Correlation of MRI-Lesion Targeted Biopsy vs. Systematic Biopsy Gleason Score with Final Pathological Gleason Score after Radical Prostatectomy
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Prostate Biopsy Approach and Performance
2.3. Statistical Analysis
3. Results
3.1. Descriptive Baseline Characteristics
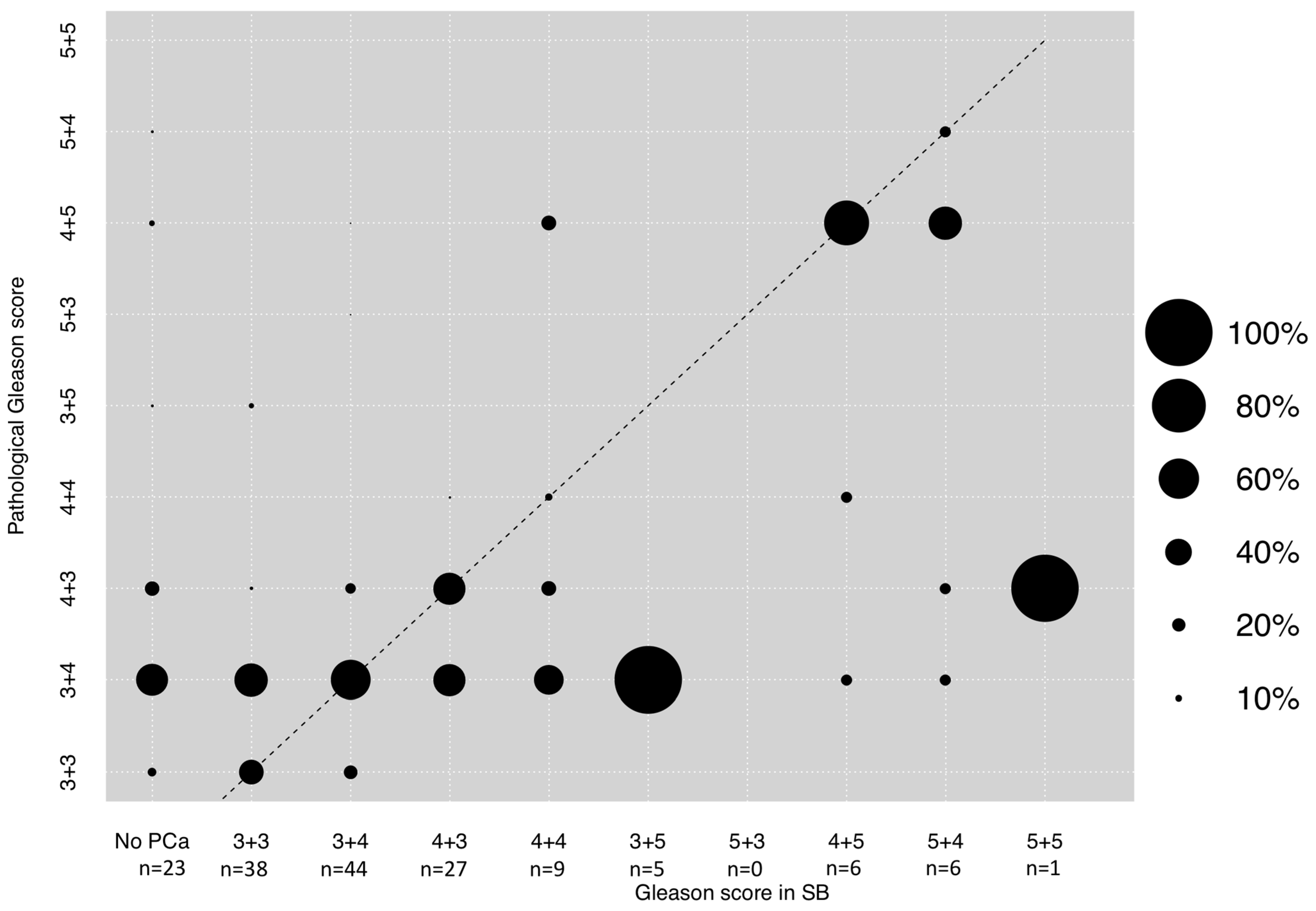
3.2. Correlation between GS in SB and Final Pathological GS after RP
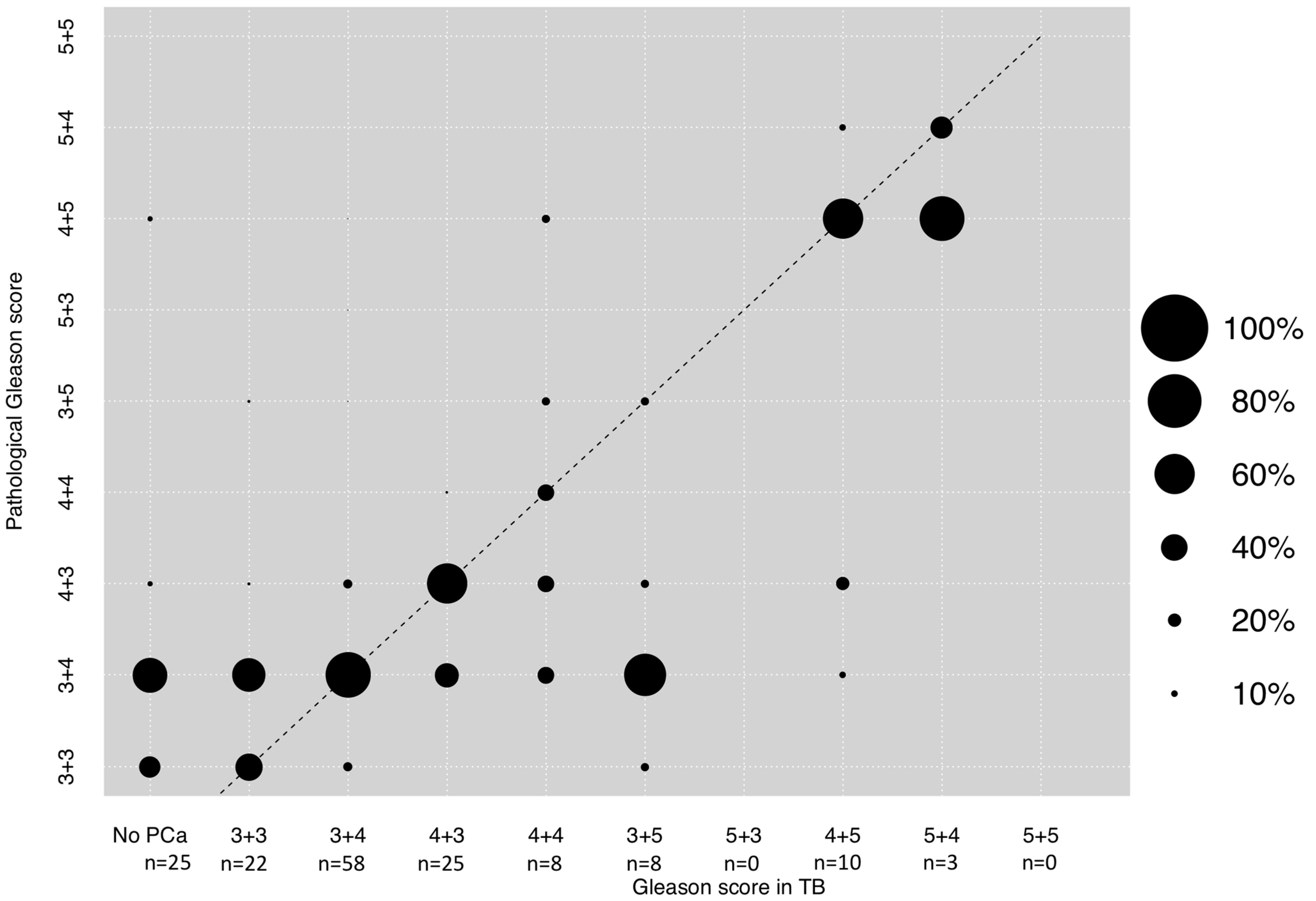
3.3. Correlation between GS in TB and Final Pathological GS after RP
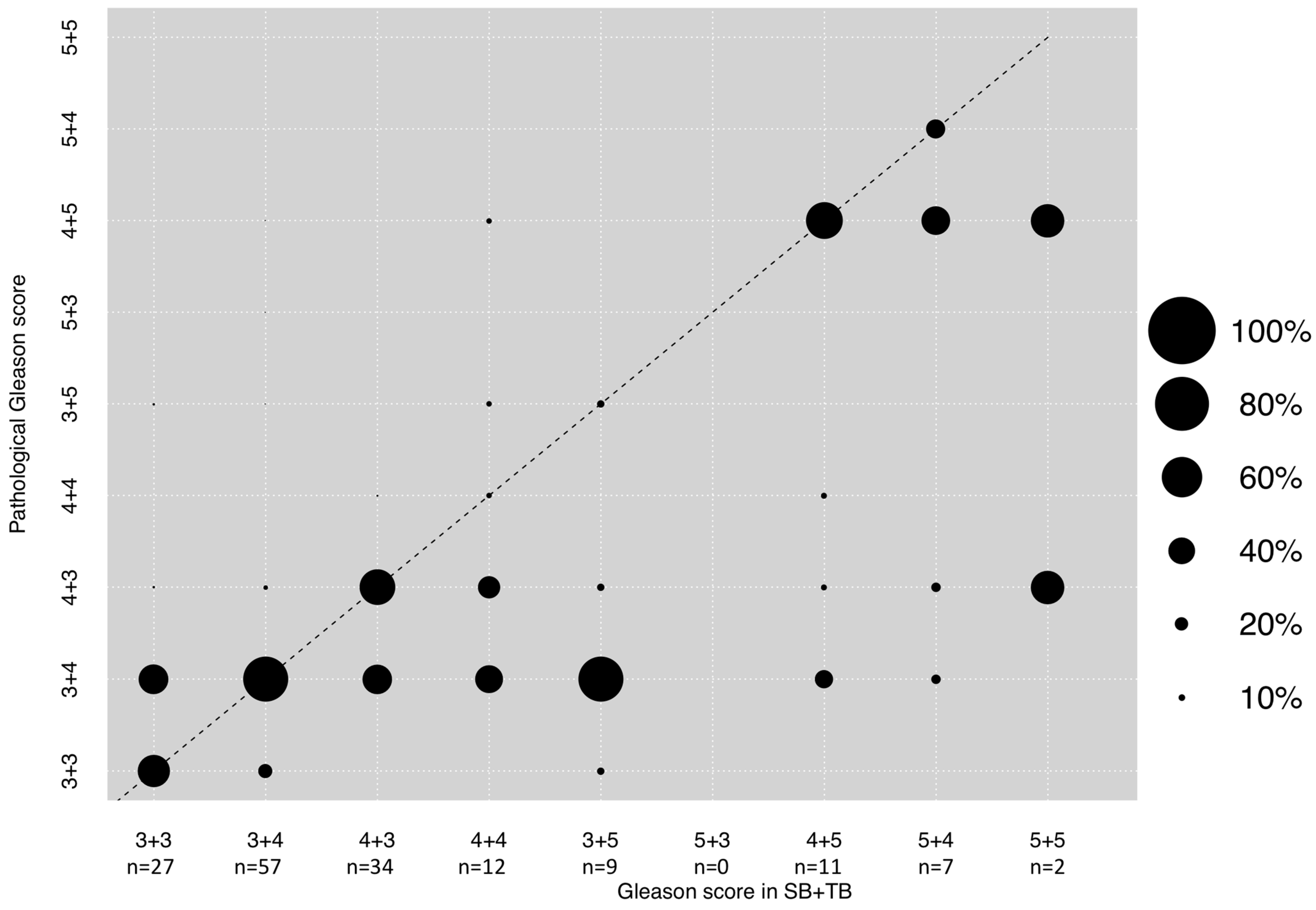
3.4. Correlation between GS in Combined SB + TB and Final Pathological GS after RP
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Ethics Approval
References
- Eeles, R.A.; Olama, A.A.; Benlloch, S.; Saunders, E.J.; Leongamornlert, D.A.; Tymrakiewicz, M.; Ghoussaini, M.; Luccarini, C.; Dennis, J.; Jugurnauth-Little, S.; et al. Identification of 23 new prostate cancer susceptibility loci using the iCOGS custom genotyping array. Nat. Genet. 2013, 45, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed]
- Malvezzi, M.; Carioli, G.; Bertuccio, P.; Boffetta, P.; Levi, F.; La Vecchia, C.; Negri, E. European cancer mortality predictions for the year 2019 with focus on breast cancer. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2019, 30, 781–787. [Google Scholar] [CrossRef] [PubMed]
- Mottet, N.; Cornford, P.; Van den Bergh, R.C.N.; Briers, E.; De Santis, M.; Fanti, S.; Gillessen, S.; Grummet, J.; Henrky, A.M.; Lam, T.B.; et al. EAU-EANM-ESTRO-ESUR-SIOG. Guidel. Prostate Cancer 2020. [Google Scholar]
- Ginsburg, K.; Cole, A.I.; Silverman, M.E.; Livingstone, J.; Smith, D.W.; Heilbrun, L.K.; Shi, D.; Mehra, R.; Sakr, W.A.; Morgan, T.M.; et al. Should all prostate needle biopsy Gleason score 4 + 4 = 8 prostate cancers be high risk? Implications for shared decision-making and patient counselling. Urol. Oncol. 2020, 38, 78.e1–78.e6. [Google Scholar] [CrossRef]
