Phenotypic Differences in a PRPH2 Mutation in Members of the Same Family Assessed with OCT and OCTA
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Initial Diagnosis
2.2. OCT and OCTA
3. Results
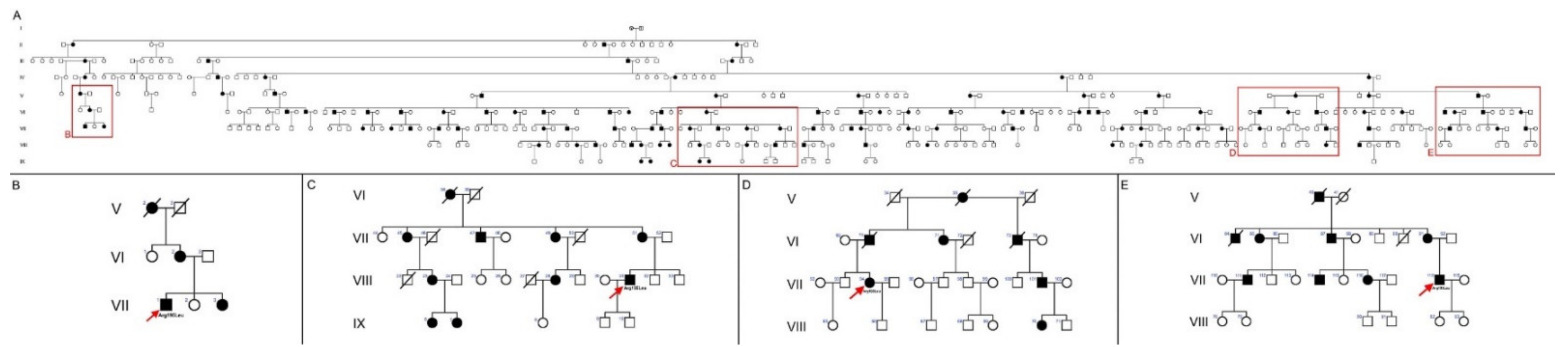
3.1. Pedigree of the Spanish Family with the PRPH2 Gene Mutation
3.2. Clinical Findings and Evolution of the Disease
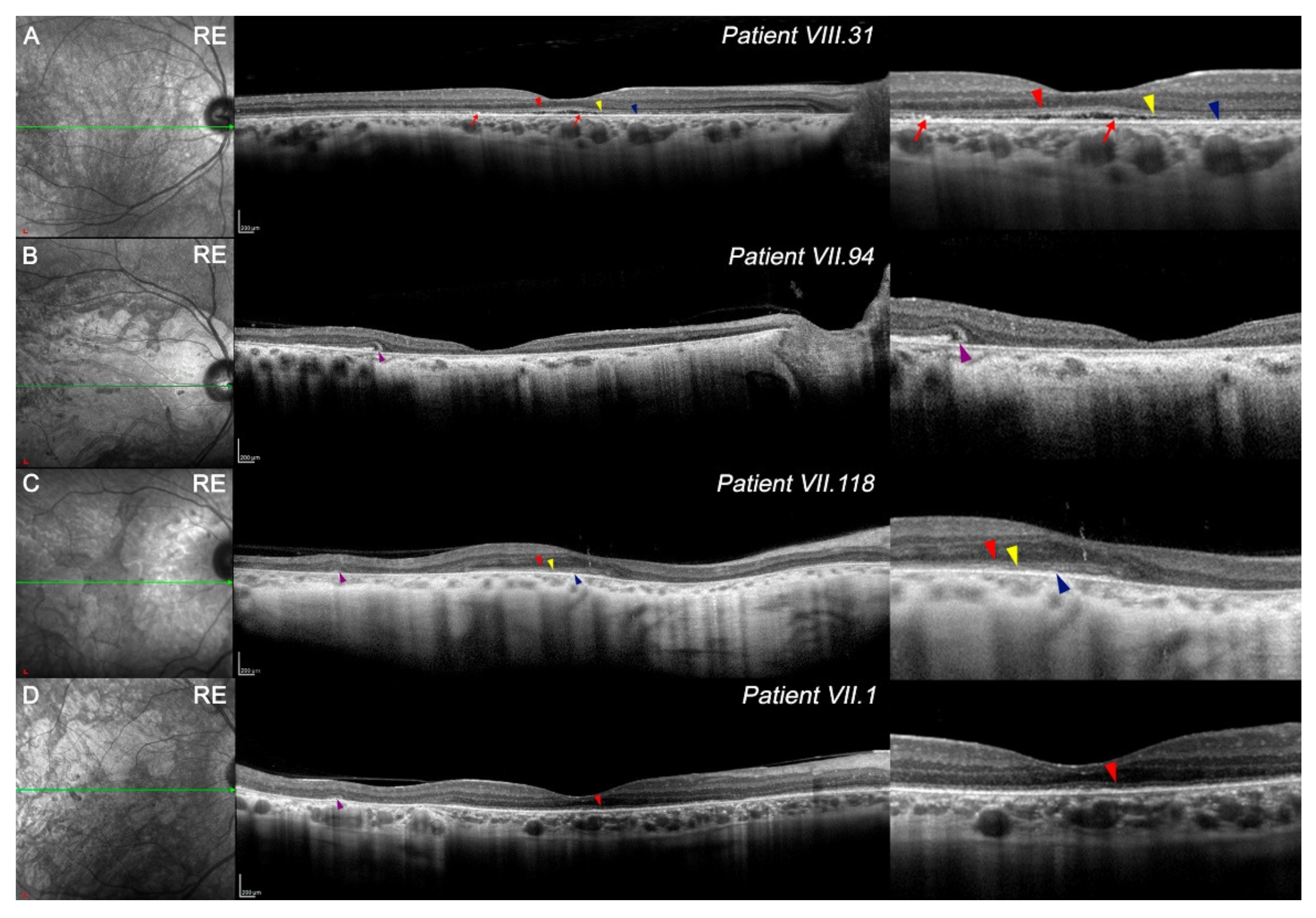
3.3. Features of OCT in CACD Patients: Macular Study and Retinal Tomography
3.4. OCTA in PRPH2 Gene Mutation Patients
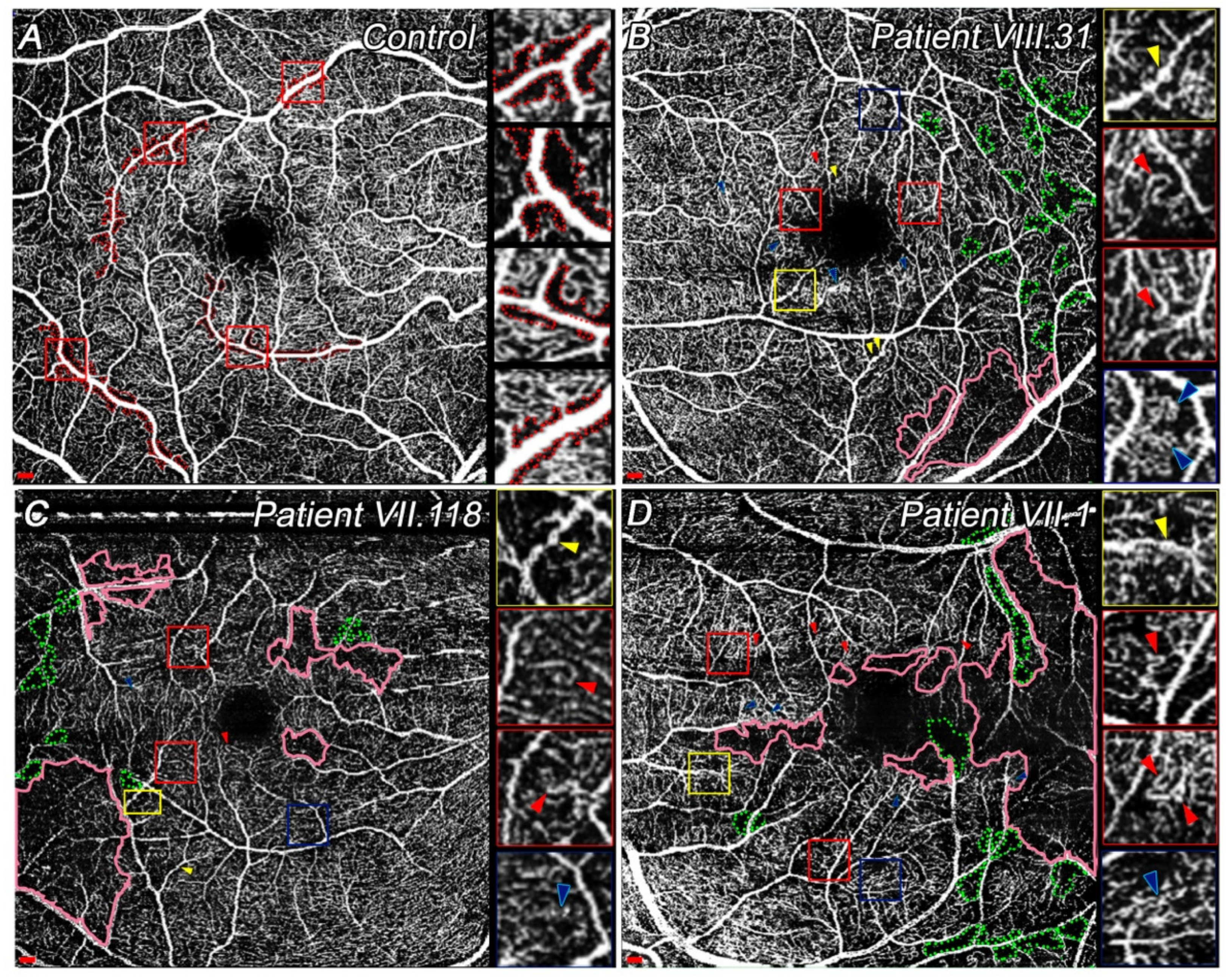
3.4.1. Quantitative and Qualitative Differences Found at SVP among Patients
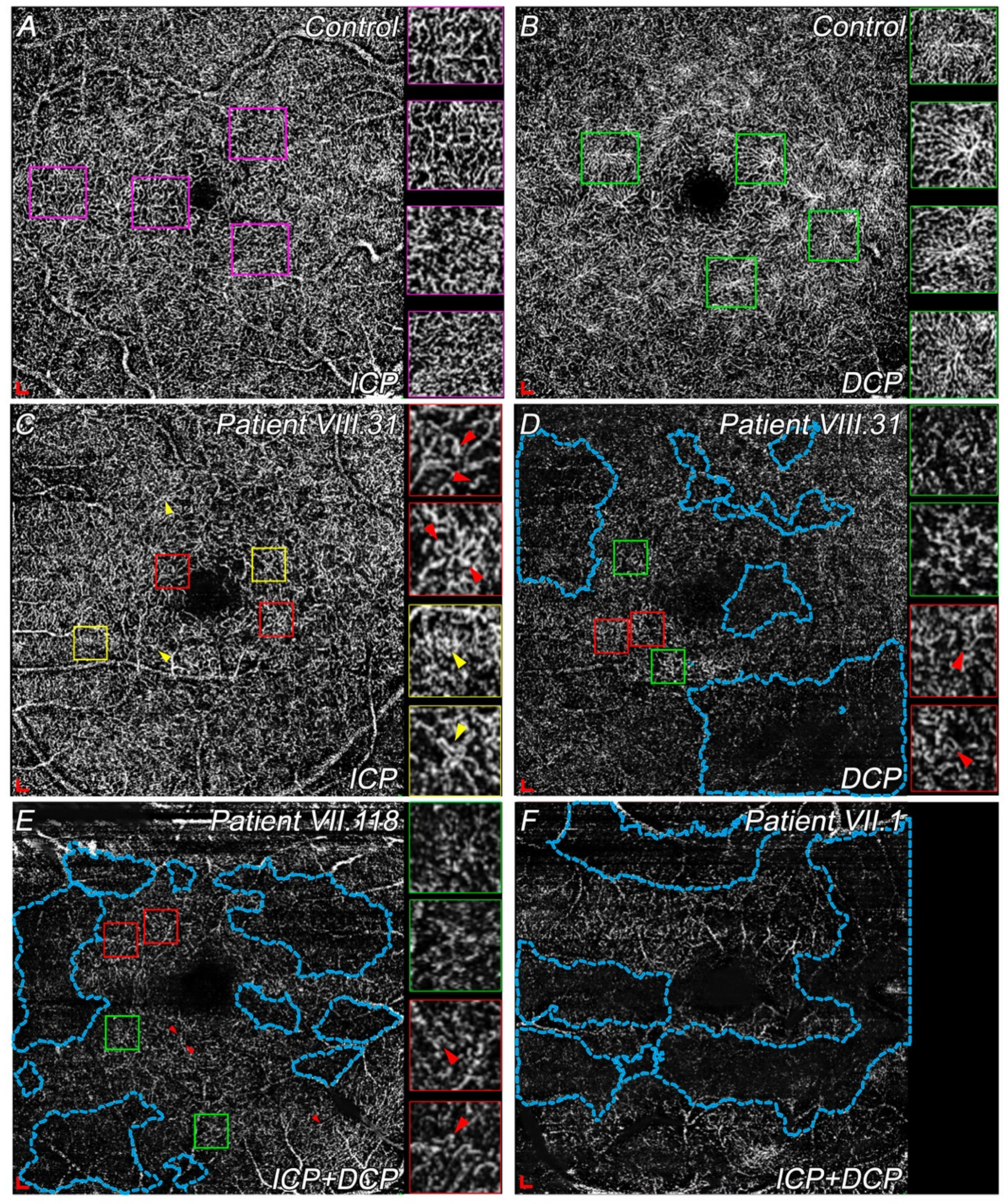
3.4.2. Differences in ICP and DCP among Choroidal Dystrophies Patients Compared with Controls
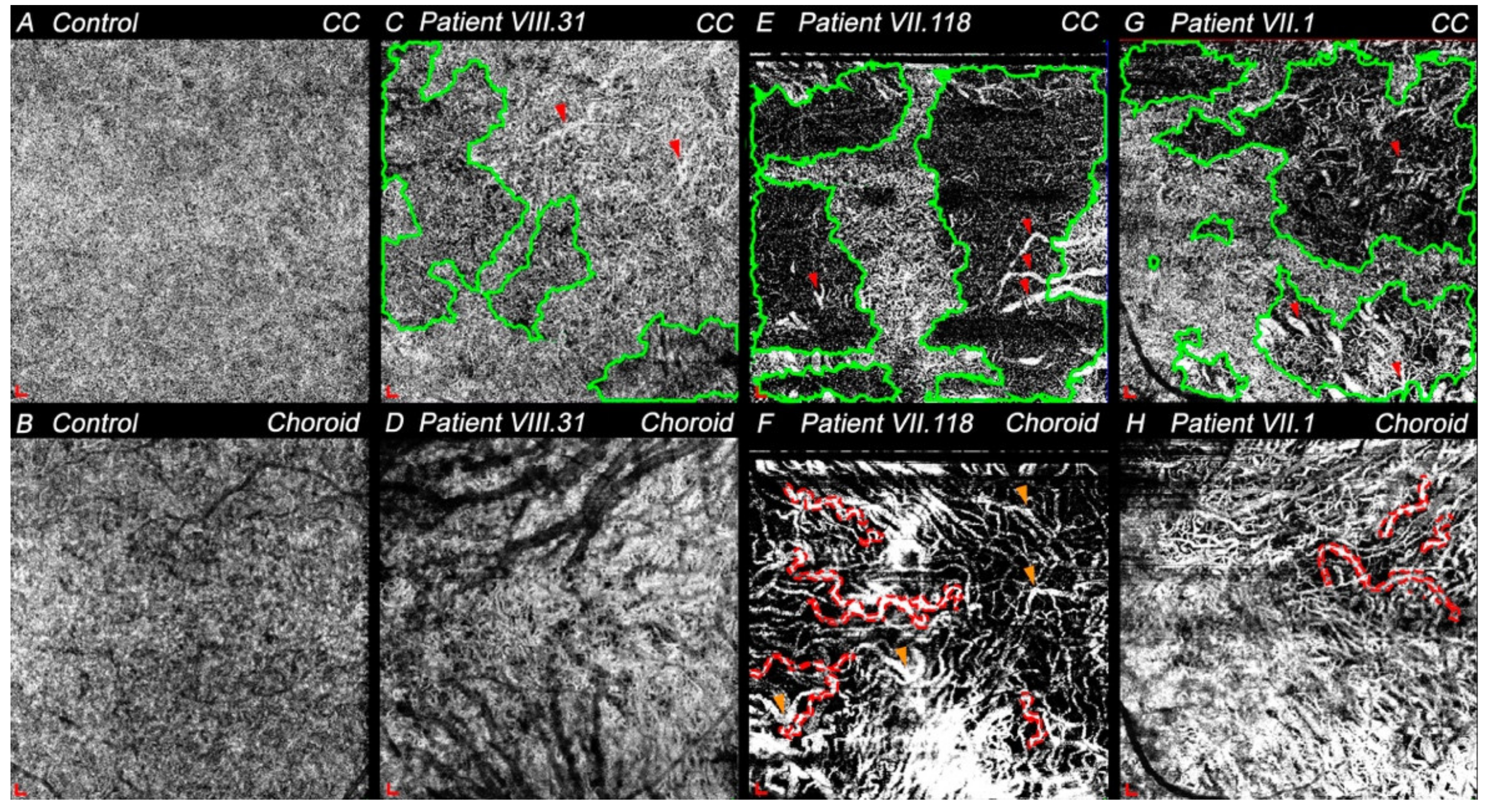
3.4.3. Choriocapillaris and Choroid Degeneration in CACD and ECA Patients
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Genead, M.A.; Fishman, G.A.; Grover, S. Hereditary Choroidal Diseases, 5th ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2012; Volume 2, ISBN 9781455707379. [Google Scholar]
- Sorsby, A. Choroidal angio-sclerosis with special reference to its hereditary character. Br. J. Ophthalmol. 1939, 23, 433–444. [Google Scholar] [CrossRef][Green Version]
- Ashton, N. Central areolar choroidal sclerosis a histo-pathological study. Br. J. Ophthalmol. 1953, 37, 140–147. [Google Scholar] [CrossRef]
- Ferry, A.P.; Llovera, I.; Shafer, D.M.; York, N. Central Areolar Choroidal Dystrophy. Arch. Ophthalmol. 1972, 88, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Hoyng, C.B.; Deutman, A.F. The development of central areolar choroidal dystrophy. Graefe’s Arch. Clin. Exp. Ophthalmol. 1996, 234, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Boon, C.J.F.; Klevering, B.J.; Cremers, F.P.M.; Zonneveld-Vrieling, M.N.; Theelen, T.; Den Hollander, A.I.; Hoyng, C.B. Central Areolar Choroidal Dystrophy. Ophthalmology 2009, 116, 771–782.e1. [Google Scholar] [CrossRef]
