/diagnostics11050884
Castellazzi M, Ferri C, Alfiero S, Lombardo I, Laudisi M, Tecilla G, Boni M, Pizzicotti S, Fainardi E, Bellini T, et al. Sex-Related Differences in Cerebrospinal Fluid Plasma-Derived Proteins of Neurological Patients. Diagnostics. 2021; 11(5):884. https://doi.org/10.3390/diagnostics11050884
Chicago/Turabian StyleCastellazzi, Massimiliano, Caterina Ferri, Sarah Alfiero, Ilenia Lombardo, Michele Laudisi, Ginevra Tecilla, Michela Boni, Stefano Pizzicotti, Enrico Fainardi, Tiziana Bellini, and et al. 2021. "Sex-Related Differences in Cerebrospinal Fluid Plasma-Derived Proteins of Neurological Patients" Diagnostics 11, no. 5: 884. https://doi.org/10.3390/diagnostics11050884
APA StyleCastellazzi, M., Ferri, C., Alfiero, S., Lombardo, I., Laudisi, M., Tecilla, G., Boni, M., Pizzicotti, S., Fainardi, E., Bellini, T., & Pugliatti, M. (2021). Sex-Related Differences in Cerebrospinal Fluid Plasma-Derived Proteins of Neurological Patients. Diagnostics, 11(5), 884. https://doi.org/10.3390/diagnostics11050884