- Charmpi, K.; Guo, T.; Zhong, Q.; Wagner, U.; Sun, R.; Toussaint, N.C.; Fritz, C.E.; Yuan, C.; Chen, H.; Rupp, N.J.; et al. Convergent network effects along the axis of gene expression during prostate cancer progression. Genome Biol. 2020, 21, 302. [Google Scholar] [CrossRef] [PubMed]
- Cicione, A.; De Nunzio, C.; Manno, S.; Damiano, R.; Posti, A.; Lima, E.; Tubaro, A.; Balloni, F. An update on prostate biopsy in the era of magnetic resonance imaging. Minerva Urol. E Nefrol. Ital. J. Urol. Nephrol. 2018, 70, 264–274. [Google Scholar] [CrossRef]
- Rührup, J.; Preisser, F.; Theißen, L.; Wenzel, M.; Roos, F.C.; Becker, A.; Kluth, L.A.; Bodelle, B.; Köllermann, J.; Chun, F.K.H.; et al. MRI-Fusion Targeted vs. Systematic Prostate Biopsy-How Does the Biopsy Technique Affect Gleason Grade Concordance and Upgrading After Radical Prostatectomy? Front Surg. 2019, 6, 55. [Google Scholar] [CrossRef] [PubMed]
- Kayano, P.P.; Carneiro, A.; Castilho, T.M.L.; Sivaraman, A.; Claros, O.R.; Baroni, R.H.; Garcia, R.G.; Mariotti, G.C.; Smaletz, O.; Filippi, R.Z.; et al. Comparison of Gleason upgrading rates in transrectal ultrasound systematic random biopsies versus US-MRI fusion biopsies for prostate cancer. Int. Braz J. Urol. Off. J. Braz Soc. Urol. 2018, 44, 1106–1113. [Google Scholar] [CrossRef]
- Aslan, G.; Çelik, S.; Sözen, S.; Akdoğan, B.; İzol, V.; Yücel Bilen, C.; Sahin, B.; Türkeri, L. Comparison of TRUS and combined MRI-targeted plus systematic prostate biopsy for the concordance between biopsy and radical prostatectomy pathology. Int. J. Clin. Pract. 2020, 75, e13797. [Google Scholar] [CrossRef]