- Smailhodzic, D.; Fleckenstein, M.; Theelen, T.; Boon, C.J.F.; van Huet, R.A.C.; van de Ven, J.P.H.; Den Hollander, A.I.; Schmitz-Valckenberg, S.; Hoyng, C.B.; Weber, B.H.F.; et al. Central areolar choroidal dystrophy (CACD) and age-related macular degeneration (AMD): Differentiating characteristics in multimodal imaging. Investig. Ophthalmol. Vis. Sci. 2011, 52, 8908–8918. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T.; Peachey, N.S.; Li, S.; Goto, Y.; Cao, Y.; Naash, M.I. The effect of peripherin/rds haploinsufficiency on rod and cone photoreceptors. J. Neurosci. 1997, 17, 8118–8128. [Google Scholar] [CrossRef] [PubMed]
- Wells, J.; Wroblewski, J.; Keen, J.; Inglehearn, C.; Jubb, C.; Eckstein, A.; Jay, M.; Arden, G.; Bhattacharya, S.; Fitzke, F.; et al. Mutations in the human retinal degeneration slow (RDS) gene can cause either retinitis pigmentosa or macular dystrophy. Nat. Genet. 1993, 3, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Daftarian, N.; Mirrahimi, M.; Sabbaghi, H.; Moghadasi, A.; Zal, N.; Dehghan Banadaki, H.; Ahmadieh, H.; Suri, F. PRPH2 mutation as the cause of various clinical manifestations in a family affected with inherited retinal dystrophy. Ophthalmic Genet. 2019, 40, 436–442. [Google Scholar] [CrossRef]
- Matioli da Palma, M.; Martin, D.; Vallin Salles, M.; Teixeira Motta, F.L.; Abujamra, S.; Ferraz Sallum, J.M. Retinal dystrophies and variants in PRPH2. Arq. Bras. Oftalmol. 2019, 82, 158–160. [Google Scholar]
- Yanagihashi, S.; Nakazawa, M.; Kurotaki, J.; Sato, M.; Miyagawa, Y.; Ohguro, H. Autosomal dominant central areolar choroidal dystrophy and a novel Arg195Leu mutation in the peripherin/RDS gene. Arch. Ophthalmol. 2003, 121, 1458–1461. [Google Scholar] [CrossRef][Green Version]
- Keilhauer, C.N.; Meigen, T.; Weber, B.H.F.F. Clinical findings in a multigeneration family with autosomal dominant central areolar choroidal dystrophy associated with an Arg195Leu mutation in the peripherin/RDS gene. Arch. Ophthalmol. 2006, 124, 1020–1027. [Google Scholar] [CrossRef] [PubMed]
- Coco, R.M.; Tellería, J.J.; Sanabria, M.R.; Rodríguez-Rúa, E.; García, M.T. PRPH2 (Peripherin/RDS) mutations associated with different macular dystrophies in a Spanish population: A new mutation. Eur. J. Ophthalmol. 2010, 20, 724–732. [Google Scholar] [CrossRef] [PubMed]