- Apfelbeck, M.; Tritschler, S.; Clevert, D.A.; Buchner, A.; Chaloupka, M.; Kretschmer, A.; Herlemann, A.; Stief, C.; Schlenker, B. Postoperative change in Gleason score of prostate cancer in fusion targeted biopsy: A matched pair analysis. Scand. J. Urol. 2020, 1–6. [Google Scholar] [CrossRef]
- De Luca, S.; Fiori, C.; Bollito, E.; Garrou, D.; Aimar, R.; Cattaneo, G.; De Cillis, S.; Manfredi, M.; Tota, D.; Federica, M.; et al. Risk of Gleason Score 3 + 4 = 7 prostate cancer upgrading at radical prostatectomy is significantly reduced by targeted versus standard biopsy. Minerva. Urol. E Nefrol. Ital. J. Urol. Nephrol. 2020, 72, 360–368. [Google Scholar] [CrossRef]
- Goel, S.; Shoag, J.E.; Gross, M.D.; Al Hussein Al Awamlh, B.; Robinson, B.; Khani, F.; Baltich Nelson, B.; Margolis, D.J.; Hu, J.C. Concordance Between Biopsy and Radical Prostatectomy Pathology in the Era of Targeted Biopsy: A Systematic Review and Meta-analysis. Eur. Urol. Oncol. 2020, 3, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Luzzago, S.; Petralia, G.; Maresca, D.; Sabatini, I.; Cordima, G.; Brescia, A.; Verweij, F.; Garelli, G.; Mistretta, F.A.; Cioffi, A.; et al. Pathological findings at radical prostatectomy of biopsy naïve men diagnosed with MRI targeted biopsy alone without concomitant standard systematic sampling. Urol. Oncol. 2020, 38, 929.e11–929.e19. [Google Scholar] [CrossRef]
- Le, J.D.; Stephenson, S.; Brugger, M.; Lu, D.Y.; Lieu, P.; Sonn, G.A.; Natarajan, S.; Dorey, F.J.; Huang, J.; Margolis, D.J.; et al. Magnetic resonance imaging-ultrasound fusion biopsy for prediction of final prostate pathology. J. Urol. 2014, 192, 1367–1373. [Google Scholar] [CrossRef] [PubMed]
- Porpiglia, F.; De Luca, S.; Passera, R.; Manfredi, M.; Mele, F.; Bollito, E.; DE Pascale, A.; Cossu, M.; Aimar, R.; Veltri, A. Multiparametric-Magnetic Resonance/Ultrasound Fusion Targeted Prostate Biopsy Improves Agreement Between Biopsy and Radical Prostatectomy Gleason Score. Anticancer Res. 2016, 36, 4833–4839. [Google Scholar] [CrossRef] [PubMed]
- Elkhoury, F.F.; Felker, E.R.; Kwan, L.; Sisk, A.E.; Delfin, M.; Natarajan, S.; Marks, L.S. Comparison of Targeted vs Systematic Prostate Biopsy in Men Who Are Biopsy Naive: The Prospective Assessment of Image Registration in the Diagnosis of Prostate Cancer (PAIREDCAP) Study. JAMA Surg. 2019, 154, 811–818. [Google Scholar] [CrossRef] [PubMed]
- Diamand, R.; Oderda, M.; Al Hajj Obeid, W.; Albisinni, S.; Van Velthoven, R.; Fasolis, G.; Simone, G.; Ferriero, M.; Roche, J.B.; et al. A multicentric study on accurate grading of prostate cancer with systematic and MRI/US fusion targeted biopsies: Comparison with final histopathology after radical prostatectomy. World J. Urol. 2019, 37, 2109–2117. [Google Scholar] [CrossRef] [PubMed]
- Borkowetz, A.; Platzek, I.; Toma, M.; Renner, T.; Herout, R.; Baunacke, M.; Laniado, M.; Baretton, G.; Froehner, M.; Zastrow, S.; et al. Direct comparison of multiparametric magnetic resonance imaging (MRI) results with final histopathology in patients with proven prostate cancer in MRI/ultrasonography-fusion biopsy. BJU Int. 2016, 118, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Leyh-Bannurah, S.R.; Kachanov, M.; Karakiewicz, P.I.; Beyersdorff, D.; Pompe, R.S.; Oh-Hohenhorst, S.J.; Fisch, M.; Maurer, T.; Graefen, M.; Budäus, L. Combined systematic versus stand-alone multiparametric MRI-guided targeted fusion biopsy: Nomogram prediction of non-organ-confined prostate cancer. World J. Urol. 2021, 39, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Radtke, J.P.; Schwab, C.; Wolf, M.B.; Freitag, M.T.; Alt, C.D.; Kesch, C.; Popeneciu, I.V.; Huettenbrink, C.; Gasch, C.; Klein, T.; et al. Multiparametric Magnetic Resonance Imaging (MRI) and MRI-Transrectal Ultrasound Fusion Biopsy for Index Tumor Detection: Correlation with Radical Prostatectomy Specimen. Eur. Urol. 2016, 70, 846–853. [Google Scholar] [CrossRef]