- Coco-Martin, R.M.; Sanchez-Tocino, H.T.; Desco, C.; Usategui-Martín, R.; Tellería, J.J. PRPH2-related retinal diseases: Broadening the clinical spectrum and describing a new mutation. Genes 2020, 11, 773. [Google Scholar] [CrossRef]
- Mcculloch, D.L.; Marmor, M.F.; Brigell, M.G.; Hamilton, R.; Holder, G.E.; Tzekov, R.; Bach, M. ISCEV STANDARDS ISCEV Standard for full-field clinical electroretinography (2015 update). Doc. Ophthalmol. 2015, 130, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Zudaire, E.; Gambardella, L.; Kurcz, C.; Vermeren, S. A computational tool for quantitative analysis of vascular networks. PLoS ONE 2011, 6, e27385. [Google Scholar] [CrossRef] [PubMed]
- Piguet, B.; Hon, E.; Munier, F.L.; Grounauer, P.A.; Niemeyer, G.; Butler, N.; Schorderet, D.F.; Sheffield, V.C.; Stone, E.M. Full characterization of the maculopathy associated with an Arg-172-Trp mutation in the RDS/peripherin gene. Ophthalmic Genet. 1996, 17, 175–186. [Google Scholar] [CrossRef]
- Downes, S.M.; Fitzke, F.W.; Holder, G.E.; Payne, A.M.; Bessant, D.A.R.R.; Bhattacharya, S.S.; Bird, A.C. Clinical Features of Codon 172 RDS Macular Dystrophy. Arch. Ophthalmol. 1999, 117, 1373–1383. [Google Scholar] [CrossRef] [PubMed]
- Boon, C.J.F.F.; den Hollander, A.I.; Hoyng, C.B.; Cremers, F.P.M.M.; Klevering, B.J.; Keunen, J.E.E.E.; Den Hollander, A.I.; Hoyng, C.B.; Cremers, F.P.M.M.; Klevering, B.J.; et al. The spectrum of retinal dystrophies caused by mutations in the peripherin/RDS gene. Prog. Retin. Eye Res. 2008, 27, 213–235. [Google Scholar] [CrossRef]
- Tsujikawa, M.; Wada, Y.; Sukegawa, M.; Sawa, M.; Gomi, F.; Nishida, K.; Tano, Y. Age at Onset Curves of Retinitis Pigmentosa. Arch. Ophthalmol. 2008, 126, 337–340. [Google Scholar] [CrossRef] [PubMed]
- Kolb, H.; Nelson, R.F.; Ahnelt, P.K.; Ortuño-Lizarán, I.; Cuenca, N. The Architecture of the Human Fovea. In Webvision: The Organization of the Retina and Visual System; Kolb, H., Fernandez, E., Nelson, R., Eds.; University of Utah Health Sciences Center: Salt Lake City, UT, USA, 2020. [Google Scholar]
- Leveille, A.S.; Morse, P.H.; Kiernan, J.P. Autosomal dominant central pigment epithelial and choroidal degeneration. Ophthalmology 1982, 89, 1407–1413. [Google Scholar] [CrossRef]