- Wenzel, M.; Welte, M.N.; Theissen, L.H.; Wittler, C.; Hoeh, B.; Humke, C.; Preisser, F.; Würnschimmel, C.; Tilki, D.; Graefen, M.; et al. Comparison of Complication Rates with Antibiotic Prophylaxis with Cefpodoxime Versus Fluoroquinolones After Transrectal Prostate Biopsy. Eur. Urol. Focus 2020. [Google Scholar] [CrossRef]
- Epstein, J.I.; Egevad, L.; Amin, M.B.; Delahunt, B.; Srigley, J.R.; Humphrey, P.A. The 2014 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma: Definition of Grading Patterns and Proposal for a New Grading System. Am. J. Surg. Pathol. 2016, 40, 244–252. [Google Scholar] [CrossRef]
- Rapisarda, S.; Bada, M.; Crocetto, F.; Barone, B.; Arcaniolo, D.; Polara, A.; Imbimbo, C.; Grosso, G. The role of multiparametric resonance and biopsy in prostate cancer detection: Comparison with definitive histological report after laparoscopic/robotic radical prostatectomy. Abdom. Radiol. 2020, 45, 4178–4184. [Google Scholar] [CrossRef] [PubMed]
- Ploussard, G.; Beauval, J.B.; Lesourd, M.; Almeras, C.; Assoun, J.; Aziza, R.; Gautier, J.R.; Loison, G.; Portalez, D.; Salin, A.; et al. Added Value of Concomitant Systematic and Fusion Targeted Biopsies for Grade Group Prediction Based on Radical Prostatectomy Final Pathology on Positive Magnetic Resonance Imaging. J. Urol. 2019, 202, 1182–1187. [Google Scholar] [CrossRef] [PubMed]
- Gansler, T.; Fedewa, S.; Qi, R.; Lin, C.C.; Jemal, A.; Moul, J.W. Most Gleason 8 Biopsies are Downgraded at Prostatectomy-Does 4 + 4 = 7? J. Urol. 2018, 199, 706–712. [Google Scholar] [CrossRef] [PubMed]