- Noble, K.G.; Carr, R.E.; Siegel, I.M. Fluorescein angiography of the hereditary choroidal dystrophies. Br. J. Ophthalmol. 1977, 61, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Noble, K.G. Central areolar choroidal dystrophy. Am. J. Ophthalmol. 1977, 84, 310–318. [Google Scholar] [CrossRef]
- Guigui, B.; Semoun, O.; Querques, G.; Coscas, G.; Soubrane, G.; Souied, E.H. Indocyanine green angiography features of central areolar choroidal dystrophy. Retin. Cases Brief Rep. 2009, 3, 434–437. [Google Scholar] [CrossRef]
- Sorsby, A.; Crick, R.P. Central areolar choroidal sclerosis. Br. J. Ophthalmol. 1953, 37, 129–139. [Google Scholar] [CrossRef]
- CARR, R.E. Central Areolar Choroidal Dystrophy. Arch. Ophthalmol. 1965, 73, 32–35. [Google Scholar] [CrossRef] [PubMed]
- Battaglia Parodi, M.; Cicinelli, M.V.; Iacono, P.; Bolognesi, G.; Bandello, F. Multimodal imaging of foveal cavitation in retinal dystrophies. Graefe’s Arch. Clin. Exp. Ophthalmol. 2017, 255, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Nõupuu, K.; Lee, W.; Zernant, J.; Tsang, S.H.; Allikmets, R.; Nôupuu, K.; Lee, W.; Zernant, J.; Tsang, S.H.; Allikmets, R. Structural and Genetic Assessment of the ABCA4- Associated Optical Gap Phenotype. Investig. Ophthalmol. Vis. Sci. 2014, 55, 7217–7226. [Google Scholar] [CrossRef] [PubMed]
- Cuenca, N.; Ortuño-Lizarán, I.; Pinilla, I. Cellular Characterization of OCT and Outer Retinal Bands Using Specific Immunohistochemistry Markers and Clinical Implications. Ophthalmology 2018, 125, 407–422. [Google Scholar] [CrossRef] [PubMed]
- Cuenca, N.; Ortuño-Lizarán, I.; Sánchez-Sáez, X.; Kutsyr, O.; Albertos-Arranz, H.; Fernández-Sánchez, L.; Martínez-Gil, N.; Noailles, A.; López-Garrido, J.A.; López-Gálvez, M.; et al. Interpretation of OCT and OCTA images from a histological approach: Clinical and experimental implications. Prog. Retin. Eye Res. 2020, 77, 100828. [Google Scholar] [CrossRef] [PubMed]
- Mitamura, Y.; Mitamura-Aizawa, S.; Katome, T.; Naito, T.; Hagiwara, A.; Kumagai, K.; Yamamoto, S. Photoreceptor impairment and restoration on optical coherence tomographic image. J. Ophthalmol. 2013, 2013, 518170. [Google Scholar] [CrossRef]