- Tourinho-Barbosa, R.R.; de la Rosette, J.; Sanchez-Salas, R. Prostate cancer multifocality, the index lesion, and the microenvironment. Curr. Opin. Urol. 2018, 28, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Kasivisvanathan, V.; Rannikko, A.S.; Borghi, M.; Panebianco, V.; Mynderse, L.A.; Vaarala, M.H.; Briganti, A.; Budäus, L.; Hellawell, G.; Hindley, R.G.; et al. MRI-Targeted or Standard Biopsy for Prostate-Cancer Diagnosis. N. Engl. J. Med. 2018, 378, 1767–1777. [Google Scholar] [CrossRef]
- Ahmed, H.U.; El-Shater Bosaily, A.; Brown, L.C.; et al. Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): A paired validating confirmatory study. Lancet Lond Engl. 2017, 389, 815–822. [Google Scholar] [CrossRef]
- Gordon, L.A.N.; Kam, J.; Gilbourd, D.; Haxhimolla, H. Australian ultrasound-guided biopsy trends: A 17-year analysis of national data. Prostate Int. 2019, 7, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Saar, M.; Linxweiler, J.; Borkowetz, A.; Fussek, S.; Urbanova, K.; Bellut, L.; Kristiansen, G.; Wullich, B. Current Role of Multiparametric MRI and MRI Targeted Biopsies for Prostate Cancer Diagnosis in Germany: A Nationwide Survey. Urol. Int. 2020, 104, 731–740. [Google Scholar] [CrossRef] [PubMed]
- Arsov, C.; Becker, N.; Rabenalt, R.; Hiester, A.; Quentin, M.; Dietzel, F.; Antoch, G.; Gabbert, H.E.; Albers, P.; Schimmöller, L. The use of targeted MR-guided prostate biopsy reduces the risk of Gleason upgrading on radical prostatectomy. J. Cancer Res. Clin. Oncol. 2015, 141, 2061–2068. [Google Scholar] [CrossRef] [PubMed]
- Manceau, C.; Fromont-Hankard, G.; Beauval, J.B.; Lesourd, M.; Almeras, C.; Bajeot, A.S.; Gautier, J.R.; Soulié, M.; Loison, G.; Salin, A.; et al. The prognostic value of high-grade prostate cancer pattern on MRI-targeted biopsies: Predictors for downgrading and importance of concomitant systematic biopsies. World J. Urol. 2021. [Google Scholar] [CrossRef] [PubMed]
| Variable | Overall n = 159 | |
|---|---|---|