- Sandberg, M.A.; Brockhurst, R.J.; Gaudio, A.R.; Berson, E.L. The association between visual acuity and central retinal thickness in retinitis pigmentosa. Investig. Ophthalmol. Vis. Sci. 2005, 46, 3349–3354. [Google Scholar] [CrossRef] [PubMed]
- Campello, L.; Kutsyr, O.; Noailles, A.; Michalska, P.; Fernández-Sánchez, L.; Martínez-Gil, N.; Ortuño-Lizarán, I.; Sánchez-Sáez, X.; de Juan, E.; Lax, P.; et al. New Nrf2-Inducer Compound ITH12674 Slows the Progression of Retinitis Pigmentosa in the Mouse Model rd10. Cell Physiol. Biochem. 2020, 54, 142–159. [Google Scholar] [PubMed]
- Fernandez-Sánchez, L.; Lax, P.; Esquiva, G.; Martín-Nieto, J.; Pinilla, I.; Cuenca, N. Safranal, a Saffron Constituent, Attenuates Retinal Degeneration in P23H Rats. PLoS ONE 2012, 7, e43074. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Sánchez, L.; Bravo-Osuna, I.; Lax, P.; Arranz-Romera, A.; Maneu, V.; Esteban-Pérez, S.; Pinilla, I.; del Puebla-González, M.; Herrero-Vanrell, R.; Cuenca, N. Controlled delivery of tauroursodeoxycholic acid from biodegradable microspheres slows retinal degeneration and vision loss in P23H rats. PLoS ONE 2017, 12, e0177998. [Google Scholar] [CrossRef]
- Toto, L.; Borrelli, E.; Mastropasqua, R.; Senatore, A.; Di Antonio, L.; Di Nicola, M.; Carpineto, P.; Mastropasqua, L. Macular features in retinitis pigmentosa: Correlations among ganglion cell complex thickness, capillary density, and macular function. Investig. Ophthalmol. Vis. Sci. 2016, 57, 6360–6366. [Google Scholar] [CrossRef]
- Toto, L.; Borrelli, E.; Di Antonio, L.; Carpineto, P.; Mastropasqua, R. Retinal vascular plexuses’ changes in dry age-related macular degeneration, evaluated by means of optical coherence tomography angiography. Retina 2016, 36, 1566–1572. [Google Scholar] [CrossRef] [PubMed]
- Lavia, C.; Couturier, A.; Erginay, A.; Dupas, B.; Tadayoni, R.; Gaudric, A. Reduced vessel density in the superficial and deep plexuses in diabetic retinopathy is associated with structural changes in corresponding retinal layers. PLoS ONE 2019, 14, e0219164. [Google Scholar] [CrossRef] [PubMed]