| Age, Years | Median (IQR) | 66 (62–71) |
| Prostate volume, cc | Median (IQR) | 43 (35–60) |
| PSA, ng/mL | Median (IQR) | 7.0 (5.2–10.0) |
| Cores taken at biopsy | Median (IQR) | 14 (14–15) |
| Number of positive cores | Median (IQR) | 5 (3–8) |
| Positive cores in MRI lesion | Median (IQR) | 1 (1–2) |
| Positive cores out of lesion | Median (IQR) | 3 (2–6) |
| DRE | non-suspicous | 101 (63.5) |
| suspicous | 58 (36.5) | |
| cT-stage | cT1 | 101 (63.5) |
| cT2 | 56 (35.2) | |
| cT3-4 | 2 (1.3) | |
| Prior biopsies | 0 | 122 (76.7) |
| 1 | 31 (19.5) | |
| ≥2 | 6 (3.8) | |
| PIRADS lesion | PIRADS 3 | 23 (14.5) |
| PIRADS 4 | 68 (42.8) | |
| PIRADS 5 | 61 (38.4) | |
| Unknown | 7 (4.4) | |
| Gleason score in lesion | No PCa | 25 (15.7) |
| 3 + 3 | 22 (13.8) | |
| 3 + 4 | 58 (36.5) | |
| 4 + 3 | 25 (15.7) | |
| 4 + 4 | 8 (5) | |
| 3 + 5 | 8 (5) | |
| 4 + 5 | 10 (6.3) | |
| 5 + 4 | 3 (1.9) | |
| 5 + 5 | 0 (0) | |
| Gleason score out of lesion | No PCa | 23 (14.5) |
| 3 + 3 | 38 (23.9) | |
| 3 + 4 | 44 (27.7) | |
| 4 + 3 | 27 (17) | |
| 4 + 4 | 9 (5.7) | |
| 3 + 5 | 5 (3.1) | |
| 4 + 5 | 6 (3.8) | |
| 5 + 4 | 6 (3.8) | |
| 5 + 5 | 1 (0.6) | |
| Pathological Gleason score | 3 + 3 | 26 (16.4) |
| 3 + 4 | 80 (50.3) | |
| 4 + 3 | 31 (19.5) | |
| 4 + 4 | 3 (1.9) | |
| 3 + 5 | 4 (2.5) | |
| 5 + 3 | 1 (0.6) | |
| 4 + 5 | 12 (7.5) | |
| 5 + 4 | 2 (1.3) | |
| 5 + 5 | 0 (0) | |
| pT-stage | pT2 | 91 (57.2) |
| ≥pT3 | 68 (42.8) |
| Highest GS regardless of SB or TB (A) | Highest GS in TB (B) | Highest GS in SB (C) | p Value A vs. B vs. C | p Value A vs. B | p Value A vs. C | p Value B vs. C | |
|---|---|---|---|---|---|---|---|
| Concordance | 79 (49.7%) | 73 (45.9%) | 59 (37.1%) | 0.068 | 0.6 | 0.03 | 0.14 |
| Upgrading | 24 (15.1%) | 53 (33.3%) | 59 (37.1%) | <0.001 | <0.001 | <0.001 | 0.5 |