- Mastropasqua, R.; D’Aloisio, R.; De Nicola, C.; Ferro, G.; Senatore, A.; Libertini, D.; Di Marzio, G.; Di Nicola, M.; Di Martino, G.; Di Antonio, L.; et al. Widefield swept source OCTA in retinitis pigmentosa. Diagnostics 2020, 10, 50. [Google Scholar] [CrossRef]
- Mo, S.; Krawitz, B.; Efstathiadis, E.; Geyman, L.; Weitz, R.; Chui, T.Y.P.; Carroll, J.; Dubra, A.; Rosen, R.B. Imaging foveal microvasculature: Optical coherence tomography angiography versus adaptive optics scanning light ophthalmoscope fluorescein angiography. Investig. Ophthalmol. Vis. Sci. 2016, 57, OCT130–OCT140. [Google Scholar] [CrossRef]
- Wu, B.; Zhu, W.; Shi, F.; Zhu, S.; Chen, X. Automatic detection of microaneurysms in retinal fundus images. Comput. Med. Imaging Graph. 2017, 55, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Pappuru, R.K.R.; Ribeiro, L.; Lobo, C.; Alves, D.; Cunha-Vaz, J. Microaneurysm turnover is a predictor of diabetic retinopathy progression. Br. J. Ophthalmol. 2019, 103, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Matsunaga, D.R.; Yi, J.J.; De Koo, L.O.; Ameri, H.; Puliafito, C.A.; Kashani, A.H. Optical coherence tomography angiography of diabetic retinopathy in human subjects. Ophthalmic Surg. Lasers Imaging Retin. 2015, 46, 796–805. [Google Scholar] [CrossRef] [PubMed]
- Tam, J.; Dhamdhere, K.P.; Tiruveedhula, P.; Lujan, B.J.; Johnson, R.N.; Bearse, M.A., Jr.; Adams, A.J.; Roorda, A. Subclinical Capillary Changes in Non Proliferative Diabetic Retinopathy. Optom. Vis. Sci. 2012, 89, 692–703. [Google Scholar] [CrossRef]
- Arrigo, A.; Romano, F.; Albertini, G.; Aragona, E.; Bandello, F.; Battaglia Parodi, M. Vascular Patterns in Retinitis Pigmentosa on Swept-Source Optical Coherence Tomography Angiography. J. Clin. Med. 2019, 8, 1425. [Google Scholar] [CrossRef] [PubMed]
- Hagag, A.M.; Wang, J.; Lu, K.; Harman, G.; Weleber, R.G.; Huang, D.; Yang, P.; Pennesi, M.E.; Jia, Y. Projection-Resolved Optical Coherence Tomographic Angiography of Retinal Plexuses in Retinitis Pigmentosa. Am. J. Ophthalmol. 2019, 204, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Bax, N.M.; Valkenburg, D.; Lambertus, S.; Klevering, B.J.; Boon, C.J.; Holz, F.G.; Lindner, M. Foveal Sparing in Central Retinal Dystrophies. Investig. Ophthalmol. Vis. Sci. 2019, 60, 3456–3467. [Google Scholar] [CrossRef] [PubMed]