| Downgrading | 56 (35.2%) | 33 (20.8%) | 41 (25.8%) | 0.01 | <0.01 | 0.09 | 0.4 |
| Significant upgrading | 5 (3.0%) | 5 (3.0%) | 8 (4.8%) | 0.6 | 1 | 0.6 | 0.6 |
| Significant downgrading | 19 (11.5%) | 11 (6.7%) | 13 (7.9%) | 0.3 | 0.2 | 0.4 | 0.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wenzel, M.; Preisser, F.; Wittler, C.; Hoeh, B.; Wild, P.J.; Tschäbunin, A.; Bodelle, B.; Würnschimmel, C.; Tilki, D.; Graefen, M.; et al. Correlation of MRI-Lesion Targeted Biopsy vs. Systematic Biopsy Gleason Score with Final Pathological Gleason Score after Radical Prostatectomy. Diagnostics 2021, 11, 882. https://doi.org/10.3390/diagnostics11050882
Wenzel M, Preisser F, Wittler C, Hoeh B, Wild PJ, Tschäbunin A, Bodelle B, Würnschimmel C, Tilki D, Graefen M, et al. Correlation of MRI-Lesion Targeted Biopsy vs. Systematic Biopsy Gleason Score with Final Pathological Gleason Score after Radical Prostatectomy. Diagnostics. 2021; 11(5):882. https://doi.org/10.3390/diagnostics11050882
Chicago/Turabian StyleWenzel, Mike, Felix Preisser, Clarissa Wittler, Benedikt Hoeh, Peter J. Wild, Alexandra Tschäbunin, Boris Bodelle, Christoph Würnschimmel, Derya Tilki, Markus Graefen, and et al. 2021. "Correlation of MRI-Lesion Targeted Biopsy vs. Systematic Biopsy Gleason Score with Final Pathological Gleason Score after Radical Prostatectomy" Diagnostics 11, no. 5: 882. https://doi.org/10.3390/diagnostics11050882
APA StyleWenzel, M., Preisser, F., Wittler, C., Hoeh, B., Wild, P. J., Tschäbunin, A., Bodelle, B., Würnschimmel, C., Tilki, D., Graefen, M., Becker, A., Karakiewicz, P. I., Chun, F. K. H., Kluth, L. A., Köllermann, J., & Mandel, P. (2021). Correlation of MRI-Lesion Targeted Biopsy vs. Systematic Biopsy Gleason Score with Final Pathological Gleason Score after Radical Prostatectomy. Diagnostics, 11(5), 882. https://doi.org/10.3390/diagnostics11050882