- Gould, D.J.; Vadakkan, T.J.; Poché, R.A.; Dickinson, M.E. Multifractal and lacunarity analysis of microvascular morphology and remodeling. Microcirculation 2011, 18, 136–151. [Google Scholar] [CrossRef] [PubMed]
| Patient/Sex | Age at Diagnosis | VA at Diagnosis | Current Age | Current VA | Visual Field Results | Age at ERG/ERG Results | |||
|---|---|---|---|---|---|---|---|---|---|
| RE | LE | RE | LE | 4–5 Years after Diagnosis (RE/LE) (dB) | 12–15 Years after Diagnosis (RE/LE) (dB) | ||||
| VII.1/M | 39 | 20/40 | 20/50 | 46 | 20/100 | 20/100 | 24-2 MD: −27.22/−26.25 PSD: 6.91/7.30 VFI: 16%/18% | – | 39/Scotopic ERG reduced. Photopic responses decreased (absent in red light) |
| VIII.31/M | 33 | 20/50+2 | 20/20−2 | 42 | 20/125−1 | 20/50 | – | – | 33/Scotopic ERG acceptable answer. Photopic responses notably reduced. |
| VII.94/F | 33 | 20/100 | 20/63−1 | 57 | 20/125 | 20/200−2 | 24-2 MD: −14/−14.12 PSD: 13.6/14.06 | 24-2 MD: −17.81/−15.51 PSD: 11.77/10.81 | 37/Scotopic ERG with a minimal reduction in amplitude. Photopic ERG and Flicker highly reduced. |
| VII.118/M | 37 | 20/40+1 | 20/32+3 | 50 | 20/63 | 20/25 | – | 24-2 MD: −28.58/−28.49 VFI: 10%/11.5% | 37/Scotopic ERG with a reduction in amplitude and latencies. Photopic and Flicker ERG absent. |
| Patients | Total Retinal Thickness (µm) | Outer Retina (µm) | Inner Retina (µm) | Choroid (µm) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Measurements at FAZ | From FAZ limits (500 µm) to 1.2 mm (Parafovea Limits) | From 1.2 mm to 2.8 mm (Perifovea) | From FAZ Limits (500 um) to 2.8 mm (Perifovea Limits) | From Foveola (0 um) to 2.8 mm (Perifovea Limits) | |||||
| Temporal | Nasal | Temporal | Nasal | Temporal | Nasal | Temporal | Nasal | ||
| Control group | 230 ± 20 | 194 ± 8 | 194 ± 7 | 150 ± 10 | 160 ± 10 | 141 ± 6 | 165 ± 9 | 280 ± 40 | 260 ± 40 |
| VIII.31 | 137.7 ± 0.5 | 94 ± 2 | 94 ± 1 | 87 ± 2 | 98 ± 7 | 130 ± 10 | 150 ± 6 | 230 ± 60 | 290 ± 50 |
| VII.94 | 20 ± 20 | 4 ± 5 | 13 ± 2 | 40 ± 20 | 4 ± 8 | 70 ± 10 | 90 ± 10 | 230 ± 50 | 120 ± 50 |
| VII.118 | 173 ± 5 | 120 ± 10 | 30 ± 30 | 30 ± 30 | 20 ± 20 | 110 ± 20 | 145 ± 9 | 140 ± 10 | 140 ± 20 |
| VII.1 | 119 ± 2 | 89 ± 4 | 90 ± 10 | 40 ± 30 | 50 ± 10 | 140 ± 20 | 210 ± 20 | 240 ± 20 | 220 ± 30 |
| Patients | SVP Parameters | ICP-DCP Parameters | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Avascular Area (mm2) | Angio-Tool Measurements | FAZ (mm2) | ||||||||
| RE | LE | Mean ± SD | Vessel Density (% Vessels) | Total Vessels Length (mm) | Average Vessels Length (mm) | Mean Lacunarity (Λ) | RE | LE | Mean ± SD | |
| Control group | 0.4 ± 0.1 | 0.4 ± 0.2 | 0.370 ± 0.001 | 53 ± 2 | 530 ± 30 | 10 ± 10 | 0.022 ± 0.003 | 0.3 ± 0.1 | 0.3 ± 0.1 | 0.25 ± 0.01 |
| VIII.31 | 0.61 | 0.63 | 0.62 ± 0.01 | 47 ± 3 | 504 ± 7 | 4.0 ± 0.4 | 0.040 ± 0.003 | 0.39 | 0.47 | 0.43 ± 0.06 |
| VII.118 | 0.54 | 0.34 | 0.4 ± 0.1 | 39.4 ± 0.7 | 450 ± 10 | 1.6 ± 0.5 | 0.049 ± 0.001 | 0.42 | 0.32 | 0.37 ± 0.07 |
| VII.1 | 0.57 | 0.58 | 0.575 ± 0.007 | 41 ± 2 | 430 ± 30 | 2.40 ± 0.04 | 0.057 ± 0.001 | 0.46 | 0.47 | 0.465 ± 0.007 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Albertos-Arranz, H.; Sánchez-Sáez, X.; Martínez-Gil, N.; Pinilla, I.; Coco-Martin, R.M.; Delgado, J.; Cuenca, N. Phenotypic Differences in a PRPH2 Mutation in Members of the Same Family Assessed with OCT and OCTA. Diagnostics 2021, 11, 777. https://doi.org/10.3390/diagnostics11050777
Albertos-Arranz H, Sánchez-Sáez X, Martínez-Gil N, Pinilla I, Coco-Martin RM, Delgado J, Cuenca N. Phenotypic Differences in a PRPH2 Mutation in Members of the Same Family Assessed with OCT and OCTA. Diagnostics. 2021; 11(5):777. https://doi.org/10.3390/diagnostics11050777
Chicago/Turabian StyleAlbertos-Arranz, Henar, Xavier Sánchez-Sáez, Natalia Martínez-Gil, Isabel Pinilla, Rosa M. Coco-Martin, Jesús Delgado, and Nicolás Cuenca. 2021. "Phenotypic Differences in a PRPH2 Mutation in Members of the Same Family Assessed with OCT and OCTA" Diagnostics 11, no. 5: 777. https://doi.org/10.3390/diagnostics11050777
APA StyleAlbertos-Arranz, H., Sánchez-Sáez, X., Martínez-Gil, N., Pinilla, I., Coco-Martin, R. M., Delgado, J., & Cuenca, N. (2021). Phenotypic Differences in a PRPH2 Mutation in Members of the Same Family Assessed with OCT and OCTA. Diagnostics, 11(5), 777. https://doi.org/10.3390/diagnostics11050777








